Abstract
It was previously demonstrated in isolated renal vascular smooth muscle cells (VSMCs) that integrin-mediated mechanotransduction triggers intracellular Ca2+ mobilization, which is the hallmark of myogenic response in VSMCs. To test directly whether integrin-mediated mechanotransduction results in the myogenic response-like behavior in renal VSMCs, cell traction force microscopy was used to monitor cell traction force when the cells were pulled with fibronectin-coated or low density lipoprotein (LDL)-coated paramagnetic beads. LDL-coated beads were used as a control for nonintegrin-mediated mechanotransduction. Pulling with LDL-coated beads increased the cell traction force by 61 ± 12% (9 cells), which returned to the prepull level after the pulling process was terminated. Pulling with noncoated beads had a minimal increase in the cell traction force (12 ± 9%, 8 cells). Pulling with fibronectin-coated beads increased the cell traction force by 56 ± 20% (7 cells). However, the cell traction force was still elevated by 23 ± 14% after the pulling process was terminated. This behavior is analogous to the changes of vascular resistance in pressure-induced myogenic response, in which vascular resistance remains elevated after myogenic constriction. Fibronectin is a native ligand for α5β1-integrins in VSMCs. Similar remanent cell traction force was found when cells were pulled with beads coated with β1-integrin antibody (Ha2/5). Activation of β1-integrin with soluble antibody also triggered variations of cell traction force and Ca2+ mobilization, which were abolished by the Src inhibitor. In conclusion, mechanical force transduced by α5β1-integrins triggered a myogenic response-like behavior in isolated renal VSMCs.
Keywords: myogenic response, paramagnetic bead, cell traction force microscopy, calcium signaling
myogenic vasoconstriction is an autoregulatory mechanism for adjusting local vascular resistance to the increase in arterial pressure, which fluctuates over a wide range under different physiological and pathophysiological conditions (27, 43). In the renal circulation, pressure-induced myogenic constriction in the afferent arteriole is an important protective mechanism to prevent the transmission of elevated systemic pressure to the glomerular capillaries, a critical determinant in the progression of glomerulosclerosis in diabetes, hypertension, and end-stage renal disease. However, the mechanisms that transduce the changes in perfusion pressure into vascular smooth muscle cells (VSMCs) to initiate myogenic constriction are still elusive (7, 15, 17, 28). Many studies on mechanotransduction of myogenic response have focused on the mechanosensitive gating of stretch-sensitive channels in triggering membrane depolarization and Ca2+ influx. However, stretch-sensitive ion channels cannot provide a sustained error signal to maintain the myogenic constriction when the stretch vanishes as the vessel contracts (9, 10, 42). In contrast, integrins are known to transduce extracellular mechanical force into intracellular biochemical signals to affect VSMC contractility (1, 20, 21, 32). We have previously shown that application of mechanical force to renal VSMCs with paramagnetic beads coated with integrin ligands activates subcellular Ca2+ release from ryanodine receptors in the form of Ca2+ sparks (1). The coupling of Ca2+ sparks to the Ca2+-activated Cl− channel may account for the membrane depolarization in VSMCs required for contraction (22). However, it has not been determined whether the integrin-mediated mechanotransduction is sufficient to elicit VSMC contraction for the myogenic response. Cells are anchored to the substratum via focal adhesions, which are composed of cytoskeleton, integrins ,and extracellular matrix. VSMCs exert cell traction force through focal adhesions (34). In the present study, cell traction-force microscopy was used to monitor the changes in cell traction exerted by renal VSMCs on a flexible substrate to examine integrin-mediated contraction at the single cell level.
MATERIALS AND METHODS
Isolation and culture of renal VSMCs.
All experiments were performed under protocols approved by the University of South Florida's Animal Care and Use Committee. Male Sprague-Dawley rats from Harlan Laboratories (120–200 g) were euthanized by anesthetic overdose (5% isoflurane in a chamber through a vaporizer). Renal vascular smooth muscle cells were isolated enzymatically from dissected arcuate arteries and cortical radial arteries, which are capable of responding to changes in perfusion pressure (3), and the yield of isolated cells from these arterioles was much higher than the afferent arterioles (14). The arteries were first digested with 0.15% papain (Sigma) for 6 min and then digested with 0.2% collagenase type 4 (Worthington), 0.1% trypsin inhibitor (Sigma), and 0.05% elastase (Sigma) for 4–6 min.
All digestions were performed in low calcium dissociation solution containing the following (in mM): 119 NaCl, 4.7 KCl, 1.2 MgSO4, 1.18 KH2PO4, 24 NaHCO3, 5.5 glucose, 10 HEPES, and 50 μM CaCl2, pH 7.4 at 37°C. The vessels were then triturated, and the freshly isolated VSMCs were plated onto glass-bottomed Petri dishes coated with Matrigel (BD Biosciences). Some VSMCs were resuspended in DMEM with 10% FBS and cultured in T25 culture flask at 37°C in 5% CO2-95% air.
Cell traction force microscopy.
Renal VSMCs were grown on flexible substrate impregnated with fluorescent microspheres. Cell traction force exerted on the substrate (by individual VSMC) was determined from measured substrate displacements using the LIBTRC analysis library developed by Micah Dembo of the Boston University, who is also the inventor of the basic theory that underlies traction force microscopy (8, 24, 25). In brief, polyacrylamide gels (5% acrylamide, 0.1% bis-acrylamide, 75-μm thickness, and Young's modulus = 28 kN/m2) were prepared on glass coverslips with fluorescent microspheres (0.2-μm diameter, yellow-green FluoSpheres; Invitrogen) impregnated under the top surface. The Young's modulus of polyacrylamide sheets was determined with an improved method based on the Hertz theory (4, 23, 26) and confirmed with atomic force microscopy. The surface was then coated with collagen type I (0.2 mg/ml; BD Biosciences) after photoactivation of the surface molecular linker, sulfosuccinimidyl-6-(4′-azido-2′-nitrophenylamino) hexanoate (Sulfo-SANPAH; Pierce Chemical) (2), which reacted nonspecifically to the polyacrylamide gel on one end and to the primary amines on the other end, linking the collagen to the polyacrylamide gel. Renal VSMCs were then seeded and cultured overnight. Fluorescent images of the impregnated microspheres right under the cell of interest were captured with a cooled CCD camera (MicroMax:512BFT; Princeton Instruments) mounted on a Zeiss microscope (Axiovert 200M) equipped with an environmental chamber. Images were collected with a ×20 objective lens (Plan-Apochromat, N.A. 0.75) at 37° C. To improve the temporal resolution in measuring cell traction force, fluorescent images and transmitted light images in some experiments were collected simultaneously with a Leica TCS SP5 confocal imaging system at 37° C.
To measure the cell traction force, the image of the cell of interest and the impregnated fluorescent microspheres underneath was first collected, followed by capturing a single image or a series of images during and after the cell was exposed to experimental maneuvers. The cell was then removed from the flexible substrate, and an image of the fluorescent microspheres without the cell adhered was collected. The last image provides the location reference for each fluorescent microsphere when there is no cell traction force. LIBTRC was then applied to the images to tract the displacement of microspheres and to compute the cell traction force. Before the availability of LIBTRC for in-house image processing, images of traction force microscopy were processed and analyzed in Boston University.
Application of electromechanical force on renal VSMCs.
Tosyl-activated paramagnetic microbeads (2.8- or 4.5-μm diameter; Dynal) were coated with fibronectin (Sigma), low-density lipoprotein (LDL; Sigma), or β1-integrin antibody (Ha2/5; Pharmingen) using the protocol provided by the manufacturer. Only cells in solitude were used for cell traction force study. After the initial cell image without beads was acquired, ligand-coated or noncoated paramagnetic beads were added to the cell chamber and incubated with VSMCs for 45 min to allow the formation of focal adhesions. The prepull image was then collected before turning on the electromagnet. The cell traction force measured from the prepull image was used as the baseline to normalize the cell traction force measured in the same experiment/time series. Controlled force was applied to VSMCs by attracting the paramagnetic beads with a miniaturized needle-shaped electromagnet (tip diameter of 420 μm, 100–150 μm away) mounted on a manipulator as described and characterized previously (1). The force applied to each cell was in the range of 0.5–2 nN. Images were then collected during and after the pulling process. Fibronectin, the predominant native ligand for α5β1-integrins in VSMCs (31, 40, 46), was used to examine the integrin-dependent response, and an LDL-coated bead was used as the control for nonintegrin-mediated mechanical signal transduction. Cell surface receptors can transmit mechanical stresses to extracellular matrix at focal adhesions (19).
Intracellular Ca2+ concentration measurement in renal VSMCs.
Renal VSMCs grown on collagen-coated Petri dishes with glass bottoms were loaded with fluo-4/AM (10 μM; Invitrogen) in HBSS (Mediatech) for 30 min, followed by 20 min for deesterification of the dye. Fluo-4 was excited at 488 nm, and its emission was collected with a bandpass filter at 522/35 nm. Fluorescent images were collected with either a Bio-Rad MRC-1000 confocal unit coupled to a Zeiss Axiovert 100TV inverted microscope or a Leica TCS SP5 confocal imaging system using water immersion objective lens (×63, N.A. 1.2). Global Ca2+ mobilization in VSMCs was monitored at 0.5 Hz in the presence of an activating antibody specific to β1-integrin (50 μg/ml, Ha2/5, hamster IgM; Pharmingen). Subcellular Ca2+ transience was monitored at 500 Hz (2 ms/scan line) in XT line scanning mode.
Immunohistochemistry.
The β1-integrin on renal VSMCs was first activated with the activating antibody (50 μg/ml, Ha2/5) for 20 min, then fixed with 4% paraformaldehyde in PBS for 20 min, and permeabilized with 0.2% Triton-X in PBS for 30 min. The cells were then incubated with an antibody specific to activated β1-integrin (10 μg/ml, HUTS-4, mouse IgG; Chemicon) overnight at 4°C, followed by incubation of DyLight 549-conjugated secondary antibodies (3 μg/ml; Jackson ImmunoResearch) for 2 h. An isotype of hamster IgM antibody (50 μg/ml; Santa Cruz) was used as control. Confocal fluorescence images were collected with a water immersion objective lens (×63, N.A. 1.2).
Data analysis.
Normalized cell traction force was reported as means ± SE. Statistical significance (P < 0.05) was assessed by paired or unpaired Student's t-test whenever applicable. Ca2+ sparks were detected in confocal images with a custom-made algorithm written in Interactive Data Language software (IDL; Research Systems, Boulder, CO). The program identified Ca2+ sparks on the basis of their statistical deviation from the background noise (5, 47). The duration and width of Ca2+ sparks were quantified as the full duration half-maximum and full-width half-maximum, respectively (36). The spark frequency of each cell was defined as the number of sparks detected per second in a scan line of 25 μm.
The time series of fluo-4 emission variations in individual renal VSMCs was sampled at 0.5 Hz for spectral analysis. Each time series was normalized with respect to the baseline before β1-integrin activation and was subjected to linear trend removal. The 256 data points were used to calculate the power spectrum with an algorithm based on Fast Fourier Transform (44). Mean normalized power spectrum was reported as means ± SE.
RESULTS
Nonintegrin-mediated mechanotransduction in renal VSMCs.
To study myogenic response at the single cell level, cell traction force was determined in renal VSMCs grown on a deformable polyacrylamide substrate impregnated with fluorescent microspheres before, during, and after mechanical force was applied by dragging cell-adherent paramagnetic beads under an electromagnetic field generated by a pointed-tip source positioned 100–150 μm away. Under resting conditions, the averaged two-dimensional cell area was 23.7 ± 16 × 10−6 cm2, the total cell traction force was 0.20 ± 0.04 dyn/cell, and normalized cell traction force was 8,260.6 ± 1,749.3 dyn/cm2 (1 Pascal = 1 N/m2 = 10 dyn/cm2), with a ratio of force along the long axis vs. short axis of 2.51 ± 0.26 (n = 39 cells).
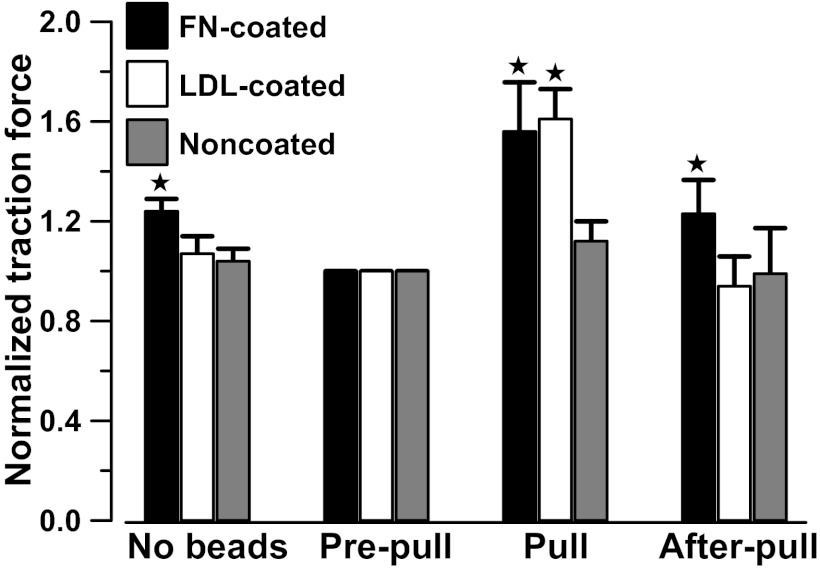
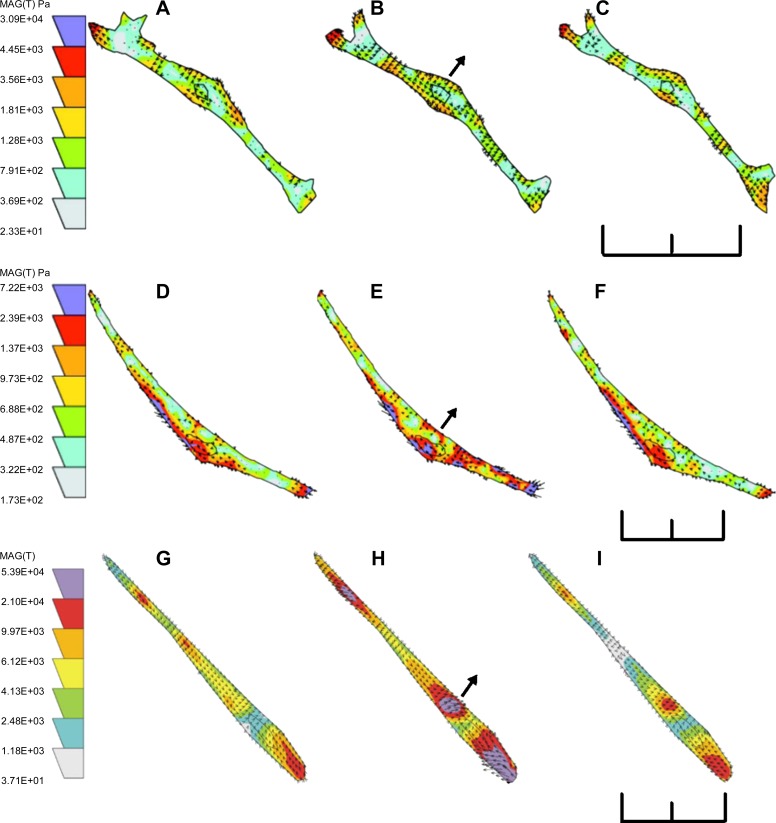
To monitor changes in cell traction forces induced by mechanical force, changes in cell traction force were monitored before bead attachment (no bead), after bead attachment (prepull), 30 s after initiation of the pulling process (pull), and 30 s after the termination of the pulling process (after pull). Attachment of fibronectin beads resulted in a drop of cell traction force, because the formation of new dorsal adhesion sites and redistribution of force (Fig. 1). The decrease in cell traction force was less with LDL beads than with fibronectin beads (19), because LDL beads did not form focal adhesion via integrin. Electromagnetic attraction of noncoated beads caused a small increase in traction force (12 ± 9%, nonsignificant; n = 8 cells), which disappeared when the pulling process was terminated (Fig. 1). Force maps showing the distribution, magnitude, and the direction of the traction force before, during, and after a renal VSMC was pulled with noncoated beads are shown in Fig. 2, A–C. The nonspecific adhesion was apparently ineffective for transducing the applied mechanical force into the VSMCs, presumably due to the lack of strong specific interactions between the cell and the paramagnetic beads.
Fig. 1.
Changes in mean normalized total cell traction force before and after attachment of paramagnetic beads to renal vascular smooth muscle cells (VSMCs), and when renal VSMCs were pulled with fibronectin-coated (FN-coated), LDL-coated, and noncoated paramagnetic beads. Cell traction force was normalized with the traction force measured after paramagnetic beads attached (prepull). The mean total cell traction force is 0.28 ± 0.09 dyn/cell at prepull (24 cells). Cell traction force was restored to prepull level after the pulling process was terminated except in cells pulled by fibronectin-coated paramagnetic beads. Error bars are SE. *Significant difference compared with the prepull control (P < 0.05).
Fig. 2.
Force maps of a renal VSMC pulled by uncoated paramagnetic beads (A, B, and C), LDL-coated paramagnetic beads (D, E, and F), and fibronectin-coated paramagnetic beads (G, H, I). Traction forces were measured before pulling (A, D, and G), 30 s after initiation of pulling (B, E, and H), and 30 s after termination of pulling (C, F, and I). Scale bars = 90 μm. Arrows indicate the direction of pulling force and the position of the electromagnet. Colored scales of cell traction force are in Pascal.
In contrast, electromagnetic pulling of LDL-coated beads increased cell traction force by 61 ± 12% (Fig. 1; n = 9 cells). The cell traction force returned to the prepull level after the pulling process was terminated, and no remanent cell traction force was detected. The force maps of a cell pulled with LDL-coated beads are shown in Fig. 2, D–F. LDL beads were more efficient than noncoated bead in transmitting mechanical force to the focal adhesion because LDL beads interacted with LDL receptors on plasma membrane.
Integrin-mediated mechanotransduction.
To examine the possible role of integrin in the myogenic response, fibronectin-coated paramagnetic beads were used to transduce mechanical force via α5β1-integrins. Pulling of fibronectin-coated beads increased the cell traction force by 56 ± 20% (Fig. 1; n = 7 cells), which was similar to the effects of pulling LDL-coated beads. However, the cell traction force did not return to the prepull level after the electromagnet was turned off and moved away. The cell traction force remained elevated at 23 ± 14% (n = 7 cells) of the prepull level. The force maps of a cell pulled with fibronectin-coated beads are shown in Fig. 2, G–I. The elevated cell traction force resembled the changes of vascular resistance in pressure induced myogenic response, in which vascular resistance remains elevated after myogenic constriction.
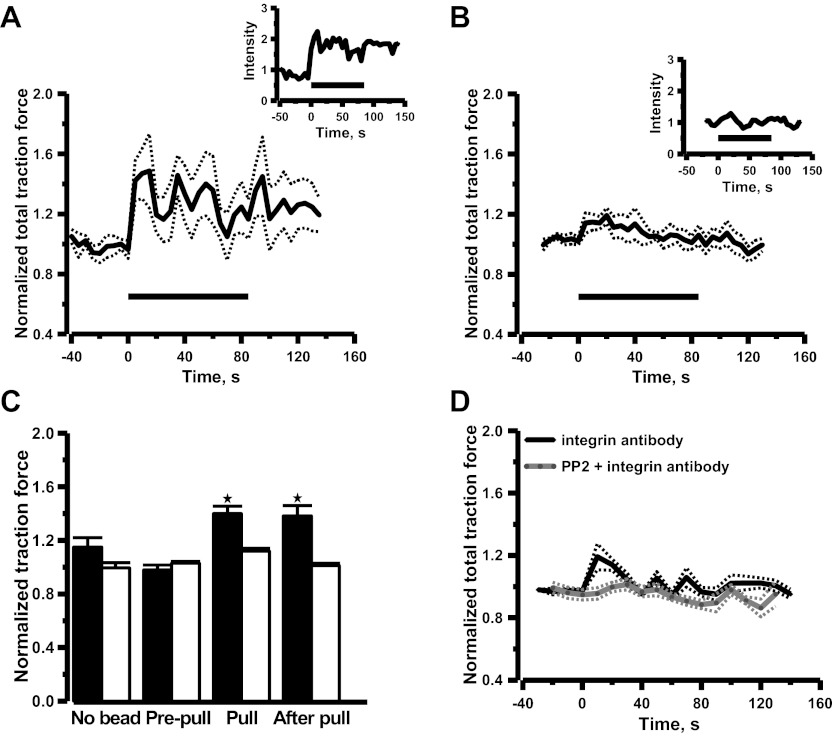
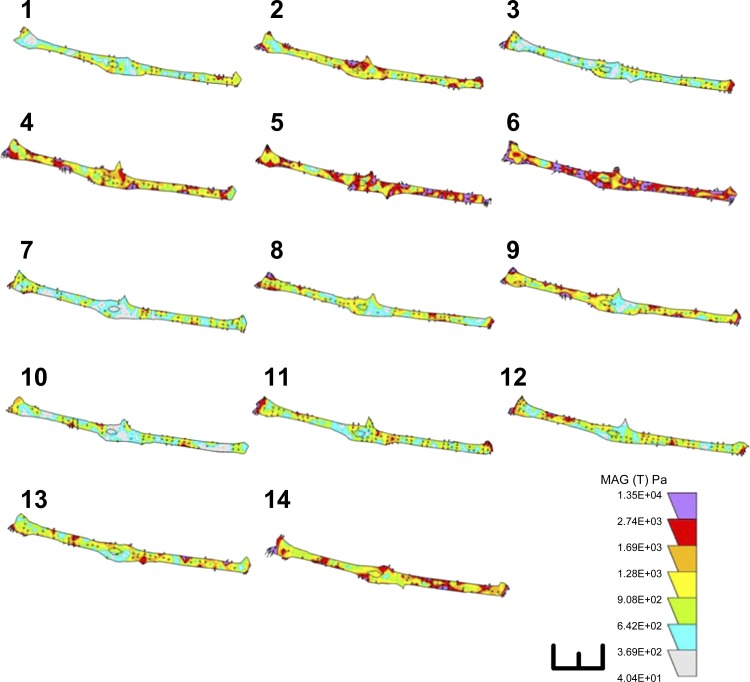
To further examine whether activation of α5β1-integrins with mechanical stimuli can account for the effects on cell traction force elicited by fibronectin-coated beads, renal VSMCs were pulled with paramagnetic beads coated with β1-integrin antibody. Attachment of β1-integrin antibody-coated beads resulted in a reduction of cell traction force as observed in fibronectin beads (Fig. 3C), which suggested that beads attachment alone did not activate integrins and generate cell traction force. The changes of cell traction force were monitored continuously during and after the pulling process. Application of mechanical force caused an immediate increase in cell traction force by 40 ± 6% (Fig. 3A; n = 6 cells), when the cells were pulled with the antibody-coated beads, and remanent cell traction force was observed after the pulling process was terminated. Application of mechanical force via beads coated with control isotype antibody only caused a transient increase of cell traction force, which returned to prepull level after the pulling process was terminated (Fig. 3B). The summary of the mean normalized data was shown in Fig. 3C. The nonspecific adhesion between VSMCs and isotype antibody-coated beads was ineffective for transducing applied mechanical force into the VSMCs as observed in noncoated beads. These observations suggest that mechanical activation of β1-integrin might account for the remanence of cell traction force, consistent with the notion that integrin-mediated mechanotransduction is an integral part of the myogenic response in VSMCs. The rhythmic variations of cell traction force induced by β1-integrin antibody-coated beads are shown in Fig. 4.
Fig. 3.
Mean time courses of changes in cell traction force induced by pulling paramagnetic beads coated with β1-integrin antibody (A), paramagnetic beads coated with control isotype antibody (B), and soluble β1-integrin antibody alone without paramagnetic beads (D). Time courses from individual cells are shown in insets. Horizontal lines indicate duration of pulling. Dotted lines are ± SE. Cell traction force was normalized with the traction force measured after paramagnetic beads attached (prepull). Mean normalized data are shown in histograms (C). Solid bars and opened bars are β1-integrin antibody and control isotype antibody, respectively. Error bars are SE. *Significant difference compared with the prepull control (P < 0.05, n = 6 cells). The β1-integrin antibody was added to the bath at t = 0 at (D). Preincubation of Src inhibitor PP2 (5 μM) abolished the increase of cell traction force induced by soluble β1-integrin antibody ( n = 6 cells).
Fig. 4.
Rhythmic variations of remanent cell traction force. A sequence of consecutive force maps (1–14) sampled at 0.2-Hz display rhythmic variations in cell traction force induced by pulling with β1-integrin antibody-coated beads. Images shown were collected after the pulling process was terminated. Cell traction force in the colored scale is 1.35E + 04 Pascal. Scale bar = 50 μm.
To examine whether activation of β1-integrins is causally linked to generation of cell traction force, cell traction force was monitored when VSMCs were incubated with activating β1-integrin antibody (Ha2/5, 50 μg/ml). Activation of β1-integrins triggered a sustained increase of cell traction force, which was abolished by pretreatment of the Src inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2; Fig. 3D). It has been shown that mechanical force transduced by β1-integrin (pulling of magnetic beads coated with activating β1-integrin antibody) activates Src in smooth muscle cells at real time (33). Taking together, these observations suggest that activation of β1-integrins with antibody and mechanical force transduced by β1-integrins share a common signaling pathway to generate cell traction force.
Activation of β1-integrins and Ca2+ mobilization.
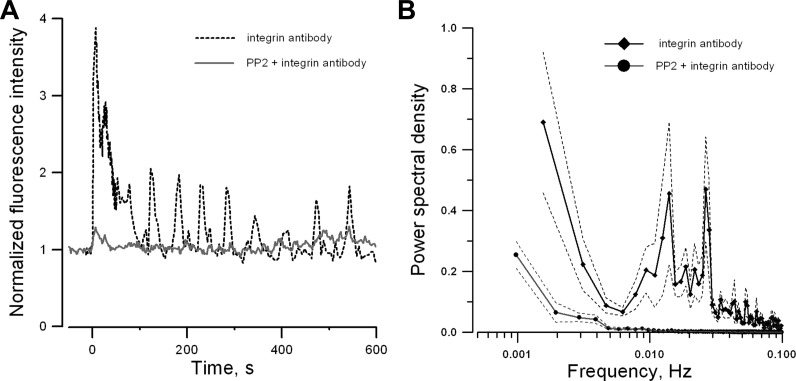
It is interesting to note that the cell traction force is oscillatory during and after mechanical stimulation using the β1-integrin antibody-coated beads. Since increase of intracellular Ca2+ concentration is a major determinant of myogenic contraction (29), the kinetic properties of β1-integrin-mediated Ca2+ mobilization was further examined in renal VSMCs. The activating antibody specific to β1-integrin (Ha2/5, 50 μg/ml) was used to stimulate β1-integrin and its effect on intracellular Ca2+ was monitored with confocal fluorescence microscopy. Activation of β1-integrin triggered a large initial Ca2+ spike followed by periodic Ca2+ oscillations (Fig. 5A). Power spectrum analysis from 45 VSMCs showed that most of the Ca2+ oscillations occurred at a frequency <0.03 Hz. (Fig. 5B), which is comparable for the rhythmic variations found in cell traction force (Fig. 4). The Ca2+ mobilization induced by activating the β1-integrin antibody was also abolished by SRC inhibitor PP2 (Fig. 5, A and B) as observed in cell traction force study (Fig. 3D).
Fig. 5.
Ca2+ mobilization in renal VSMCs induced by soluble β1-integrin antibody. Normalized time courses of fluo-4 emission in a single cultured renal VSMC (A) and the mean normalized power spectra of oscillations in fluo-4 emission (B). The β1-integrin antibody was added to the bath at t = 0. Solid lines are mean normalized power spectra (45 cells/3 experiments). Dotted lines are ± SE. Preincubation of Src inhibitor (5 μM PP2) attenuates β1-integrin antibody induced Ca2+ oscillations.
In addition to global Ca2+ mobilization, local subcellular Ca2+ release (Ca2+ sparks) triggered by β1-integrins was also monitored in freshly isolated renal VSMCs using confocal fluorescence microscopy at 500 Hz in linescan mode. It was shown previously that mechanical force transduced by integrin triggers Ca2+ sparks in renal VSMCs (1). Ca2+ sparks can initiate or modulate VSMCs contractility via Ca2+-activated channels on the plasma membrane. Application of β1-integrin antibody (Ha2/5) to VSMCs enhanced the occurrence of Ca2+ sparks (Fig. 6A) compared with the control using β2-integrin antibody (mouse, monoclonal IgG, Pharmingen, 50 μg/ml). Since β2-integrin is not expressed in VSMCs, the Ca2+ sparks detected in the presence of β2-integrin antibody could be considered as spontaneous sparks. Similarly, stimulating the α5-integrin with antibody (HMα5–1, 25 μg/ml; Pharmingen) could also trigger Ca2+ sparks as predicted, because α5β1-integrin heterodimers are the predominant fibronectin receptors in VSMCs.
Fig. 6.
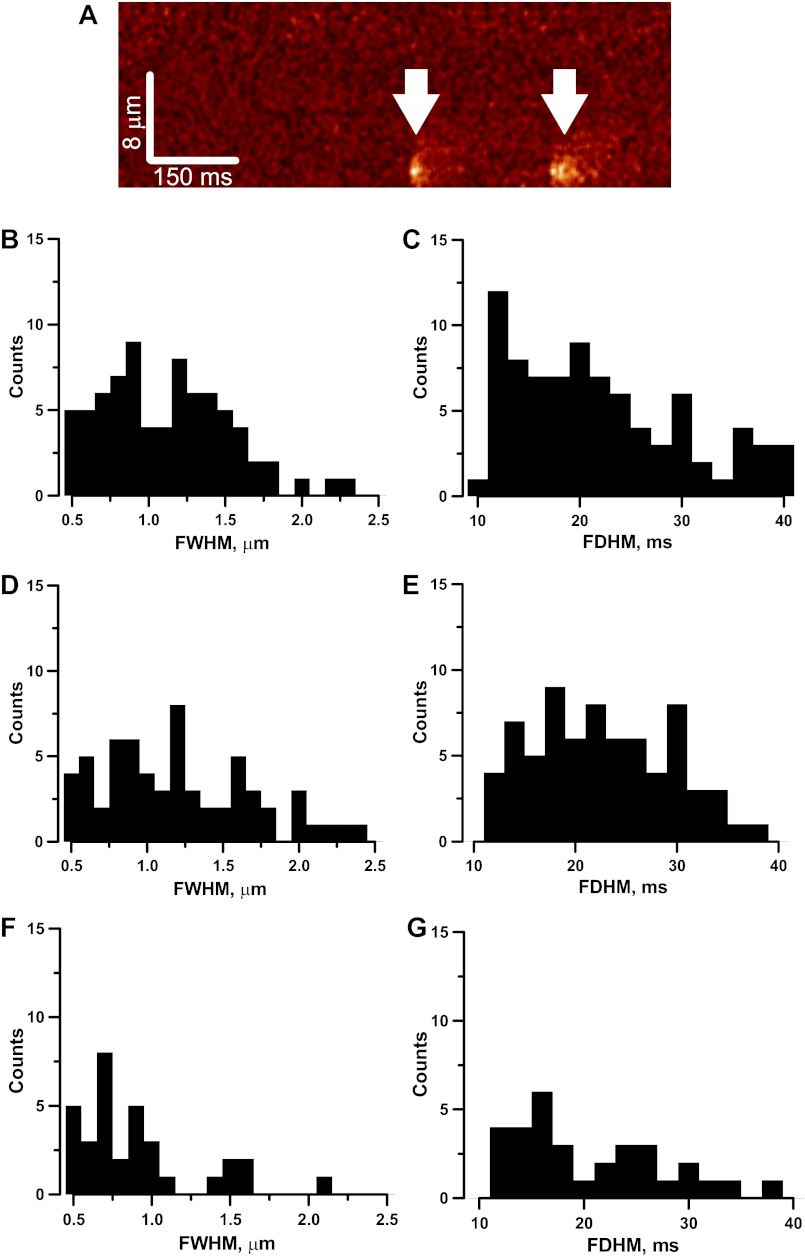
Occurrence and characterization of Ca2+ sparks induced by soluble integrin antibody. XT line scan image of a single VSMC exhibiting Ca2+ sparks (white arrowheads) in the presence of β1-integrin antibody (A). The whole image is composed of 512 scan lines collected at 500 Hz. Distribution of full width at half the maximum (FWHM, amplitude) and full duration at half maximum (FDHM, duration) of Ca2+ sparks in the presence of α5-integrin antibody (B and C, 83 Ca2+ sparks), β1-integrin antibody (D and E, 71 Ca2+ sparks), and β2-integrin antibody (F and G, 33 Ca2+ sparks) were similar. Medians of FWHM are 0.9, 1.2, and 0.8 μm, respectively. Corresponding medians FDHM are 20, 22, and 18 μs.
The mean Ca2+ spark frequency in the presence of α5-, β1-, and β2-integrin antibodies were 0.5 ± 0.07 sparks/s (n = 36 cells), 0.6 ± 0.08 sparks/s (n = 35 cells), and 0.04 ± 0.02 sparks/s (n = 30 cells). The frequency of sparks triggered in renal VSMCs by α5- and β1-antibodies was significantly higher (P < 0.05) than those triggered by β2-integrin antibody. The frequency distribution of the spatiotemporal parameters of Ca2+ sparks triggered by α5-, β1-, and β2-integrin antibodies is shown in Fig. 6.
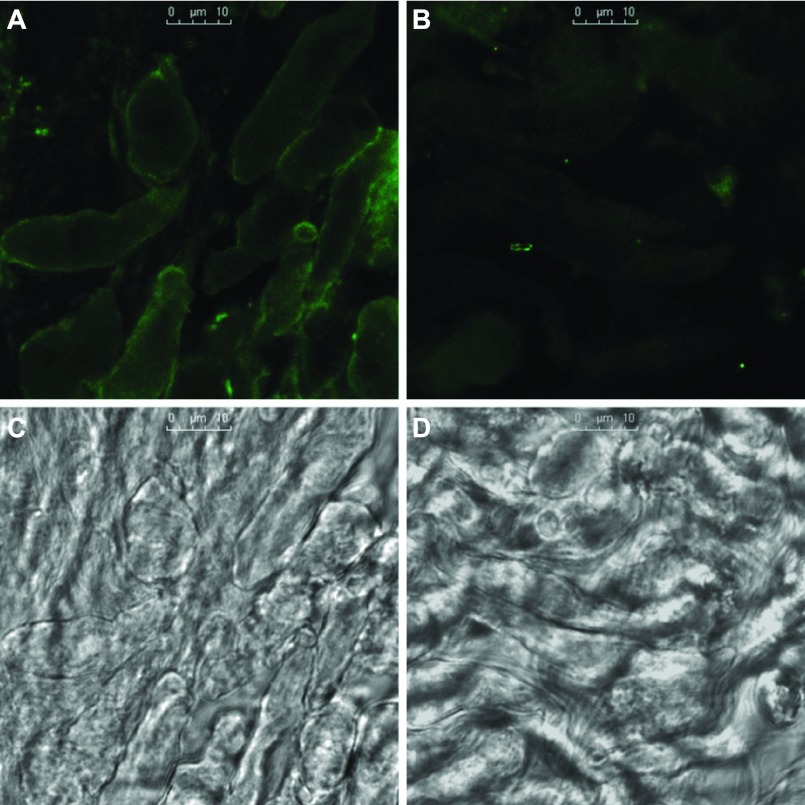
Integrins can exist in active or inactive state. Figure 7 is the immunofluorescence of activated β1-integrin in freshly isolated renal VSMCs with and without activation by Ha2/5 antibody. An isotype of hamster IgM antibody was used as the control for Ha2/5 antibody. Immunofluorescence of β1-integrin in activated form was enhanced on the plasma membrane after exposure to Ha2/5 antibody but not in the control. The mean immunofluorescence intensity of β1-integrin in the activated form was increased by 110 ± 8% (3 experiments, 42 cells) after activation compared with the control.
Fig. 7.
Immunofluorescence of activated β1-integrin detected with antibody HUTS-4 in freshly isolated renal VSMCs after activation by antibody Ha2/5 (A) and control isotype antibody (B). Corresponding transmitted light images are shown in C and D, respectively. Strong immunofluorescence of activated β1-integrin was localized on the plasma membrane after activation. Scale bars in C and D = 10 μm. Mean immunofluorescence intensity was increased by 110 ± 8% (3 experiments, 42 cells) after activation compared with the control.
DISCUSSION
Myogenic response and tubuloglomerular feedback are the two major mechanisms to autoregulate renal blood flow to prevent the fluctuations of arterial blood pressure from being transmitted into the kidneys. The sensor for tubuloglomerular feedback resides on the macula densa of distal tubule in each nephron, which senses the flow-dependent changes in the luminal NaCl concentration. However, the sensing mechanisms for detecting the changes of transmural pressure in myogenic response are still elusive. Interactions between integrins and extracellular matrix might contribute to this mechanotransduction process. It was previously demonstrated that the magnetic pulling of fibronectin-coated paramagnetic beads in renal VSMCs triggers intracellular Ca2+ release from ryanodine receptors as Ca2+ sparks (1). Coupling of local Ca2+ sparks to adjacent Ca2+-activated Cl− channels might lead to membrane depolarization, Ca2+ influx, and myogenic response (22). However, direct evidence establishing the link between integrin-mediated mechanotransduction and myogenic response is still missing. In the present study, cell traction force microscopy was used to monitor the contractile reaction of the entire cell when mechanical force was applied via integrin-dependent and integrin-independent adhesion and when β1-integrin was activated with antibody. Magnetic pulling of fibronectin-coated paramagnetic beads elicited an immediate sizable increase of cell traction force, indicating efficacious transduction of force from paramagnetic beads to the cells. Moreover, the cell traction force remained elevated after the pulling process was terminated. The remanent cell traction force is not due to the residual magnetization of the paramagnetic beads, which are not ferromagnetic. It was not observed when noncoated beads or LDL-coated beads were used, suggesting that the remanent cell traction force is specific to integrin-mediated mechanotransduction. This persistently sustained cell traction force was also found when paramagnetic beads were coated with β1-integrin antibodies. Addition of fibronectin-coated beads and β1-integrin antibody-coated beads to cell induced a small decrease in the cell traction force instead of an increase of cell traction (Figs. 1 and 3C), which suggested that the beads attachment alone did not activate integrin and generate cell traction force. By using atomic force microscopy to directly measure and apply nanoscale force to live cultured VSMCs via fibronectin-coated microbeads, Sun et al. (38) demonstrated that upward mechanical force applied to fibronectin-integrin adhesion sites induces an actin-dependent, myogenic response-like counteracting downward pull. Activation of β1-integrins with antibody triggered a Src-dependent increase of cell traction force. It is known that mechanical force transduced by β1-integrin activates Src in smooth muscle cells at real time (33). Taken together, these observations suggest that activation of β1-integrins and mechanical force transduced by β1-integrin share a common signaling pathway to generate cell traction force.
Integrins exist in both the active and inactive state. Ligand recognition has been shown to induce conformational change in integrins, leading to the exposure of neo-epitopes in ligand-induced binding sites (12, 13). A number of monoclonal antibodies have been shown to activate integrins directly, and a subset of this class of antibodies can recognize ligand-induced binding sites (13, 18). These antibodies can be used to activate specific integrins and to differentiate the active and inactive states of integrins. In this study, the functional antibody Ha2/5 was used to activate β1-integrins, and β1-integrin in activated conformation after Ha2/5 incubation was successfully detected with antibody HUTS-4, confirming our experimental conditions for β1-integrin activation. It is noteworthy that activation of β1-integrins triggered periodic Ca2+ oscillations in renal VSMCs. By sampling cell traction force at 0.2 Hz, the oscillatory remanent cell traction force triggered by β1-integrin-coated bead was detected. The increase of intracellular Ca2+ is a hallmark of myogenic response (30). Hence, the periodic Ca2+ spikes and oscillatory remanent cell traction force induced by the activation of β1-integrins are consistent with this general consensus of myogenic response, as well as the oscillatory nature of myogenic response in renal arterioles (6, 45). Myogenic response in the renal microcirculation of the rat kidney is oscillating at 0.1–0.2 Hz, which is an order of magnitude faster than that of cell traction force oscillation. The kinetic difference of myogenic response in intact renal arterioles and renal VSMCs in culture is mostly likely due to the difference of experimental conditions.
The mechanism for integrin-induced Ca2+ mobilization is unclear, but insights might be gained from the local Ca2+ signaling at the subcellular level. Magnetic pulling of fibronectin-coated beads triggers Ca2+ sparks in freshly isolated renal VSMCs (1). This process is independent of Ca2+ influx since removal of extracellular Ca2+ did not inhibit Ca2+ sparks induced by the pulling force exerted via fibronectin-coated paramagnetic beads (1). Even though it has been shown that Ca2+ influx via L-type Ca2+ channels contributes to global cytosolic Ca2+ in myogenic response, which in turn slowly increases luminal sarcoplasmic reticulum Ca2+ load and Ca2+ sparks activity (11), this process is not closely coupled to the integrin-dependent Ca2+ sparks activation in rat renal VSMCs. Moreover, pulling of fibronectin-coated beads or activation of β1-integrins with antibodies triggered Ca2+ sparks within a few seconds. Hence, it is unlikely that the increase in Ca2+ spark activity is the result of a slow increase of sarcoplasmic reticulum Ca2+ load. Alternatively, integrin-dependent Ca2+ spark activation could be related to cADP-ribose, an endogenous agonist of ryanodine receptors. It has been shown that application of local mechanical force via integrins in smooth muscle activates Src rapidly (<0.3 s) at a remote cytoplasmic site (39), and activation of Src can stimulate ADP-ribose cyclases for cyclic ADP-ribose synthesis (16). This notion is supported by our previous finding in pulmonary VSMCs that the integrin ligand RGD-peptide causes a rapid increase in the level of cyclic ADP-ribose (40). In addition, stretch-induced Ca2+ sparks can be mediated by NO via S-nitrosylation of ryanodine receptors (35, 37). Since integrin stimulation for example by RGD peptides induced phosphorylation of focal adhesion kinase, intracellular Ca2+ release, and NO production (41), integrins might enhance Ca2+ release via FAK/NOS pathways.
In summary, mechanical force applied via the fibronection-α5β1-integrin interactions not only increased the cell traction force of renal VSMCs during but also after the removal of the stimulus. The remanent cell traction force was specific to integrin-mediated mechanotransduction, which was not found with LDL-coated beads. Direct activation of β1-integrins with soluble antibodies triggered periodic Ca2+ spikes and increase of cell traction force, which might account for the oscillatory cell traction force induced by mechanical pulling with β1-integrin antibody-coated beads. In conclusion, mechanical force transduction by α5β1-integrins triggers a myogenic response-like behavior in isolated renal VSMCs.
GRANTS
This study was supported by National Institutes of Health Grants 1R03-CA-123621-01A1 (to C.-M. Lo) and HL-071835 (to J. S. K. Sham) a Grant-In-Aid (toK.-P. Yip) from the American Heart Association Greater Southeast.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.B., C.-M.L., J.S.S., and K.-P.Y. conception and design of research; L.B., C.-M.L., and K.-P.Y. performed experiments; L.B., C.-M.L., J.S.S., and K.-P.Y. analyzed data; L.B., C.-M.L., J.S.S., and K.-P.Y. interpreted results of experiments; L.B., C.-M.L., and K.-P.Y. prepared figures; L.B. and K.-P.Y. drafted manuscript; L.B., C.-M.L., J.S.S., and K.-P.Y. edited and revised manuscript; L.B., C.-M.L., J.S.S., and K.-P.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. M. Dembo for helpful suggestions and technical assistance.
REFERENCES
- 1.Balasubramanian L, Ahmed A, Lo CM, Sham JS, Yip KP. Integrin-mediated mechanotransduction in renal vascular smooth muscle cells: activation of calcium sparks. Am J Physiol Regul Integr Comp Physiol 293: R1586–R1594, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Beningo KA, Lo CM, Wang YL. Flexible polyacrylamide substrata for the analysis of mechanical interactions at cell-substratum adhesions. Methods Cell Biol 69: 325–339, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Carmines PK, Inscho EW, Gensure RC. Arterial pressure effects on preglomerular microvasculature of juxtamedullary nephrons. Am J Physiol Renal Fluid Electrolyte Physiol 258: F94–F102, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Chao JT, Gui P, Zamponi GW, Davis GE, Davis MJ. Spatial association of the Cav1.2 calcium channel with α5β1-integrin. Am J Physiol Cell Physiol 300: C477–C489, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 262: 740–744, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Chon KH, Raghavan R, Chen YM, Marsh DJ, Yip KP. Interactions of TGF-dependent and myogenic oscillations in tubular pressure. Am J Physiol Renal Physiol 288: F298–F307, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Davis GE, Hill MA, Meininger GA. Integrins and mechanotransduction of the vascular myogenic response. Am J Physiol Heart Circ Physiol 280: H1427–H1433, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J 76: 2307–2316, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drummond HA, Grifoni SC, Jernigan NL. A new trick for an old dogma: ENaC proteins as mechanotransducers in vascular smooth muscle. Physiology (Bethesda) 23: 23–31, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Essin K, Welling A, Hofmann F, Luft FC, Gollasch M, Moosmang S. Indirect coupling between Cav1.2 channels and ryanodine receptors to generate Ca2+ sparks in murine arterial smooth muscle cells. J Physiol 584: 205–219, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frelinger AL, 3rd, Cohen I, Plow EF, Smith MA, Roberts J, Lam SC, Ginsberg MH. Selective inhibition of integrin function by antibodies specific for ligand-occupied receptor conformers. J Biol Chem 265: 6346–6352, 1990 [PubMed] [Google Scholar]
- 13.Frelinger AL, Du XP, 3rd, Plow EF, Ginsberg MH. Monoclonal antibodies to ligand-occupied conformers of integrin alpha IIb beta 3 (glycoprotein IIb-IIIa) alter receptor affinity, specificity, and function. J Biol Chem 266: 17106–17111, 1991 [PubMed] [Google Scholar]
- 14.Gordienko DV, Clausen C, Goligorsky MS. Ionic currents and endothelin signaling in smooth muscle cells from rat renal resistance arteries. Am J Physiol Renal Fluid Electrolyte Physiol 266: F325–F341, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Gui P, Chao JT, Wu X, Yang Y, Davis GE, Davis MJ. Coordinated regulation of vascular Ca2+ and K+ channels by integrin signaling. Adv Exp Med Biol 674: 69–79, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Gul R, Kim SY, Park KH, Kim BJ, Kim SJ, Im MJ, Kim UH. A novel signaling pathway of ADP-ribosyl cyclase activation by angiotensin II in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol 295: H77–H88, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Hill MA, Davis MJ, Meininger GA, Potocnik SJ, Murphy TV. Arteriolar myogenic signalling mechanisms: implications for local vascular function. Clin Hemorheol Microcirc 34: 67–79, 2006 [PubMed] [Google Scholar]
- 18.Humphries JD, Schofield NR, Mostafavi-Pour Z, Green LJ, Garratt AN, Mould AP, Humphries MJ. Dual functionality of the anti-beta1 integrin antibody, 12G10, exemplifies agonistic signalling from the ligand binding pocket of integrin adhesion receptors. J Biol Chem 280: 10234–10243, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J 20: 811–827, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res 91: 877–887, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci USA 98: 1042–1046, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji G, Barsotti RJ, Feldman ME, Kotlikoff MI. Stretch-induced calcium release in smooth muscle. J Gen Physiol 119: 533–544, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Hu Z, Li C. New method for measuring Poisson's ratio in polymer gels. J Appl Polym Sci 50: 1107–1111, 1993 [Google Scholar]
- 24.Lo CM, Buxton DB, Chua GC, Dembo M, Adelstein RS, Wang YL. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol Biol Cell 15: 982–989, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo CM, Glogauer M, Rossi M, Ferrier J. Cell-substrate separation: effect of applied force and temperature. Eur Biophys J 27: 9–17, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J 79: 144–152, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsh DJ, Osborn JL, Cowley AW., Jr 1/f fluctuations in arterial pressure and regulation of renal blood flow in dogs. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1394–F1400, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Lemus LA, Wu X, Wilson E, Hill MA, Davis GE, Davis MJ, Meininger GA. Integrins as unique receptors for vascular control. J Vasc Res 40: 211–233, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP. Calcium measurement in isolated arterioles during myogenic and agonist stimulation. Am J Physiol Heart Circ Physiol 261: H950–H959, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP. Calcium measurement in isolated arterioles during myogenic and agonist stimulation. Am J Physiol Heart Circ Physiol 261: H950–H959, 1991 [DOI] [PubMed] [Google Scholar]
- 31.Mori T, Cowley AW., Jr Role of pressure in angiotensin II-induced renal injury: chronic servo-control of renal perfusion pressure in rats. Hypertension 43: 752–759, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Muranyi A, MacDonald JA, Deng JT, Wilson DP, Haystead TA, Walsh MP, Erdodi F, Kiss E, Wu Y, Hartshorne DJ. Phosphorylation of the myosin phosphatase target subunit by integrin-linked kinase. Biochem J 366: 211–216, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci USA 105: 6626–6631, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagayama K, Adachi A, Matsumoto T. Heterogeneous response of traction force at focal adhesions of vascular smooth muscle cells subjected to macroscopic stretch on a micropillar substrate. J Biomech 44: 2699–2705, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Petroff MG, Kim SH, Pepe S, Dessy C, Marban E, Balligand JL, Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol 3: 867–873, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Remillard CV, Zhang WM, Shimoda LA, Sham JS. Physiological properties and functions of Ca2+ sparks in rat intrapulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 283: L433–L444, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Salama G, Menshikova EV, Abramson JJ. Molecular interaction between nitric oxide and ryanodine receptors of skeletal and cardiac sarcoplasmic reticulum. Antioxid Redox Signal 2: 5–16, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Sun Z, Martinez-Lemus LA, Hill MA, Meininger GA. Extracellular matrix-specific focal adhesions in vascular smooth muscle produce mechanically active adhesion sites. Am J Physiol Cell Physiol 295: C268–C278, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Z, Martinez-Lemus LA, Trache A, Trzeciakowski JP, Davis GE, Pohl U, Meininger GA. Mechanical properties of the interaction between fibronectin and α5β1-integrin on vascular smooth muscle cells studied using atomic force microscopy. Am J Physiol Heart Circ Physiol 289: H2526–H2535, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Umesh A, Thompson MA, Chini EN, Yip KP, Sham JS. Integrin ligands mobilize Ca2+ from ryanodine receptor-gated stores and lysosome-related acidic organelles in pulmonary arterial smooth muscle cells. J Biol Chem 281: 34312–34323, 2006 [DOI] [PubMed] [Google Scholar]
- 41.van der Wees CG, Bax WH, van der Valk EJ, van der Laarse A. Integrin stimulation induces calcium signalling in rat cardiomyocytes by a NO-dependent mechanism. Pflügers Arch 451: 588–595, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Yip KP, Holstein-Rathlou NH. Chaos and non-linear phenomena in renal vascular control. Cardiovasc Res 31: 359–370, 1996 [PubMed] [Google Scholar]
- 44.Yip KP, Holstein-Rathlou NH, Marsh DJ. Chaos in blood flow control in genetic and renovascular hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 261: F400–F408, 1991 [DOI] [PubMed] [Google Scholar]
- 45.Yip KP, Holstein-Rathlou NH, Marsh DJ. Mechanisms of temporal variation in single-nephron blood flow in rats. Am J Physiol Renal Fluid Electrolyte Physiol 264: F427–F434, 1993 [DOI] [PubMed] [Google Scholar]
- 46.Yip KP, Marsh DJ. An Arg-Gly-Asp peptide stimulates constriction in rat afferent arteriole. Am J Physiol Renal Physiol 273: F768–F776, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol 285: L680–L690, 2003 [DOI] [PubMed] [Google Scholar]