Abstract
The serotonin (5-HT) transporter (SERT) facilitates clearance of extracellular 5-HT by its uptake and internalization. Decreased expression of SERT and consequent high 5-HT levels have been implicated in various diarrheal disorders. Thus, appropriate regulation of SERT is critical for maintenance of 5-HT homeostasis in health and disease. Previous studies demonstrated that SERT is regulated via posttranslational and transcriptional mechanisms. However, the role of epigenetic mechanisms in SERT regulation is not known. Current studies investigated the effects of histone deacetylase (HDAC) inhibition on SERT expression and delineated the mechanisms. Treatment of Caco-2 cells with the pan-HDAC inhibitors butyrate (5 mM) and trichostatin (TSA, 1 μM) decreased SERT mRNA and protein levels. Butyrate- or TSA-induced decrease in SERT was associated with decreased activity of human SERT (hSERT) promoter 1 (upstream of exon 1a), but not hSERT promoter 2 (upstream of exon 2). Butyrate + TSA did not show an additive effect on SERT expression, indicating that mechanisms involving histone hyperacetylation may be involved. Chromatin immunoprecipitation assays demonstrated enrichment of the hSERT promoter 1 (flanking nt −250/+2) with tetra-acetylated histone H3 or H4, which was increased (∼3-fold) by butyrate. Interestingly, specific inhibition of HDAC2 (but not HDAC1) utilizing small interfering RNA decreased SERT mRNA and protein levels. The decrease in SERT expression by HDAC inhibition was recapitulated in an in vivo model. SERT mRNA levels were decreased in the ileum and colon of mice fed pectin (increased availability of butyrate) compared with controls fed a fiber-free diet (∼50–60%). Our results identify a novel role of HDAC2 as a regulator of SERT gene expression in intestinal epithelial cells.
Keywords: SERT, histone deacetylase inhibition, trichostatin A, butyrate epigenetics
serotonin (5-HT) plays diverse roles in the gastrointestinal (GI) tract, ranging from modulation of electrolyte absorption, maintenance of fluid homeostasis, alterations in GI motility, and regulation of gut permeability (9, 20, 23, 24, 39, 44). High 5-HT levels are implicated in the pathophysiology of carcinoid syndrome, dumping syndrome, inflammation, or enteric infections (40). Since 5-HT mediates its actions via various receptor subtypes, it is critical to maintain optimal extracellular availability of 5-HT in the gut to facilitate its physiological actions and prevent receptor desensitization. In this regard, the intestinal 5-HT transporter (SERT, SLC6A4) plays a key role in clearance of 5-HT by its rapid uptake through an apically localized, fluoxetine-sensitive NaCl-dependent transport process (19, 35, 36). Several lines of evidence support downregulation of SERT in inflammatory or diarrheal disorders. For example, SERT expression is decreased in ulcerative colitis patients and in several experimental models of colitis (10, 13, 30). Similarly, targeted deletion of SERT in mice results in increased stool water content (indicative of diarrheal phenotype), an abnormal pattern of motility, and exacerbation of inflammatory responses (3, 8). Despite these reports acknowledging the importance of SERT as a novel target for GI disorders, not much is known regarding the cellular and molecular mechanisms underlying the regulation of SERT in health or disease.
Emerging studies from our laboratory and others have shown that SERT activity is regulated rapidly via posttranslational mechanisms, such as cellular protein kinases or protein tyrosine phosphatases, or via alterations in SERT surface levels (12, 37). In addition, recent studies have demonstrated SERT regulation via alterations in its gene expression. Foley et al. (14) demonstrated SERT downregulation in response to proinflammatory agents such as TNF and IFNγ. Our recent findings demonstrated that SERT expression is upregulated by EGF via transcriptional mechanisms (17). Whether epigenetic mechanisms are involved in modulating SERT expression in intestinal epithelial cells (IECs) is not known.
Epigenetic modifications occur via direct modification on DNA (such as methylation at CpG sites) or involve alterations in the methylation or acetylation status of chromatin-associated histones. Histone acetylation is an essential epigenetic mechanism that controls chromatin structure, DNA accessibility to transcription factors, and modulation of gene expression. Dysregulated expression or activity of histone deacetylase (HDAC) has been implicated in pathogenesis of cancer and inflammatory and autoimmune diseases (1). Thus, HDAC inhibitors have emerged as potential candidates for treatment of cancer or inflammatory bowel diseases (1, 21, 32, 42, 43). The present studies utilize in vitro and in vivo murine models to examine the effects of HDAC inhibition on SERT.
Our results, for the first time, demonstrate that SERT expression is decreased by treatment of Caco-2 cells with the HDAC inhibitors butyrate and trichostatin A (TSA). This inhibition in SERT expression was mimicked by small interfering RNA (siRNA) inhibition of HDAC2, but not HDAC1. The SERT gene is under the regulation of two alternate promoters: human SERT (hSERT) promoter 1 (hSERTp1, upstream of exon 1a) and an alternate, hSERT promoter 2 (hSERTp2, upstream of exon 2) (17, 31). Interestingly, HDAC inhibition by butyrate or TSA decreased SERT gene promoter activity (hSERTp1, upstream of exon 1a). Progressive deletions of hSERTp1 indicated that the putative butyrate- or TSA-responsive elements are present in the region flanking nucleotides −272/+2 of hSERTp1. Consistent with these data, chromatin immunoprecipitation (ChIP) assays indicated an enrichment of the region flanking nucleotides −250/+2 with acetylated histone H3 or H4 that was further increased by butyrate. Similar to the in vitro model, in mice, pectin feeding (which results in high colonic butyrate levels by anaerobic fermentation) decreased SERT mRNA expression in the ileum and distal colon. These data indicate a novel mechanism of downregulation of intestinal SERT by epigenetic mechanisms involving HDAC2 inhibition and increased association of histone H3 or H4 acetylation with hSERTp1.
METHODS
Cell culture.
Caco-2 cells were grown in 75-cm2 plastic flasks at 37°C in a 5% CO2 environment. The culture medium consisted of high-glucose MEM, 20% FBS, 20 mM HEPES, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Cells at passages 25–45 were plated on 24-well plastic supports or 12-well Transwell filters at a density of 2 × 104 cells/well. Cells were used for experiments at days 10–12 postplating. Fully differentiated Caco-2 monolayers were treated with HDAC inhibitors for 24 h in MEM supplemented with 0.1% FBS.
Animal models.
In vivo studies utilizing C57BL/6J mice (Jackson Laboratory) were approved by the Animal Care Committees of University of Illinois at Chicago and Jesse Brown Veterans Affairs Medical Center. Male mice (25–28 g body wt) were divided into control and pectin-fed groups. The control group received a fiber-free elemental diet (catalog no. TD97201, Harlan Laboratories), while the pectin-fed group was given a pectin-containing pellet diet (6% diet; catalog no. TD97202, Harlan Laboratories). After 1 wk of feeding, mice were euthanized under anesthesia utilizing a bottled CO2 source and cervical dislocation. Immediately thereafter, intestines were resected, and the mucosa was scraped for RNA extraction from different regions of the intestine and stored at −80°C.
Real time RT-PCR studies.
The relative abundance of SERT mRNA from control and butyrate-treated Caco-2 cells or from mouse intestine was quantitated using real-time RT-PCR. Briefly, RNA was extracted from Caco-2 cells or mouse tissues with use of a total RNA kit according to the manufacturer's instructions (Stratagene, La Jolla, CA). cDNA formation and PCR amplification were carried out by SYBR Green One-Step Real-Time PCR Master Mix utilizing the Mx3000P system (Stratagene). The primers for hSERT and β-actin are described elsewhere (17, 19).
Western blotting.
Control or treated Caco-2 cells were washed with ice-cold 1× PBS and lysed in 20 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, and 1× protease cocktail inhibitor mixture. The cells were lysed by sonication, and the lysate was centrifuged at 7,000 rpm for 7 min at 4°C. Protein concentration was determined by the Bradford assay. To examine the expression levels of SERT, 100 μg of cell lysates were loaded on SDS-polyacrylamide gels and transblotted to nitrocellulose membranes. After 1 h in 1× PBS-5% nonfat dry milk blocking buffer, the membranes were probed with monoclonal SERT antibody (Abcam; 1:75 dilution) or GAPDH antibody (1:1,000 dilution) in 1× PBS and 2.5% nonfat dry milk overnight at 4°C. The membranes were washed four times with the wash buffer containing 1× PBS and 0.1% Tween 20 for 5 min. Finally, the membranes were probed with horseradish peroxidase-conjugated goat anti-rabbit (for GAPDH) or goat anti-mouse (for SERT) IgG antibody (1:2,000 dilution) for 1 h, and the bands were visualized with enhanced chemiluminescence detection reagents.
Promoter cloning.
The SERT gene is under the control of two alternate promoters: hSERTp1 (upstream of exon 1a) and hSERTp2 (upstream of exon 2 and extending upstream of exon 1c) (31). The 5′-flanking regions of the hSERT gene were cloned [nt −872/+2 relative to the transcription initiation site (+1) for hSERTp1 and nt −1195/+123 for hSERTp2, where +1 is the start of exon 2] by PCR utilizing human genomic DNA, gene-specific primers, and Elongase enzyme mix (Invitrogen, Carlsbad, CA). The sequences for cloning full-length promoter and progressive deletions have been described by us previously (17).
Transient transfection and luciferase assay.
For transfection studies, Caco-2 cells were transfected with Lipofectamine reagent with hSERTp-luciferase constructs and pCMVβ (β-galactosidase mammalian expression vector; Clontech, Mountain View, CA). At 24 h posttransfection, cells were treated apically with butyrate or TSA for 24 h in serum-free medium containing 0.1% FBS. Cells were then plated and, at 24 h posttransfection, lysed in passive lysis buffer (Promega), and the activities of firefly luciferase (Promega) and β-galactosidase were measured by luminometer according to the manufacturer's instructions utilizing commercially available kits. The promoter activity was expressed as the ratio of luciferase to β-galactosidase activity (relative luciferase activity) in each sample.
ChIP assays.
ChIP assay was performed utilizing the commercially available CHIP One-Day Kit essentially according to the manufacturer's instructions (Qiagen). Briefly, untreated cells or cells treated with butyrate (5 mM) were fixed in 1% (vol/vol) formaldehyde, and chromatin was sonicated; then immunoprecipitated with 5 μg of specific antibodies against tetra-acetylated histone H3 or H4 or tetra-methylated histone H3 or H4 (overnight) at room temperature (Upstate Biotechnology). Normal rabbit IgG was used as a control (Upstate Biotechnology). After reverse cross-linking and DNA extraction, immunoprecipitated chromatin was used as the template for real-time quantitative PCR utilizing the primers spanning region flanking nucleotides −872/+2 of hSERTp1. At the end of amplification, PCR products were separated on 1% agarose gel containing ethidium bromide and run using 0.5× Tris-borate-EDTA buffer.
siRNA experiments.
Expression of HDAC1 or HDAC2 was selectively silenced utilizing predesigned siRNAs (Qiagen). Scrambled siRNA was used as nontargeting control. Caco-2 cells were transiently transfected with 100 pmol of siRNA duplexes for 24–72 h using Lipofectamine (Roche). Silencing was validated by real-time PCR and Western blotting utilizing HDAC1- or HDAC2-specific primers and specific antibodies (Abcam).
Statistical analysis.
Values are means ± SE. One-way ANOVA or Student's t-test was utilized for statistical analysis. P ≤ 0.05 was considered statistically significant.
RESULTS
Butyrate and TSA decrease SERT expression.
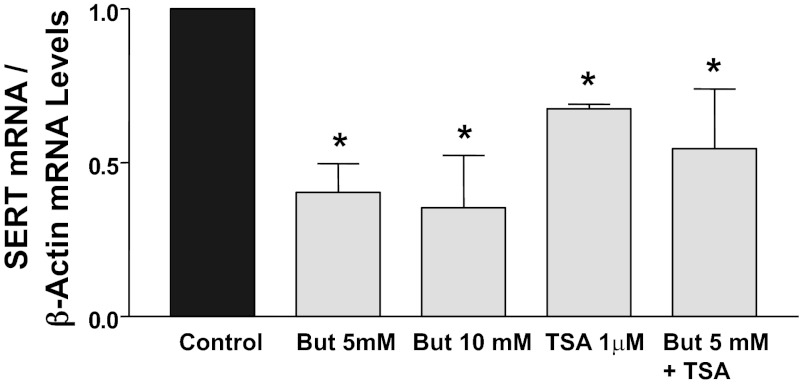
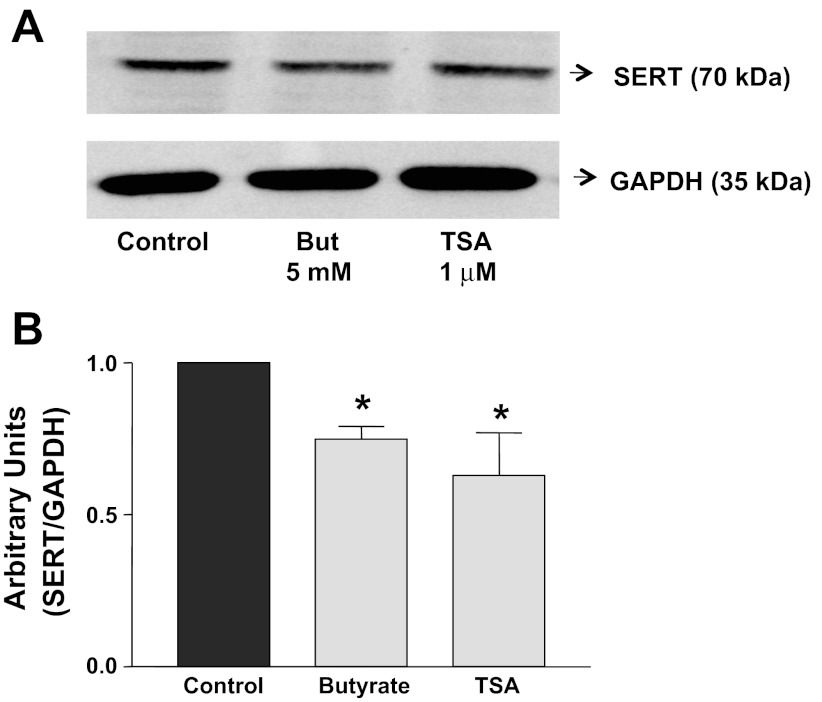
Butyrate and TSA are two well-known and classical inhibitors of HDACs. To determine whether alterations in histone acetylation modulate SERT expression, we investigated the endogenous expression of SERT following HDAC inhibition. Figure 1 demonstrates that treatment of Caco-2 cells with 5 or 10 mM sodium butyrate for 24 h significantly decreased SERT mRNA levels to a similar magnitude (∼60%). Thus 5 mM butyrate was used for subsequent studies. TSA (1 μM, 24 h) also decreased SERT mRNA levels (∼45%) compared with control (Fig. 1). The effects of TSA + butyrate (5 mM) were not additive, indicating that hyperacetylation of histones may be involved in decreasing SERT mRNA expression. To examine whether butyrate or TSA decreases SERT protein expression, Western blotting was performed utilizing a specific anti-SERT antibody. Consistent with mRNA, butyrate or TSA decreased SERT protein expression (Fig. 2). In addition, Caco-2 cells treated with 5 mM butyrate or 1 μM TSA showed no significant alterations in viability as measured by lactate dehydrogenase release (data not shown).
Fig. 1.
Treatment of Caco-2 cells with histone deacetylase (HDAC) inhibitors decreases 5-HT transporter (SERT) mRNA expression. Caco-2 cells grown on plastic supports for 10–12 days were treated for 24 h with butyrate (But, 5–10 mM), or trichostatin A (TSA, 1 μM), or butyrate (5 mM) + TSA (1 μM) in culture medium supplemented with 0.1% FBS. Total RNA was extracted, and quantitative real-time RT-PCR was performed utilizing SYBR Green fluorescent dye. SERT mRNA levels were normalized to levels of β-actin mRNA. Values are means ± SE from ≥3 different experiments performed in triplicate. *P < 0.05 vs. control. Similar results were obtained from Caco-2 cells grown on Transwell inserts for 12 days (data not shown).
Fig. 2.
Butyrate or TSA decreases SERT protein expression. Fully differentiated Caco-2 cells (grown for 10–12 days) were treated for 24 h with 5 mM butyrate, 1 μM TSA, or 5 mM butyrate + 1 μM TSA in cell culture medium supplemented with 0.1% FBS. Protein lysates were prepared, run on SDS-PAGE, and transblotted, and Western blotting was performed utilizing anti-SERT antibody. A: representative blot of 3 different experiments. B: densitometric analysis showing relative expression of SERT normalized to GAPDH. *P < 0.05 vs. control.
Effect of butyrate and TSA on the promoter activities of hSERT.
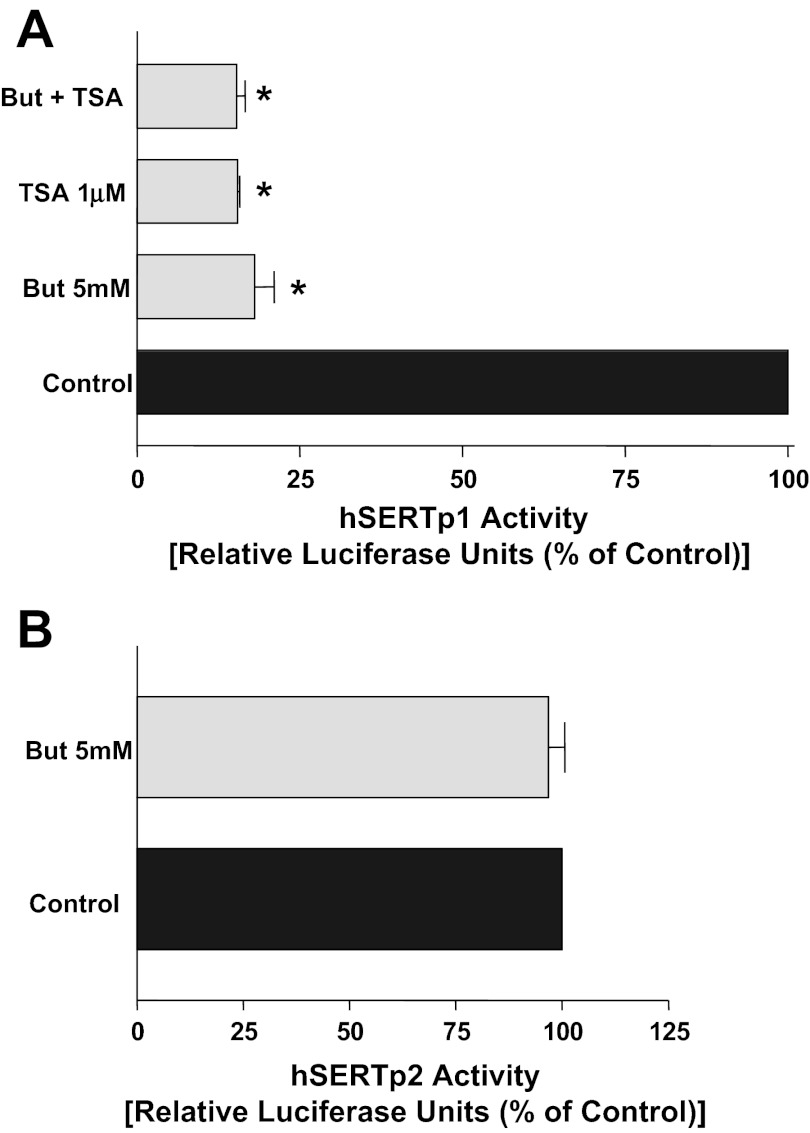
To elucidate the molecular mechanisms underlying decreased SERT expression by butyrate or TSA, we next examined whether SERT promoter activity is altered. The SERT gene is under the regulation of two alternate promoters: hSERTp1 (upstream of exon 1a) and the recently identified intestinal-specific promoter hSERTp2 (upstream of exon 2 and extending upstream of exon 1c). The promoter activity was assessed by transient cotransfection of promoter constructs in Caco-2 cells, along with the β-galactosidase mammalian expression vector as an internal control to adjust for transfection efficiency. Results demonstrated that butyrate or TSA had differential effects on hSERTp1 and hSERTp2. Interestingly, 5 mM butyrate decreased hSERTp1 (flanking nt −872 and +2) activity (Fig. 3A), with no effect at 1 mM butyrate (not shown). In addition, TSA mimicked the effects of butyrate on hSERTp1 activity, with no additive effect observed with TSA + butyrate (Fig. 3A). In contrast to hSERTp1, butyrate or TSA had no effect on hSERTp2 activity (Fig. 3B). These results suggest that inhibition of SERT by butyrate or TSA occurs specifically via suppression of hSERTp1.
Fig. 3.
Effects of butyrate or TSA on human SERT (hSERT) promoter (hSERTp1 and hSERTp2) activities in Caco-2 cells. A: effect of 5 mM butyrate or 1 μM TSA on hSERTp1 activity. Caco-2 cells were transiently cotransfected with the hSERTp1 fragment, along with β-galactosidase vector to correct for transfection efficiency. At 24 h posttransfection, cells were incubated with butyrate, TSA, or butyrate + TSA for another 24 h. Butyrate or TSA decreased hSERTp1 activity, with no additive effect on simultaneous addition. Values are means ± SE of 4–5 different experiments performed in triplicate. *P < 0.001 vs. control. B: effect of 5 mM butyrate on hSERTp2 activity. Caco-2 cells were transiently cotransfected with the hSERTp2 fragment, along with β-galactosidase vector. At 24 h posttransfection, cells were incubated with butyrate for another 24 h. Values are means ± SE of 3 different experiments performed in triplicate.
Identification of the butyrate- or TSA-responsive region in hSERTp1.
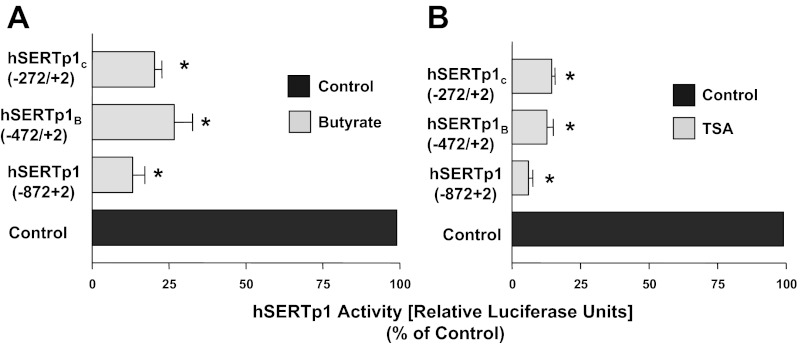
Since hSERTp1 was inhibited by HDAC inhibition, we next determined which specific region in hSERTp1 is responsive to HDAC inhibitors. For these studies, the effect of butyrate or TSA was assessed utilizing different fragments of the hSERTp1 representing 5′-progressive deletions of the promoter. Treatment of Caco-2 cells with 5 mM butyrate resulted in a marked decrease in relative luciferase activity (normalized to β-galactosidase activity) of each promoter construct (Fig. 4A). Similar to butyrate, deletion to the −472/+2 (hSERTp1B) or −272/+2 (hSERTp1c) fragment retained the TSA-mediated inhibition compared with the respective controls taken as 100% (Fig. 4B). These data indicate that the HDAC inhibitors act in the region flanking nucleotides −272/+2 of hSERTp1.
Fig. 4.
Butyrate- or TSA-responsive regions of hSERTp1 (hSERTp1B and hSERTp1C). A: progressive 5′ deletions of hSERTp1 treated with butyrate. Different promoter constructs of hSERTp1 were treated with 5 mM butyrate for 24 h. At 24 h posttreatment, cells were harvested for measurement of promoter activity by luciferase assay. Values were normalized to β-galactosidase to adjust for transfection efficiency. Inhibitory effect of butyrate was retained in all constructs. Values are means ± SE from ≥3 different experiments performed in triplicate. *P < 0.001 vs. control. B: progressive 5′-deletion constructs of hSERTp1 treated with TSA. Similar to butyrate, TSA-responsive region predominantly spans nucleotides −272/+2 of hSERTp1. Values are means ± SE from ≥3 different experiments performed in triplicate. *P < 0.001 vs. control.
Butyrate alters histone status at hSERTp1.
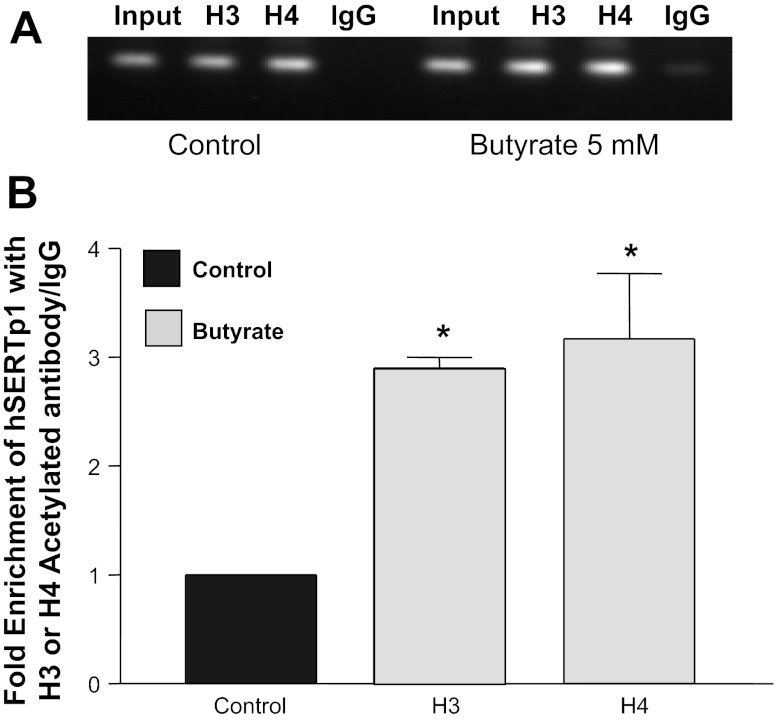
Given that butyrate and TSA inhibit HDACs, we next examined if alterations in histone status at hSERTp1 contribute to SERT regulation. We examined the association of acetylated histones with hSERTp1 by ChIP assays. Chromatin from control cells and cells treated with 5 mM butyrate was immunoprecipitated with tetra-acetylated or -methylated histone H3 or H4 antibody. Enrichment of hSERTp1 with the immunoprecipitates of histone H3 or H4 histone was assessed by real-time PCR utilizing specific primers flanking nucleotides −872/+2. Figure 5A shows the PCR products separated on 1% agarose gel depicting enrichment with the specific antibodies, but not with IgG (negative control). Quantification of the data demonstrated that, in control cells, hSERTp1 spanning nucleotides −250/+2 was mainly associated with tetra-acetylated histone H3 or H4. Treatment with butyrate resulted in stronger association (∼3-fold) of hSERTp1 with acetylated histone H3 or H4 (Fig. 5). However, no enrichment of hSERTp1 region with methylated histone H3 or H4 was observed in control or butyrate-treated cells (data not shown). These data indicate that histone at the SERT promoter is characterized by modifications involving hyperacetylation of histone H3 or H4 that may indicate activation of chromatin and modulation of transcription.
Fig. 5.
Butyrate alters histone status at hSERTp1. Control cells or cells treated with butyrate (5 mM) were fixed in formaldehyde, chromatin was sonicated, and immunoprecipitated with specific antibodies against tetra-acetylated histone H3 or H4. After reverse cross-linking and DNA extraction, immunoprecipitated chromatin was used as the template for real-time quantitative PCR utilizing primers spanning nucleotides −872/+2 of hSERTp1. A: PCR products at the end of amplification separated on 1% agarose gel containing ethidium bromide. B: enrichment of acetylated histone H3 or H4 with hSERTp1 at nucleotides −250/+2 by butyrate. Values are means ± SE from 3 different experiments. *P < 0.01 vs. control.
HDAC expression in Caco-2 cells.
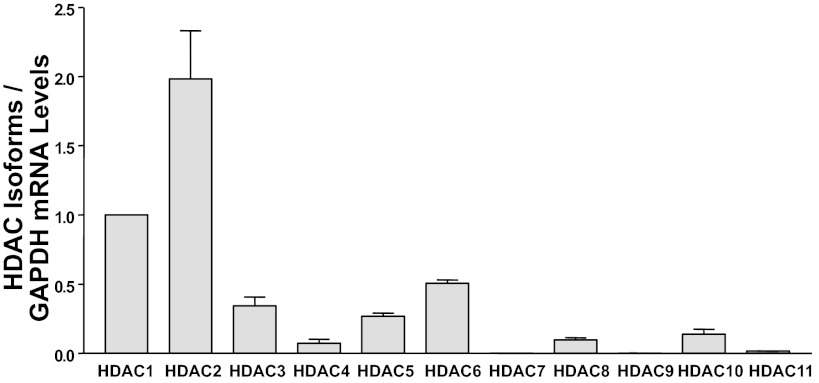
The commonly utilized HDAC inhibitors such as butyrate and TSA are nonspecific inhibitors of class I HDACs (HDAC1, HDAC2, HDAC3, and HDAC8) and class II HDACs (HDAC4, HDAC5, HDAC6, HDAC7, HDAC9, and HDAC10) (28). Therefore, to specifically dissect which HDAC inhibits SERT, we initially examined expression of HDAC1, HDAC2, HDAC3, HDAC4, HDAC5, HDAC6, HDAC7, HDAC8, HDAC9, HDAC10, and HDAC11 mRNA in Caco-2 cells. Results revealed that Caco-2 cells express all the HDACs, with HDAC7 and HDAC9 expressed at the lowest levels. Figure 6 demonstrates the expression of different HDACs relative to HDAC1. As shown in Fig. 6, HDAC1 and HDAC2 are the predominant isoforms expressed in Caco-2 cells.
Fig. 6.
Expression of HDAC1–11 in Caco-2 cells. Total RNA was extracted from Caco-2 cells, and quantitative real-time RT-PCR was performed utilizing primers specific for human HDAC1–11. HDAC mRNA levels were normalized to levels of GAPDH mRNA. Results are expressed as relative expression compared with levels of HDAC1 mRNA taken as 1. Values are means ± SE from ≥3 different experiments performed in triplicate.
HDAC2 regulates SERT mRNA expression.
To directly test the role of HDAC1 or HDAC2 in modulating SERT expression, we utilized different predesigned siRNAs to silence their individual expression in Caco-2 cells. After 48 h of treatment with 100 nM siRNA duplexes, selective downregulation of HDAC1 or HDAC2 mRNA (Fig. 7, A and B) normalized to GAPDH as an internal control was achieved compared with scrambled siRNA controls. The levels of HDAC1 mRNA remained unaltered with HDAC2 silencing, and vice versa, indicating specificity of inhibition (Fig. 7, A and B). Interestingly, SERT mRNA levels were decreased specifically (∼45%) with HDAC2 silencing, with no effect observed with HDAC1 inhibition (Fig. 7C).
Fig. 7.
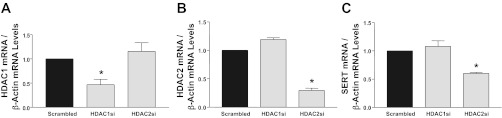
Small interfering RNA (siRNA) silencing of HDAC2 inhibits SERT mRNA expression. mRNA levels of HDAC1 (A), HDAC2 (B), or SERT (C) were measured and normalized to GAPDH in Caco-2 cells treated with scrambled, HDAC1, or HDAC2 siRNA for 48 h. Values are means ± SE of values obtained from ≥3 different experiments performed in triplicate. *P < 0.01 vs. control.
HDAC2 silencing inhibits SERT protein expression.
We next examined if the decrease in SERT mRNA by HDAC2 inhibition also occurs at the protein level. Treatment of Caco-2 cells with HDAC1- or HDAC2-specific siRNA duplexes for 72 h decreased HDAC1 or HDAC2 protein levels, respectively (Fig. 8A), demonstrating efficient knockdown. Densitometric analysis showed an ∼80% reduction in HDAC2 (but not HDAC1) protein levels after silencing of HDAC2 in Caco-2 cells (Fig. 8B). Similar to inhibition by butyrate or TSA, HDAC2 inhibition by siRNA reduced SERT protein levels by ∼50%. There was no effect on SERT protein expression in response to HDAC1 inhibition (Fig. 8C). These findings suggest a novel role of HDAC2 in modulating SERT expression.
Fig. 8.
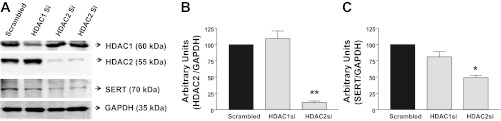
siRNA silencing of HDAC2 inhibits SERT protein levels. A: representative gel showing protein levels of HDAC1, HDAC2, SERT, and GAPDH in Caco-2 cells treated with scrambled, HDAC1, or HDAC2 siRNA for 72 h. B: densitometric analysis of HDAC2 after siRNA knockdown. C: densitometric analysis of SERT protein levels after siRNA knockdown of HDAC1 or HDAC2. Values are means ± SE of ≥3 different experiments performed in triplicate. *P < 0.01; **P < 0.001 vs. control (scrambled).
Pectin feeding to mice decreases SERT mRNA and protein levels.
Pectin is a soluble dietary fiber that increases the production of short-chain fatty acids (SCFAs) such as butyrate in the large intestine. To examine whether SERT expression is modulated by butyrate in vivo, we utilized the in vivo model of mice fed a fiber-free diet (control) or mice fed pectin for 7 days. Our previous studies demonstrated that, similar to human intestine, SERT mRNA levels were higher in mouse small intestine than colon. Interestingly, pectin feeding decreased SERT mRNA expression in the ileum and distal colon, with no alteration in the jejunum (Fig. 9). These data recapitulate the observations in the in vitro model.
Fig. 9.
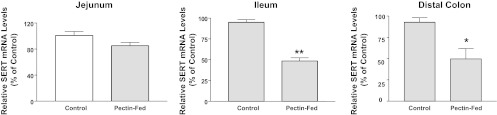
Pectin feeding decreases SERT mRNA in native mouse intestine. Total RNA was extracted from different regions of intestine isolated from control and pectin-fed mice. SERT mRNA levels were normalized to levels of β-actin/GAPDH mRNA. **P < 0.001; *P < 0.05 vs. control.
DISCUSSION
SERT is an important pharmacological target of selective 5-HT reuptake inhibitors, such as fluoxetine, frequently prescribed in anxiety disorders. In the GI tract, a decrease in SERT and consequent high 5-HT levels have been implicated in the pathogenesis of several diarrheal diseases associated with enteric infections or inflammatory bowel diseases (9, 10, 12, 13, 30). Thus, SERT is emerging as an important target for treatment of diarrheal and inflammatory diseases. However, very little is known regarding mechanisms regulating SERT in the human intestine. Our current findings present novel evidence about the role of epigenetic mechanisms involving histone modifications in regulating SERT expression in IECs. Histone acetylation is regulated by two classes of enzymes: histone acetyltransferases, which add acetyl groups to specific amino acids of histones, and HDACs, which catalyze the removal of the acetyl groups from histones or specific transcription factors. Our data demonstrate that inhibition of HDACs by the classical inhibitors butyrate and TSA decreased SERT mRNA and protein levels in human intestinal Caco-2 cells.
Interestingly, the colon is an essential site for production of the SCFAs acetate, propionate, and butyrate, produced as end products of bacterial fermentation of undigested carbohydrates (16, 25). Of the SCFAs, butyrate is the most potent HDAC inhibitor in the intestine and is known to exhibit its effect on various cellular functions via mechanisms dependent on or independent of its histone deacetylation capability (5, 16). In the GI tract, SERT is apically localized, evenly distributed across the crypt villus axis, and differentially expressed across the length of the GI tract. For example, our previous studies utilizing Northern blotting of human resected intestinal samples, as well as organ donor intestine, showed higher SERT mRNA and protein expression in the ileum and lower expression in the colonic epithelium (19). Given that the SCFAs are the most predominant anions in the colonic lumen, our data demonstrating downregulation of SERT expression in response to butyrate may explain the lower expression of SERT in the colon than in the small intestine (in which SCFAs are less available). The concentration of butyrate used in the present study (5 mM) is relevant to the physiological concentration of SCFAs in the normal colonic lumen (50–100 mM) (16, 25). The effects on SERT were specific, as previous studies have shown that butyrate (at similar concentrations) increased the expression of Na+/H+ exchanger 3 (27, 33), downregulated in adenoma (DRA) (2), and monocarboxylate transporter 1 (MCT1) (5) in IECs.
Since the effects of butyrate in IECs vary depending on the experimental model (in vitro vs. in vivo) and the doses used in the study, we further examined if the effects of butyrate can be validated in an in vivo model. Pectin is known to be almost completely fermented to SCFAs in the large intestine (15). Similar to in vitro results, in mice fed a pectin diet for 7 days, SERT mRNA levels in the ileum and distal colon were decreased. Under similar conditions, the mRNA expression of other transporters, such as MCT1, was increased in the colon in response to the pectin diet, as reported previously (29) (data not shown). Since SERT is predominantly expressed in the small intestine (19), Caco-2 monolayers represent an excellent in vitro model system to study its regulation. Although colonic in origin, Caco-2 cells on differentiation manifest anatomic and functional similarities to ileal enterocytes and are well equipped with functional serotonergic machinery. Previous studies from our laboratory and others showed suitability of Caco-2 cells for examining regulation of electrolyte and 5-HT transport processes (12, 14, 18, 20).
HDACs comprise a family of 18 genes, grouped into classes I-IV (42). Classes I, II, and IV consist of 11 family members, referred to as “classical” HDACs, whereas class III members are known as sirtuins. Classical HDACs are emerging as a promising novel class of anticancer drug targets (42). For example, vorinostat (suberoylanilide hydroxamic acid) was the first HDAC inhibitor to be approved by the US Food and Drug Administration for treatment of cutaneous T-cell lymphoma (34). However, given that HDAC inhibitors act against several or all 11 HDAC family members, clinical phase I trials have documented many side effects, such as diarrhea, electrolyte changes, weight loss, disordered clotting, fatigue, and cardiac arrhythmias (6). Similarly, recent studies have reported the use of HDAC inhibitors for amelioration of intestinal inflammation (22). However, the use of these drugs may be limited by other reports describing their effect as immunosuppressive drugs that block innate immunity and increase susceptibility to severe infections in vivo (4). Therefore, it is critical to define the role of a particular HDAC isoform in health and disease rather than harness the potential of a nonselective HDAC inhibitor. In this regard, our real-time PCR studies demonstrated that, of the 11 HDAC isoforms tested, HDAC1 and HDAC2 were the most abundantly expressed in Caco-2 cells. Utilization of siRNA technology to selectively knock down HDAC1 or HDAC2 demonstrated that SERT mRNA and protein expression was downregulated by selective inhibition of HDAC2, but not HDAC1. Thus our results document that inhibition of HDAC2 is involved in decreased SERT expression in the IECS. We speculate that a decrease in SERT expression by inhibition of HDAC2 may contribute to the side effects (such as diarrhea or electrolyte changes) of HDAC inhibitors used in clinical trials.
To understand the mechanisms of SERT inhibition, we examined the effect of butyrate or TSA on SERT promoter activity. The SERT gene is under the control of two alternate promoters, the previously identified hSERTp1 (regulating neuronal and intestinal SERT) and the recently identified intestine-specific hSERTp2. Previous studies from our laboratory and others have shown that both promoters are active in IECs, with higher activity of hSERTp1 than hSERTp2 in Caco-2 cells (17, 31). We previously showed that EGF stimulated the activities of both SERT promoters via activator protein-1, but by distinct mechanisms (17). In contrast to EGF, the butyrate-mediated decrease in SERT gene expression was reflected in a decreased activity of hSERTp1, but not hSERTp2. Studies utilizing progressive 5′ deletions of hSERTp1 narrowed the butyrate- or TSA-responsive region as spanning nucleotides −272/+2. Further studies were performed to examine if butyrate decreases SERT expression by altering the status of posttranslational modification of histones at the SERT gene in the area representing hSERTp1. The short NH2 termini of histones H4 and H3 contain conserved amino acids that undergo posttranslational modifications, such as phosphorylation, methylation, and acetylation, to influence the folding and functional state of the chromatin fiber (26). Recent studies have shown that butyrate increases intestinal mucin MUC2 expression via activator protein-1 and acetylation/or methylation of histones at the MUC2 promoter (7). Our studies utilizing ChIP assays demonstrated enrichment of acetylated histone H3 or H4 with the hSERTp1 fragment on the SERT gene, which was associated with decreased promoter activity by butyrate treatment of Caco-2 cells. However, our data showed no association of the hSERTp1 gene fragment with methylated histone H3 or H4 under control conditions or in response to butyrate. Thus the SERT promoter 1 region appears to be specifically modulated by alterations in the acetylation (but not methylation) status of histone H3 or H4.
Inhibition of HDACs has been shown to increase acetylation of histones and relax chromatin, a condition usually associated with increased transcription. In contrast, our data showed SERT transcription suppression in response to HDAC inhibition. This is not surprising, as multiple reports have shown that HDAC inhibitors also possess suppressive effects, such as effects on immune response gene induction (4, 38, 41). We speculate that the butyrate-mediated increase in hyperacetylation at hSERTp1 (which indicates active chromatin modeling) may favor binding of a transcription factor (probably a repressor) that decreases SERT transcription. Future studies will investigate this possibility.
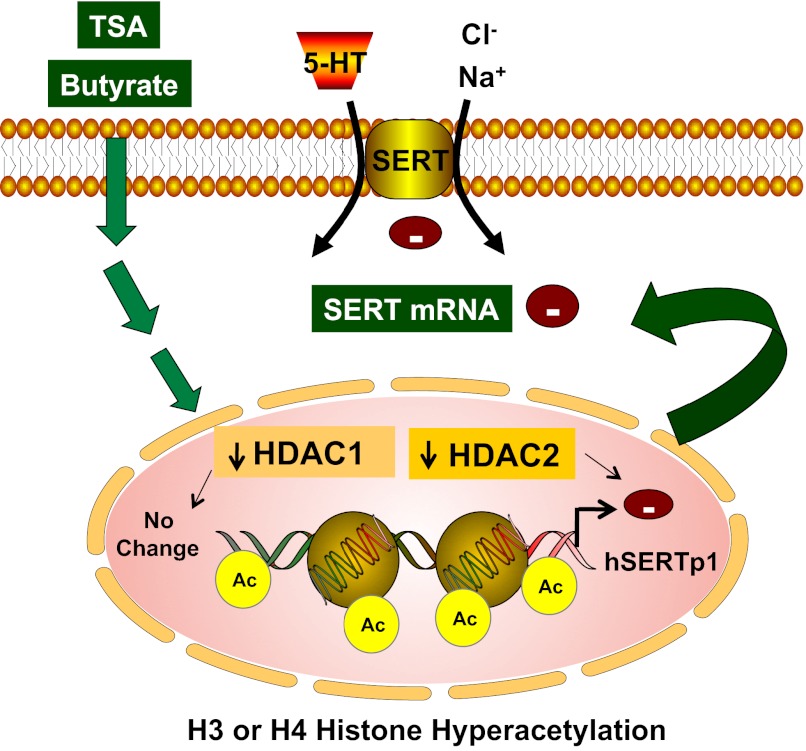
In conclusion, our results define novel regulatory mechanisms of SERT expression via epigenetic mechanisms involving HDAC2 (Fig. 10). Identification of histone acetylation or chromatin remodeling as a mechanism involved in SERT inhibition offers new insights to understand the pathophysiology of infectious or inflammatory bowel diseases, where SERT expression is decreased. These studies may also be relevant for understanding the region-specific expression of SERT across the length of the GI tract.
Fig. 10.
Schematic of proposed model of butyrate- or TSA-mediated effects on SERT. HDAC inhibitors butyrate and TSA decrease SERT expression by increasing association of acetylated histone H3 or H4 with hSERTp1 (upstream of exon 1a), resulting in decrease in promoter activity. Specific inhibition of HDAC2 (but not HDAC1) mimicked effects of butyrate or TSA in decreasing SERT expression.
GRANTS
These studies were supported by the Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-74458 and DK-096258 (R. K. Gill), DK-71596 (W. Alrefai), and DK-54016, DK-81858, and DK-92441 (P. K. Dudeja).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.K.G. is responsible for conception and design of the research; R.K.G., A.K., P.M., D.M., and V.S. performed the experiments; R.K.G. and W.A.A. analyzed the data; R.K.G. interpreted the results of the experiments; R.K.G. prepared the figures; R.K.G. drafted the manuscript; R.K.G., P.K.D., W.A.A., and S.S. edited and revised the manuscript; R.K.G., A.K., P.M., D.M., V.S., P.K.D., W.A.A., and S.S. approved the final version of the manuscript.
REFERENCES
- 1. Adcock IM. HDAC inhibitors as anti-inflammatory agents. Br J Pharmacol 150: 829– 831, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alrefai WA, Wen X, Jiang W, Katz JP, Steinbrecher KA, Cohen MB, Williams IR, Dudeja PK, Wu GD. Molecular cloning and promoter analysis of downregulated in adenoma (DRA). Am J Physiol Gastrointest Liver Physiol 293: G923– G934, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bischoff SC, Mailer R, Pabst O, Weier G, Sedlik W, Li Z, Chen JJ, Murphy DL, Gershon MD. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol 296: G685– G695, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Bode KA, Dalpke AH. HDAC inhibitors block innate immunity. Blood 117: 1102– 1103, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Borthakur A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: involvement of NF-κB pathway. J Cell Biochem 103: 1452– 1463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruserud O, Stapnes C, Ersvaer E, Gjertsen BT, Ryningen A. Histone deacetylase inhibitors in cancer treatment: a review of the clinical toxicity and the modulation of gene expression in cancer cell. Curr Pharm Biotechnol 8: 388– 400, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G, van Goudoever JB, van Seuningen I, Renes IB. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J 420: 211– 219, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. J Neurosci 21: 6348– 6361, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coates MD, Johnson AC, Greenwood-Van Meerveld B, Mawe GM. Effects of serotonin transporter inhibition on gastrointestinal motility and colonic sensitivity in the mouse. Neurogastroenterol Motil 18: 464– 471, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 126: 1657– 1664, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Esmaili A, Nazir SF, Borthakur A, Yu D, Turner JR, Saksena S, Singla A, Hecht GA, Alrefai WA, Gill RK. Enteropathogenic Escherichia coli infection inhibits intestinal serotonin transporter function and expression. Gastroenterology 137: 2074– 2083, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology 139: 249– 258, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foley KF, Pantano C, Ciolino A, Mawe GM. IFN-γ and TNF-α decrease serotonin transporter function and expression in Caco2 cells. Am J Physiol Gastrointest Liver Physiol 292: G779– G784, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Fukunaga T, Sasaki M, Araki Y, Okamoto T, Yasuoka T, Tsujikawa T, Fujiyama Y, Bamba T. Effects of the soluble fibre pectin on intestinal cell proliferation, fecal short chain fatty acid production and microbial population. Digestion 67: 42– 49, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Gill R, Alrefai W, Borthakur A, Dudeja P. Intestinal anion absorption. In: Physiology of the Gastrointestinal Tract (5th ed.). New York: Elsevier Academic, 2011, vol. 2, p. 1819–1848 [Google Scholar]
- 17. Gill RK, Anbazhagan AN, Esmaili A, Kumar A, Nazir S, Malakooti J, Alrefai WA, Saksena S. Epidermal growth factor upregulates serotonin transporter in human intestinal epithelial cells via transcriptional mechanisms. Am J Physiol Gastrointest Liver Physiol 300: G627– G636, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest 117: 428– 437, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gill RK, Pant N, Saksena S, Singla A, Nazir TM, Vohwinkel L, Turner JR, Goldstein J, Alrefai WA, Dudeja PK. Function, expression, and characterization of the serotonin transporter in the native human intestine. Am J Physiol Gastrointest Liver Physiol 294: G254– G262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, Turner JR, Ramaswamy K, Dudeja PK. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKCα in human intestinal epithelial cells. Gastroenterology 128: 962– 974, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Glauben R, Batra A, Fedke I, Zeitz M, Lehr HA, Leoni F, Mascagni P, Fantuzzi G, Dinarello CA, Siegmund B. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol 176: 5015– 5022, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Glauben R, Siegmund B. Inhibition of histone deacetylases in inflammatory bowel diseases. Mol Med 17: 426– 433, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hasler WL. Serotonin and the GI tract. Curr Gastroenterol Rep 11: 383– 391, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Haub S, Kanuri G, Volynets V, Brune T, Bischoff SC, Bergheim I. Serotonin reuptake transporter (SERT) plays a critical role in the onset of fructose-induced hepatic steatosis in mice. Am J Physiol Gastrointest Liver Physiol 298: G335– G344, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Hijova E, Chmelarova A. Short chain fatty acids and colonic health. Bratisl Lek Listy 108: 354– 358, 2007 [PubMed] [Google Scholar]
- 26. Jenuwein T, Allis CD. Translating the histone code. Science 293: 1074– 1080, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Kiela PR, Kuscuoglu N, Midura AJ, Midura-Kiela MT, Larmonier CB, Lipko M, Ghishan FK. Molecular mechanism of rat NHE3 gene promoter regulation by sodium butyrate. Am J Physiol Cell Physiol 293: C64– C74, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Kim HJ, Bae SC. Histone deacetylase inhibitors: molecular mechanisms of action and clinical trials as anti-cancer drugs. Am J Transl Res 3: 166– 179, 2011 [PMC free article] [PubMed] [Google Scholar]
- 29. Kirat D, Kondo K, Shimada R, Kato S. Dietary pectin up-regulates monocaboxylate transporter 1 in the rat gastrointestinal tract. Exp Physiol 94: 422– 433, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol 285: G207– G216, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Linden DR, White SL, Brooks EM, Mawe GM. Novel promoter and alternate transcription start site of the human serotonin reuptake transporter in intestinal mucosa. Neurogastroenterol Motil 21: 534– 541, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luhrs H, Kudlich T, Neumann M, Schauber J, Melcher R, Gostner A, Scheppach W, Menzel TP. Butyrate-enhanced TNFα-induced apoptosis is associated with inhibition of NF-κB. Anticancer Res 22: 1561– 1568, 2002 [PubMed] [Google Scholar]
- 33. Malakooti J, Saksena S, Gill RK, Dudeja PK. Transcriptional regulation of the intestinal luminal Na+ and Cl− transporters. Biochem J 435: 313– 325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol 25: 84– 90, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Martel F. Recent advances on the importance of the serotonin transporter SERT in the rat intestine. Pharmacol Res 54: 73– 76, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Martel F, Monteiro R, Lemos C. Uptake of serotonin at the apical and basolateral membranes of human intestinal epithelial (Caco-2) cells occurs through the neuronal serotonin transporter (SERT). J Pharmacol Exp Ther 306: 355– 362, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Mendoza C, Matheus N, Iceta R, Mesonero JE, Alcalde AI. Lipopolysaccharide induces alteration of serotonin transporter in human intestinal epithelial cells. Innate Immun 15: 243– 250, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Nair AR, Boersma LJ, Schiltz L, Chaudhry MA, Muschel RJ. Paradoxical effects of trichostatin A: inhibition of NF-Y-associated histone acetyltransferase activity, phosphorylation of hGCN5 and downregulation of cyclin A and B1 mRNA. Cancer Lett 166: 55– 64, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Saksena S, Gill RK, Tyagi S, Alrefai WA, Sarwar Z, Ramaswamy K, Dudeja PK. Involvement of c-Src and protein kinase Cδ in the inhibition of Cl−/OH− exchange activity in Caco-2 cells by serotonin. J Biol Chem 280: 11859– 11868, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Spiller R. Serotonin and GI clinical disorders. Neuropharmacology 55: 1072– 1080, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Tong X, Yin L, Giardina C. Butyrate suppresses Cox-2 activation in colon cancer cells through HDAC inhibition. Biochem Biophys Res Commun 317: 463– 471, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Witt O, Deubzer HE, Milde T, Oehme I. HDAC family: what are the cancer relevant targets? Cancer Lett 277: 8– 21, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Witt O, Lindemann R. HDAC inhibitors: magic bullets, dirty drugs or just another targeted therapy. Cancer Lett 280: 123– 124, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Yamada T, Inui A, Hayashi N, Fujimura M, Fujimiya M. Serotonin stimulates endotoxin translocation via 5-HT3 receptors in the rat ileum. Am J Physiol Gastrointest Liver Physiol 284: G782– G788, 2003 [DOI] [PubMed] [Google Scholar]