Abstract
Exposure to atmospheric fine particulate matter (PM2.5) is a modifiable risk factor of cardiovascular disease. Ultrafine particles (UFP, diameter <0.1 μm), a subfraction of PM2.5, promote vascular oxidative stress and inflammatory responses. Epidemiologic studies suggest that PM exposure promotes vascular calcification. Here, we assessed whether UFP exposure promotes vascular calcification via NF-κB signaling. UFP exposure at 50 μg/ml increased alkaline phosphatase (ALP) activity by 4.4 ± 0.2-fold on day 3 (n = 3, P < 0.001) and matrix calcification by 3.5 ± 1.7-fold on day 10 (n = 4, P < 0.05) in calcifying vascular cells (CVC), a subpopulation of vascular smooth muscle cells with osteoblastic potential. Treatment of CVC with conditioned media derived from UFP-treated macrophages (UFP-CM) also led to an increase in ALP activities and matrix calcification. Furthermore, both UFP and UFP-CM significantly increased NF-κB activity, and cotreatment with an NF-κB inhibitor, JSH23, attenuated both UFP- and UFP-CM-induced ALP activity and calcification. When low-density lipoprotein receptor-null mice were exposed to UFP at 359.5 μg/m3 for 10 wk, NF-κB activation and vascular calcification were detected in the regions of aortic roots compared with control filtered air-exposed mice. These findings suggest that UFP promotes vascular calcification via activating NF-κB signaling.
Keywords: air pollution, vascular calcification, ultrafine particles, NF-κB, calcifying vascular cells
exposure to ambient particulate matter (PM) is recognized as a modifiable risk factor to cardiovascular morbidity and mortality (7). Ultrafine particles (UFP, dp < 0.1 μm), highly enriched in transition metals and redox-active organic chemicals (35, 61), promote vascular oxidative stress and inflammatory responses, leading to accelerated atherosclerosis lesion size in Apo-E null mice (4, 7, 26, 27). Vascular calcification is recognized as a distinct but relevant process to atherosclerosis (1, 48, 50, 57), beginning as early as the second decade of life, and increasing with age and lesion progression (51). Intimal calcification is further characterized in the advanced atherosclerotic plaques (1), closely associated with atherosclerotic burden and cardiovascular events (1, 46, 48, 50, 54, 57). Arterial calcification is increased in individuals exposed to urban air pollution (18, 22). Furthermore, the association between atmospheric particulate matter and coronary artery calcification increased by twofold in the urban city centers (39). Chronic traffic exposure was further linked with both coronary arterial and abdominal aortic calcification (2, 17). While epidemiological studies reveal the association of PM exposure with vascular calcification, the causative relation and the underlying mechanism(s) remain to be investigated.
To investigate the effects of UFP on vascular calcification, we used calcifying vascular cells (CVC). CVC have characteristics of mesenchymal stem cell and exhibits smooth muscle cell phenotype (6). CVC also have the capacity to differentiate into the osteoblast (1, 44) and produce mineralized nodular structures (6) akin to human calcified plaque (25, 45). Oxidized low-density lipoprotein (ox-LDL) was reported to induce CVC calcification (42). Thus CVC provide an in vitro model to assess the mechanisms underlying UFP-modulated vascular calcification.
NF-κB signaling is a major signal pathway that mediates proinflammatory responses (5, 12) and is implicated in the osteogenic differentiation of smooth muscle cells during vascular calcification (11, 16). Exposure to UFP activates NF-κB signaling, leading to inflammatory responses in human aortic endothelial cells (27). Whether UFP-induced NF-κB activity is involved in vascular smooth muscle cells osteogenic differentiation remains unknown. For this reason, CVC were used to assess UFP-mediated NF-κB signaling and vascular calcification. The findings provide new insights into the role of NF-κB signaling in UFP-induced vascular cell calcification.
MATERIALS AND METHODS
Materials.
Media (DMEM, M199, and RPMI-1640) were purchased from Invitrogen. FBS was purchased from Phenix Research. Antibody against phosphorylated-NF-κB-p65 (active NF-κB) was obtained from Cell Signaling. JSH-23 was purchased from Sigma.
Cell culture.
Primary bovine smooth muscle cells (BSMC) and CVC derived by dilutional cloning from bovine aortic smooth muscle cell cultures were kindly provided by Dr. Linda Demer at University of California, Los Angeles, CA. Cells were cultured in DMEM supplemented with 15% FBS. THP-1 cells were obtained from American Type of Culture Collection and maintained in RPMI 1640 supplemented with 15% FBS and with 50 uM β-mercaptoethanol.
Collection and preparation of UFP.
UFP were collected at the University of Southern California (USC) campus near downtown Los Angeles. The particles sampled represent a mixture of pollution sources, including fresh ambient PM emitted by heavy-duty diesel trucks, light duty gasoline vehicles, and ship emissions, as well as PM formed by photochemical oxidation of primary organic vapors. The USC site is therefore representative of the dominant outdoor sources influencing numerous urban regions in the US where high concentration of PM are freshly emitted from vehicular traffic in nearby freeways (59).
UFP were collected by a High-Volume Particle Sampler (31). Following collection, UFP samples were extracted from the Zefluor PTFE membrane filters (3 μm, 28139–597; Pall Life Sciences) by soaking for 30 min in ultrapure deionized Milli Q water followed by vortexing for 5 and 30 min of sonication. The aqueous suspensions were pooled and were kept at −20°C to retain chemical stability. The PM suspension was reaerosolized by the protocol described by Morgan et al. (33) and yielded highly concentrated PM for in vivo exposure in a controlled manner.
UFP exposure.
UFP suspensions were reaerosolized by a Vortran nebulizer (Vortran Medical Technology, Sacramento, CA) using compressed filtered air. Particles were diffusion dried by passing through silica gel, and static charges were removed (Po-210 neutralizer) before entering exposure chambers. Mice were exposed to PM-aerosolized air (or control filtered air) via whole body animal exposure chambers as described previously (33). Briefly, five Ldlr−/− male mice (C57BL/6J background) at 12 wk of age in each group were placed on a high-fat diet (HFD D12492: 5.24 kcal/g, 34.9 g% fat, 26.2 g% protein, and 26.2 g% carbohydrate; Research Diets) and exposed to filtered air (FA) or UFP at a targeted concentration of ∼400 μg/m3 for 5 h/day and 3 day/wk for 10 wk. A scanning mobility particle sizer (SMPS Model 3080; TSI) was deployed in parallel with the animal chambers to continuously monitor particle sizes and concentrations. The resuspended aerosol size distribution closely approximated airborne PM measured at the USC site as previously described (58). All studies were performed in the vivarium in the Ray R. Irani Building (www.cmb.usc.edu) in compliance with USC Institutional Animal Care and Use Committee protocol.
The mean exposure concentration of the reaerosolized UFP was 359.5 μg/m3, while the number concentration was 1.91 × 105 particles/cm3. The number concentration of FA was <100 particles/cm3 on average, and the mass concentrations of FA below detection limit. The particles were generated in a manner to establish a similar size distribution to ambient particles.
Immunohistochemistry and von Kossa staining.
After exposure to FA or UFP, mice were euthanized. The heart tissues containing aortic root were embedded in OCT and cryosectioned. Standard immunohistochemistry and von Kossa staining were performed in the pathology laboratory at USC, Health Science Campus. Red-colored chromogen was used as substrate for active NF-κB staining to differentiate positive staining from lipofuscin, a brown-colored molecule commonly observed in the valvular tissue. Semiquantitative analyses of NF-κB and von Kossa staining were performed using ImageJ software (NIH v1.46r).
Preparation of conditioned media from macrophages.
THP-1 cells cultured in six-well plates were differentiated into macrophages in vitro by addition of 100 ng/ml of PMA into culture media for 3 days. The cells were then rinsed three times with PBS and treated with control buffer or UFP (50 μg/ml) in M199/2.5% FBS for 4 h. The treatment media were removed, and the cells were cultured with fresh M199/2.5% FBS for 24 h. The media were then collected and used as conditioned media (CM).
ALP activity assay.
ALP activity was used as an early osteoblastic differentiation marker (19, 29, 60). CVC were cultured in 96-well plate for 3 days and then treated with control buffer or UFP (50 μg/ml) and/or NF-κB inhibitor, JSH23 (15 uM), in M199/2.5% FBS or conditioned media for 3 days. After treatment, cells were washed with PBS for three times and lysed with 50 μl ALP lysis buffer (150 mM NaCl, 3 mM NaHCO3, and 0.2% Triton X-100) for 30 min at 37°C. Prechilled p-nitrophenyl phosphate substrate (100 μl of 2.5 mg/ml prepared in 200 mM 2-amino-1-propanol/5 mM MgCl2 at pH 10.5) was added. After incubating at 37°C for 5–10 min, absorbance was measured at 405 nm.
In vitro calcification assay.
CVC were cultured in 96-well plate for 3 days and then treated with control buffer, UFP (50 μg/ml), and/or the NF-κB inhibitor JSH23 (15 uM), in M199/2.5% FBS/5 mM of β-glycerophosphate or CM for 10 days. Treatment media were changed every 3 days during the experimental period. After treatment for 10 days, cells were washed three times with PBS and incubated with 0.6 N HCl overnight. Calcium content was determined using o-Cresolphthalein Complexone method following manufacturer's instruction (Teco Diagnostics).
NF-κB reporter gene assay.
CVC cells were grown to subconfluence in 24-well plates. The cells were infected overnight with Adenovirus-NF-κB-Luc (Vector Biolabs) at multiple of infection of 1:100. The cells were then treated overnight with agents in M199 containing 0.1% FBS or conditioned media. The cells were rinsed with PBS for three times and lysed in 100 μl of passive lysis buffer (Promega), and luciferase activities were quantified with a luminometer using Bright-Glow substrate (Promega).
Statistic analysis.
Data were expressed as means ± SD. Multiple comparisons were made by one-way ANOVA, and statistical significance for pair-wise comparison was determined using the Tukey test. A P value < 0.05 was considered statistically significant.
RESULTS
Characteristics of UFP.
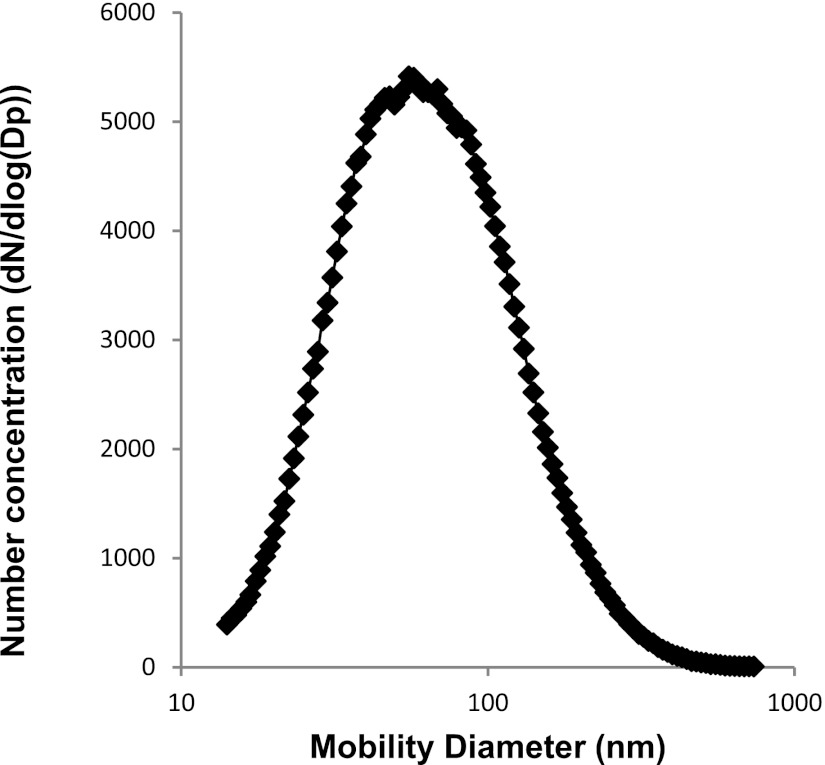
The UFPs were collected from USC campus near downtown Los Angeles as previously reported (58). The main chemical constituents in UFP were analyzed in terms of inorganic ions of primary interest (NO3 −, SO42−, and NH4+); inorganic, organic, elemental, and total carbon content; and selected metal species (Table 1). The size distribution of the UFP was comparable to the representative UFP collected downtown Los Angeles (Fig. 1). As shown in Fig. 1, the median diameter of the aerosol size distribution is ∼45 nm (with a geometric standard deviation = 1.9).
Table 1.
Chemical composition of UFP
| Fraction of the Selected Chemical Constituents of the UFP | ||
|---|---|---|
| Concentration | SD | |
| Inorganic ions, fraction in total PM mass, μg/μg | ||
| Nitrate | 0.095 | 0.012 |
| Sulfate | 0.142 | 0.020 |
| Ammonium | 0.026 | 0.004 |
| EC/OC, fraction in total PM mass, μg/μg | ||
| Organic carbon | 0.190 | 0.010 |
| Elemental carbon | 9.67E-03 | 4.83E-04 |
| Total carbon | 0.200 | 0.010 |
| Selected elements and metals, fraction in total PM mass, ng/μg | ||
| Na | 35.37 | 1.05 |
| Mg | 4.96 | 0.26 |
| Al | 0.49 | 0.03 |
| K | 9.37 | 1.01 |
| Ca | 28.59 | 1.52 |
| Ti | 1.80E-03 | 1.71E-03 |
| V | 1.06E-01 | 5.29E-03 |
| Cr | 1.68E-02 | 1.73E-03 |
| Mn | 0.287 | 0.014 |
| Fe | 0.283 | 0.019 |
| Ni | 0.082 | 0.006 |
| Cu | 1.112 | 0.052 |
| Zn | 2.68 | 0.14 |
| Mo | 4.45E-02 | 1.37E-03 |
| Cd | 8.47E-03 | 7.54E-04 |
| Ba | 0.80 | 0.05 |
| Pt | 5.53E-05 | 2.42E-04 |
| Pb | 0.026 | 0.001 |
UFP, ultrafine particles; PM, particulate matter; OC, organic carbon; EC, elemental carbon.
Fig. 1.
Size distribution of ultrafine particles (UFP). Size distribution of UFP was comparable to the representative UFP collected in downtown Los Angeles with a median diameter of ∼45 nm (geometric standard deviation = 1.9).
Direct effects of UFP on osteoblastic differentiation and matrix calcification of vascular smooth muscle cells.
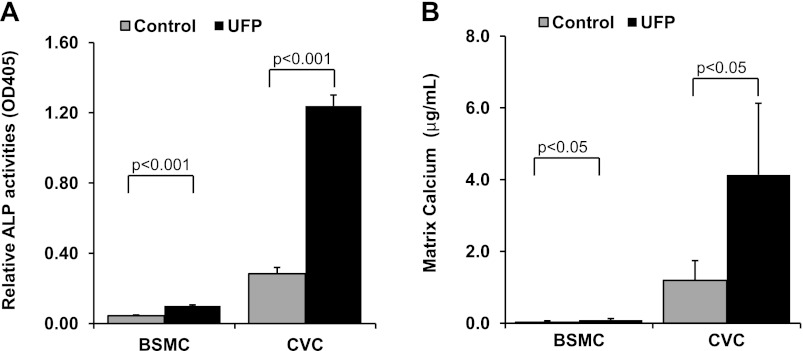
The effects of UFP on the calcification of vascular smooth muscle cells were examined in both BSMC and CVC. ALP activity, an early osteoblastic differentiation marker, was significantly increased in both BSMC and CVC in response to UFP exposure for 3 days (BSMC: control = 0.044 ± 0.004, UFP = 0.100 ± 0.006, n = 5, P < 0.001; CVC: control = 0.28 ± 0.04, UFP = 1.24 ± 0.06, n = 3, P < 0.001; Fig. 2A). Prolonged exposure to UFP (10 days) increased calcification in both cell types as measured by matrix calcium deposition (BSMC: control = 0.030 ± 0.036 μg/ml, UFP = 0.087 ± 0.041 μg/ml, n = 5, P < 0.05; CVC: control = 1.2 ± 0.6 μg/ml, UFP = 4.1 ± 2.0 μg/ml, n = 4, P < 0.05; Fig. 2B). The basal levels of ALP activity and calcium deposition were minimal in BSMC, and the induction of ALP activity and calcification by UFP were significantly less compared with that of CVC. For this reason, we used CVC in the ensuing studies.
Fig. 2.
UFP increased alkaline phosphatase (ALP) activity and promoted calcification in vascular smooth muscle cells. A: ALP activity was measured in the whole cell lysates of bovine smooth muscle cells (BSMC) and calcifying vascular cells (CVC) after treatment with control buffer or UFP (50 μg/ml) for 3 days. B: matrix calcium deposition was measured in BSMC and CVC after treatment with control buffer or UFP (50 μg/ml) for 10 days with media change every 3 days.
Paracrine effects of UFP on osteoblastic differentiation and matrix calcification.
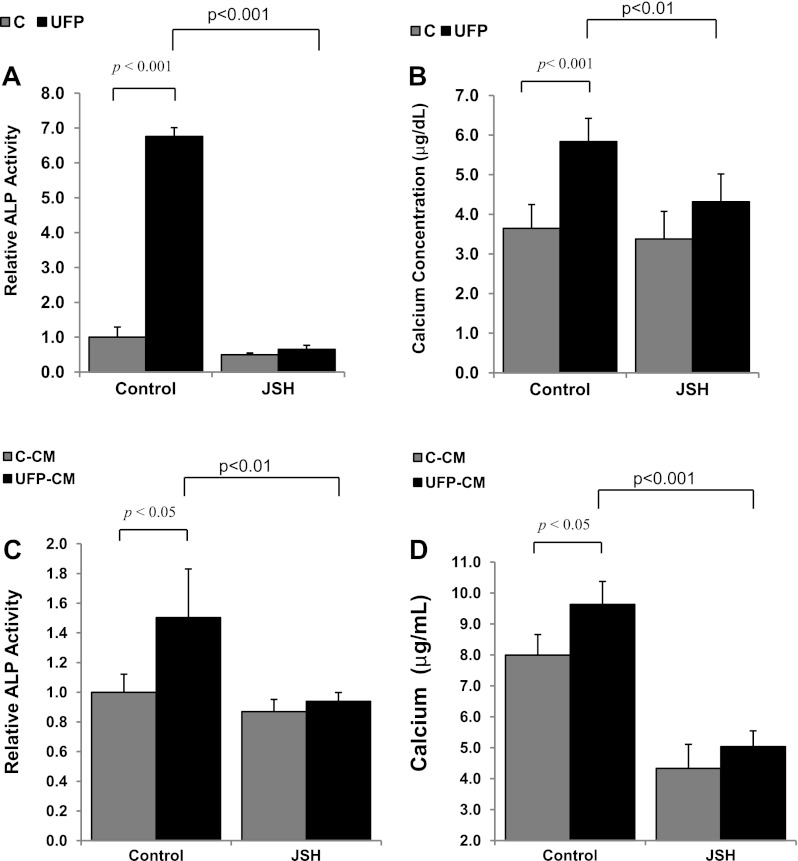
UFP exposure activate macrophages (3) to secret cytokines that were implicated in calcification of smooth muscle cells (55, 56). Here, we assessed the paracrine effects of macrophages exposed to UFP. UFP-conditioned macrophage media (UFP-CM) significantly increased both ALP activity (control-CM = 1.0 ± 0.1, UFP-CM = 1.5 ± 0.3, P < 0.01, n = 6; see Fig. 4C) and matrix calcification (control-CM = 8.0 ± 0.7 μg/ml, UFP-CM = 9.6 ± 0.7 μg/ml, P < 0.05, n = 4; see Fig. 4D) in CVC.
Fig. 4.
NF-κB signaling mediated UFP-induced osteoblastic differentiation and calcification. CVC were treated for 3 days with control buffer, UFP (50 μg/ml), and/or the NF-κB inhibitor JSH23 (A and B) or for 10 days with control conditioned media (C-CM), UFP-conditioned media (UFP-CM), and/or JSH23 (C and D). ALP activity (A and C) or matrix calcium deposition (B and D) was measured. Inhibition of NF-κB abrogated the effects of UFP and UFP conditioned media.
Effects of UFP on NF-κB signaling in CVC.
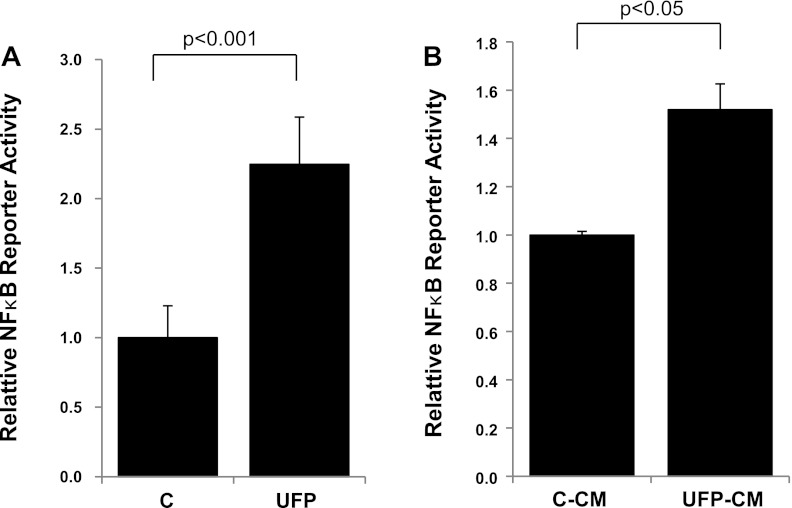
Diesel UFP was previously reported to activate NF-κB signaling in human aortic endothelial cells (27). In this study, we assessed whether ambient UFP induced NF-κB activity in CVC. UFP exposure significantly activated NF-κB reporter activity in CVC (control = 1.0 ± 0.2, UFP = 2.3 ± 0.3, n = 4, P < 0.001; Fig. 3A). In parallel, UFP-conditioned media also increased the NF-κB reporter activity (control-CM = 1.0 ± 0.0, UFP-CM = 1.5 ± 0.1, n = 3, P < 0.05; Fig. 3B).
Fig. 3.
UFP- and UFP-conditioned media activated the NF-κB pathway. CVC cells were infected with adenoviruses carrying recombinant NF-κB reporter and subsequently treated overnight with control buffer or UFP (A; 50 μg/ml) or with control conditioned media (B; C-CM) or UFP-conditioned media (UFP-CM) derived from macrophages. Reporter gene (luciferase) activities were measured by a luminometer.
Effects of NF-κB signaling in UFP-promoted calcification.
To investigate whether UFP induced calcification via NF-κB signaling, we cotreated the CVC cells with UFP and/or an NF-κB inhibitor, JSH23. JSH23 significantly attenuated UFP-induced ALP activity (control = 1.0 ± 0.3, UFP = 6.4 ± 0.6, JSH = 0.4 ± 0.1, JSH-UFP = 0.5 ± 0.2; JSH-UFP vs. UFP, n = 3, p < 0.001; Fig. 4A). Similarly, UFP-induced calcification was significantly attenuated by treatment with JSH23 (control = 3.6 ± 0.6 μg/ml, UFP = 5.8 ± 0.6 μg/ml, JSH = 3.4 ± 0.7 μg/ml, UFP + JSH = 4.3 ± 0.7 μg/ml; UFP + JSH vs. UFP: P < 0.01, n = 5; Fig. 4B).
JSH23 also attenuated UFP-conditioned media-induced ALP activity (control-CM = 1.0 ± 0.1, UFP-CM = 1.5 ± 0.3, control-CM + JSH = 0.9 ± 0.1, UFP-CM + JSH = 0.9 ± 0.1; UFP-CM + JSH vs. UFP-CM, n = 3, P < 0.01; Fig. 4C) and calcification (control-CM = 8.0 ± 0.7, UFP-CM = 9.6 ± 0.7, control-CM + JSH = 4.3 ± 0.8, UFP-CM + JSH = 5.0 ± 0.5; UFP-CM + JSH vs. UFP-CM, P < 0.001, n = 4; Fig. 4D). These data support the role of NF-κB signaling in UFP-mediated calcification in CVC cells.
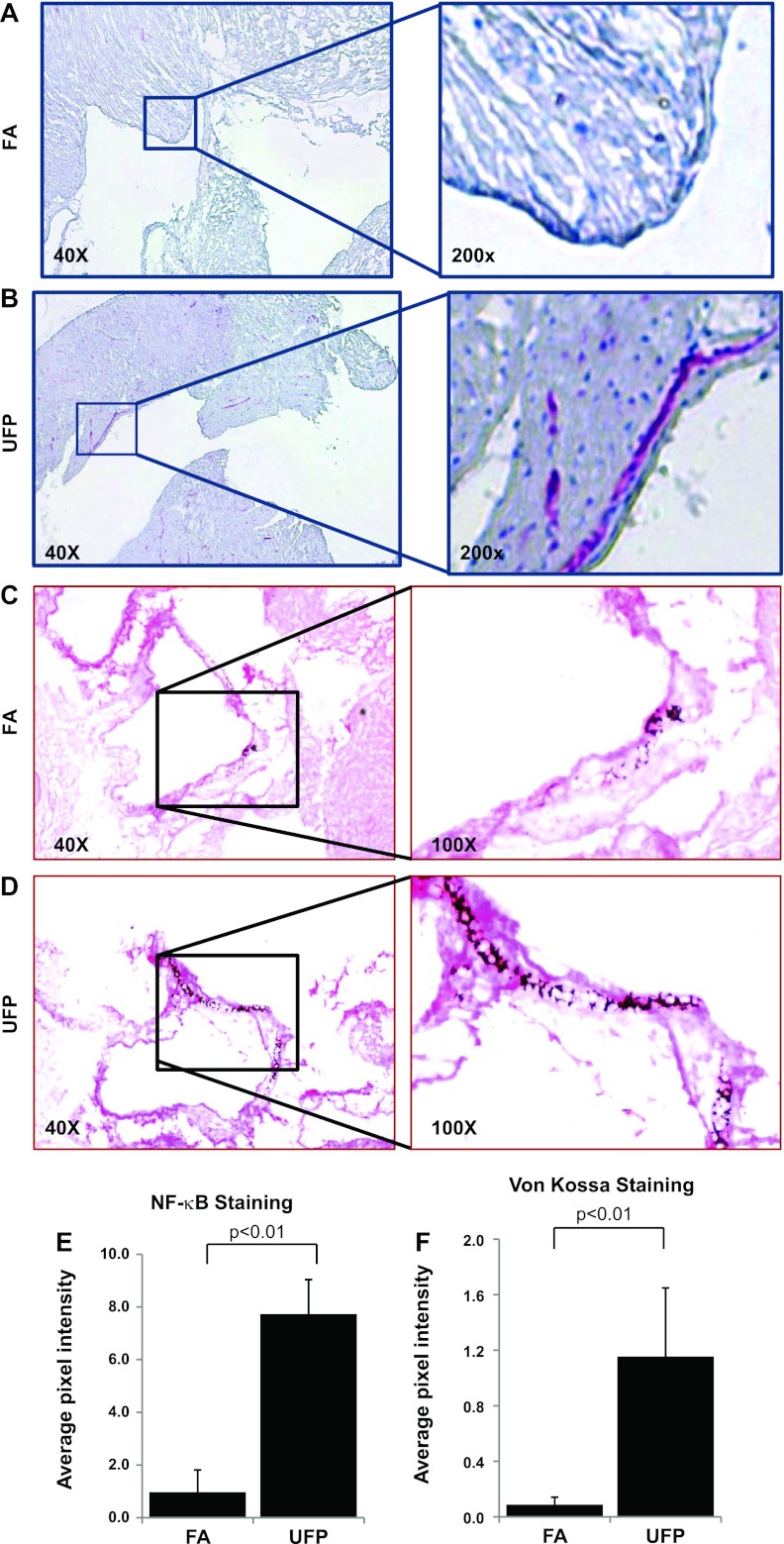
Effects of UFP exposure on vascular calcification in Ldlr−/− mice.
Ldlr−/− mice (48, 49) on a high-fat diet were exposed to UFP or FA (control) for 10 wk, and NF-κB activation and vascular calcification were assessed in the aortic root. In the UFP-exposed mice, NF-κB (phosphor-p65) was activated in the junction between endocardiac and aortic valve (Fig. 5, A and B). In the aortic root region, the control mice developed scattered foci of calcium deposits (Fig. 5C), whereas UFP-exposed mice developed more prominent calcification (Fig. 5D). Semiquantitative analysis showed that UFP significantly increased both active NF-κB (P < 0.01; Fig. 5E) and von Kossa staining (P < 0.01; Fig. 5F).
Fig. 5.
UFP exposure activates NF-κB and promoted vascular calcification in Ldlr−/− mice. Aortic root sections from Ldlr−/− mice exposed to control filtered air (FA) or UFP for 10 wk were immunostained for NF-κB activation with anti-NF-κB (phospho-p65) antibody (A and B) or histochemical stained for calcium deposition by von Kossa (C and D). Representative pictures revealed prominent active NF-κB staining (red) and increased calcium deposition in the aortic root region in mice exposed to UFP. The control mice showed only scattered loci of calcification in the aortic root areas. Semiquantitative analysis indicated that active NF-κB (E) and von Kossa (F) staining were significantly increased in response to UFP exposure.
DISCUSSION
Our findings provide a mechanistic insight into UFP-mediated vascular calcification. We demonstrated that exposure to ambient UFP promoted vascular calcification in CVC cells via NF-κB activation. Exposure to UFP- or UFP-conditioned macrophage media significantly increased ALP activity and calcium deposition in CVC. Both of these effects were abrogated by a NF-κB inhibitor, JSH23. We further recapitulated our in vitro study by demonstrating prominent presence of aortic calcification and NF-κB activation in the Ldlr−/− mice exposed to UFP.
Due to their high surface to volume ratios per unit mass, UFP display biochemical characteristics in favor of adsorption or absorption of potentially toxic organic compounds and their potential distribution to pulmonary and cardiovascular systems (14, 36, 40). Approximately 1% of inhaled nano-sized particles are believed to transmigrate across human pulmonary epithelium into systemic arterial circulation (37, 52, 53). When these UFP accumulate to a high concentration in “hot spots” (23), cytotoxicity develops (21). In this study, we showed that UFP is able to promote vascular calcification directly and indirectly via macrophages. In atherosclerotic lesions, SMC may be exposed due to damaged endothelial cells. Even in the vessels with intact endothelium, where smooth muscle cells are not directly exposed to UFP, our results suggest that UFP may be able to affect in a paracrine manner. Macrophages are activated upon exposure to particulate matter in the pulmonary alveoli (3). Plasma from subjects exposed to air pollution has been reported to affect vascular cells, in part, through the paracrine effects involving cytokines, microparticles, and/or oxidatively modified proteins or lipids (10). In this study, conditioned media derived from UFP-exposed macrophages were used to recapitulate the paracrine effect of lung-infiltrated macrophages. The conditioned media, analogous to the direct UFP exposure, activated NF-κB signaling and increased ALP activity and calcium deposition in CVC. These data suggest that secreted factors from UFP-exposed macrophages are implicated in UFP-promoted vascular calcification. However, arterial wall may not be directly exposed to the concentration of PM used in the current study, as merely a small amount of PM may transmigrate through the epithelial cells into the systemic circulation. In addition, the surface chemistry of PM may also be altered during interactions with the lung microenvironment. Thus the in vitro conditions may not exactly recapitulate those of in vivo. Nevertheless, the current study paved the basis for future assessment of circulating cytokines secreted by activated macrophages or by lung epithelial cells in response to UFP exposure.
Emerging evidence supports the role of NF-κB in PM-induced proinflammatory responses. Diesel exhaust particles activate nuclear translocation of NF-κB in human bronchial epithelium (43). Diesel exhaust particles activate the NF-κB-mediated inflammatory chemokines such as IL-8, monocyte chemoattractant protein-1, and adhesion molecules (27). Furthermore, NF-κB activation is implicated in receptor activator of NF-κB ligand (RANKL)-mediated osteogenic differentiation of smooth muscle cells (11, 16). Specifically, RANKL or TNF-α induces NF-κB activation to promote calcification in aortic smooth muscle cells (41, 62). A previous study by our group demonstrated that diesel UFP activated NF-κB in endothelial cells (27). In the current study, we demonstrated that UFP exposure activated NF-κB reporter in CVC and increased active NF-κB staining in the heart-aortic vasculature of Ldlr−/− mice. We also demonstrated that both ambient UFP- and UFP-conditioned media-induced ALP activity and calcification were abrogated by an NF-κB inhibitor, JSH23, suggesting NF-κB as an important UFP-mediated signaling molecule in the promotion of vascular calcification.
The mechanisms underlying UFP- and UFP-conditioned media-induced NF-κB activation are poorly understood. While direct UFP treatment may induce TNF-α to activate NF-κB (15), UFP-conditioned media may harbor macrophage-secreted cytokines to activate NF-κB. In addition, UFP-induced oxidative stress may contribute to vascular calcification via NF-κB (9, 20, 24) and/or BMP-Msx2-Wnt signaling pathway (8, 32, 47) in vascular smooth muscle cells. The detailed mechanism underlying UFP-promoted vascular calcification via NF-κB signaling awaits further investigation.
Hyperlipidemic Ldlr−/− mice are a well-accepted model for atherosclerosis (28, 30, 38) and vascular calcification (13, 34, 49). Araujo et al. (4) reported that Apoe−/− mice, another model of atherosclerosis, developed accelerated atherosclerotic lesion size in exposure to ambient UFP (4). In the current study, we demonstrated that UFP-exposed Ldlr−/− mouse developed prominent positive von Kossa staining, suggesting calcium deposition in the aortic root region. Our in vitro and in vivo findings suggest that ambient atmospheric UFP exposure promotes vascular calcification via the NF-κB signaling pathway.
GRANTS
This project was supported by the National Heart Lung and Blood Institute Grants R01-HL-083015 (to T. Hsiai) and R21-HL-091302 (to T. Hsiai) and by the Southern California Particle Center, funded by Environmental Protection Agency under the STAR program through Award No. 2145 G GB139 (to C. Sioutas) and South Coast Air Quality Management District Award No. 11527 (to C. Sioutas).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.L., C.S., and T.H. conception and design of research; R.L., D.M., W.K., Y.D., and M.N. performed experiments; R.L., D.M., C.S., and T.H. analyzed data; R.L., P.P., Y.T., M.N., C.S., and T.H. interpreted results of experiments; R.L. and D.M. prepared figures; R.L. and D.M. drafted manuscript; R.L., D.M., P.P., Y.T., M.N., C.S., and T.H. edited and revised manuscript; R.L., D.M., W.K., P.P., Y.D., Y.T., M.N., C.S., and T.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Linda L. Demer for providing CVC and BSMC and Dr. Todd Morgan and Dr. Caleb Finch for technical assistance in the animal study.
REFERENCES
- 1.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 24: 1161–1170, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Allen RW, Criqui MH, Diez Roux AV, Allison M, Shea S, Detrano R, Sheppard L, Wong ND, Stukovsky KH, Kaufman JD. Fine particulate matter air pollution, proximity to traffic, and aortic atherosclerosis. Epidemiology 20: 254–264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre E, Stoeger T, Takenaka S, Bahnweg M, Ritter B, Karg E, Lentner B, Reinhard C, Schulz H, Wjst M. Inhalation of ultrafine carbon particles triggers biphasic pro-inflammatory response in the mouse lung. Eur Respir J 28: 275–285, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res 102: 589–596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336: 1066–1071, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest 91: 1800–1809, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brook RD, Rajagopalan S, Pope CA, Brook JR, 3rd, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121: 2331–2378, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem 283: 15319–15327, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canty TG, Jr, Boyle EM, Jr, Farr A, Morgan EN, Verrier ED, Pohlman TH. Oxidative stress induces NF-kappaB nuclear translocation without degradation of IkappaBalpha. Circulation 100: II361–364, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Channell MM, Paffett ML, Devlin RB, Madden MC, Campen MJ. Circulating factors induce coronary endothelial cell activation following exposure to inhaled diesel exhaust and nitrogen dioxide in humans: evidence from a novel translational in vitro model. Toxicol Sci 127: 179–186, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho HH, Shin KK, Kim YJ, Song JS, Kim JM, Bae YC, Kim CD, Jung JS. NF-kappaB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J Cell Physiol 223: 168–177, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J 9: 899–909, 1995 [PubMed] [Google Scholar]
- 13.Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, Zapol WM, Bloch KD, Yu PB. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler Thromb Vasc Biol 32: 613–622, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frampton MW. Systemic and cardiovascular effects of airway injury and inflammation: ultrafine particle exposure in humans. Environ Health Perspect 109: 529–532, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartz AM, Bauer B, Block ML, Hong JS, Miller DS. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J 22: 2723–2733, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heymann MF, Herisson F, Davaine JM, Charrier C, Battaglia S, Passuti N, Lambert G, Goueffic Y, Heymann D. Role of the OPG/RANK/RANKL triad in calcifications of the atheromatous plaques: Comparison between carotid and femoral beds. Cytokine 58: 300–306, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann B, Moebus S, Dragano N, Mohlenkamp S, Memmesheimer M, Erbel R, Jockel KH. Residential traffic exposure and coronary heart disease: results from the Heinz Nixdorf Recall Study. Biomarkers 14, Suppl 1: 74–78, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann B, Moebus S, Mohlenkamp S, Stang A, Lehmann N, Dragano N, Schmermund A, Memmesheimer M, Mann K, Erbel R, Jockel KH. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation 116: 489–496, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Iba K, Takada J, Yamashita T. The serum level of bone-specific alkaline phosphatase activity is associated with aortic calcification in osteoporosis patients. J Bone Miner Metab 22: 594–596, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Kratsovnik E, Bromberg Y, Sperling O, Zoref-Shani E. Oxidative stress activates transcription factor NF-κB-mediated protective signaling in primary rat neuronal cultures. J Mol Neurosci 26: 27–32, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Kreyling WG, Semmler-Behnke M, Moller W. Ultrafine particle-lung interactions: does size matter? J Aerosol Med 19: 74–83, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Lambrechtsen J, Gerke O, Egstrup K, Sand NP, Norgaard BL, Petersen H, Mickley H, Diederichsen AC. The relation between coronary artery calcification in asymptomatic subjects and both traditional risk factors and living in the city centre: a DanRisk substudy. J Intern Med 271: 444–450, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Li N, Hao M, Phalen RF, Hinds WC, Nel AE. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin Immunol 109: 250–265, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Li N, Karin M. Is NF-kappaB the sensor of oxidative stress? FASEB J 13: 1137–1143, 1999 [PubMed] [Google Scholar]
- 25.Li R, Mittelstein D, Lee J, Fang K, Majumdar R, Tintut Y, Demer LL, Hsiai TK. A dynamic model of calcific nodule destabilization in response to monocyte- and oxidized lipid-induced matrix metalloproteinases. Am J Physiol Cell Physiol 302: C658–C665, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R, Ning Z, Cui J, Khalsa B, Ai L, Takabe W, Beebe T, Majumdar R, Sioutas C, Hsiai T. Ultrafine particles from diesel engines induce vascular oxidative stress via JNK activation. Free Radic Biol Med 46: 775–782, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li R, Ning Z, Majumdar R, Cui J, Takabe W, Jen N, Sioutas C, Hsiai T. Ultrafine particles from diesel vehicle emissions at different driving cycles induce differential vascular pro-inflammatory responses: Implication of chemical components and NF-κB signaling. Part Fibre Toxicol 7: 6–17, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Li D, Chen J, Xie J, Bandyopadhyay S, Zhang D, Nemarkommula AR, Liu H, Mehta JL, Hermonat PL. Inhibition of atherogenesis in LDLR knockout mice by systemic delivery of adeno-associated virus type 2-hIL-10. Atherosclerosis 188: 19–27, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Mathieu P, Voisine P, Pepin A, Shetty R, Savard N, Dagenais F. Calcification of human valve interstitial cells is dependent on alkaline phosphatase activity. J Heart Valve Dis 14: 353–357, 2005 [PubMed] [Google Scholar]
- 30.Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, Satoh H, Inoue K, Kawase Y, Jishage K, Suzuki H, Takeya M, Schnackenberg L, Beger R, Hermonat PL, Thomas M, Sawamura T. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res 100: 1634–1642, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Misra C, Kim S, Shen S, Sioutas C. A high flow rate, very low pressure drop impactor for inertial separation of ultrafine from accumulation mode particles. J Aerosol Sci 33: 735–752, 2002 [Google Scholar]
- 32.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med 31: 509–519, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Morgan TE, Davis DA, Iwata N, Tanner JA, Snyder D, Ning Z, Kam W, Hsu YT, Winkler JW, Chen JC, Petasis NA, Baudry M, Sioutas C, Finch CE. Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environ Health Perspect 119: 1003–1009, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(−/−) mice. Circulation 117: 411–420, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science 311: 622–627, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation 105: 411–414, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Nemmar A, Hoylaerts MF, Hoet PH, Nemery B. Possible mechanisms of the cardiovascular effects of inhaled particles: systemic translocation and prothrombotic effects. Toxicol Lett 149: 243–253, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Neven E, D'Haese PC. Vascular calcification in chronic renal failure: what have we learned from animal studies? Circ Res 108: 249–264, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Nicoll R, Henein MY. Air pollution and its cardiovascular and other risks. J Intern Med 271: 429–432, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Oberdorster G. Significance of particle parameters in the evaluation of exposure-dose-response relationships of inhaled particles. Particul Sci Technol 14: 135–151, 1996 [PubMed] [Google Scholar]
- 41.Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lopez-Ongil S, Coll B, Fernandez E, Valdivielso JM. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res 104: 1041–1048, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol 17: 680–687, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Pourazar J, Blomberg A, Kelly FJ, Davies DE, Wilson SJ, Holgate ST, Sandstrom T. Diesel exhaust increases EGFR and phosphorylated C-terminal Tyr 1173 in the bronchial epithelium. Part Fibre Toxicol 5: 8, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 107: 2181–2184, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubin MR, Silverberg SJ. Vascular calcification and osteoporosis–the nature of the nexus. J Clin Endocrinol Metab 89: 4243–4245, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Schinke T, Karsenty G. Vascular calcification–a passive process in need of inhibitors. Nephrol Dial Transplant 15: 1272–1274, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, Behrmann A, Towler DA. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann NY Acad Sci 1117: 40–50, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol 26: 1423–1430, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest 115: 1210–1220, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Speer MY, Giachelli CM. Regulation of cardiovascular calcification. Cardiovasc Pathol 13: 63–70, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Stary HC. The sequence of cell and matrix changes in atherosclerotic lesions of coronary arteries in the first forty years of life. Eur Heart J 11, Suppl E: 3–19, 1990 [DOI] [PubMed] [Google Scholar]
- 52.Takenaka S, Karg E, Kreyling WG, Lentner B, Moller W, Behnke-Semmler M, Jennen L, Walch A, Michalke B, Schramel P. Distribution pattern of inhaled ultrafine gold particles in the rat lung. Inhal Toxicol 18: 733–740, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Takenaka S, Karg E, Kreyling WG, Lentner B, Schulz H, Ziesenis A, Schramel P, Heyder J. Fate and toxic effects of inhaled ultrafine cadmium oxide particles in the rat lung. Inhal Toxicol 16: 83–92, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Thompson GR, Partridge J. Coronary calcification score: the coronary-risk impact factor. Lancet 363: 557–559, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation 105: 650–655, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Trion A, van der Laarse A. Vascular smooth muscle cells and calcification in atherosclerosis. Am Heart J 147: 808–814, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab 286: E686–E696, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Verma V, Ning Z, Cho AK, Schauer JJ, Shafer MM, Sioutas C. Redox activity of urban quasi-ultrafine particles from primary and secondary sources. Atmospheric Environ 43: 6360–6368, 2009 [Google Scholar]
- 59.Verma V, Polidori A, Schauer JJ, Shafer MM, Cassee FR, Sioutas C. Physicochemical and toxicological profiles of particulate matter in Los Angeles during the October 2007 southern California wildfires. Environ Sci Technol 43: 954–960, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Xue W, Comes N, Borras T. Presence of an established calcification marker in trabecular meshwork tissue of glaucoma donors. Invest Ophthalmol Vis Sci 48: 3184–3194, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y, Schauer JJ, Shafer MM, Hannigan MP, Dutton SJ. Source apportionment of in vitro reactive oxygen species bioassay activity from atmospheric particulate matter. Environ Sci Technol 42: 7502–7509, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Zhao G, Xu MJ, Zhao MM, Dai XY, Kong W, Wilson GM, Guan Y, Wang CY, Wang X. Activation of nuclear factor-kappa B accelerates vascular calcification by inhibiting ankylosis protein homolog expression. Kidney Int 82: 34–44, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]