Abstract
The objective of this study was to develop a novel in vitro model for smooth muscle cell (SMC) differentiation from human embryonic stem cell-derived mesenchymal cells (hES-MCs). We found that hES-MCs were differentiated to SMCs by transforming growth factor-β (TGF-β) in a dose- and time-dependent manner as demonstrated by the expression of SMC-specific genes smooth muscle α-actin, calponin, and smooth muscle myosin heavy chain. Under normal growth conditions, however, the differentiation capacity of hES-MCs was very limited. hES-MC-derived SMCs had an elongated and spindle-shaped morphology and contracted in response to the induction of carbachol and KCl. KCl-induced calcium transient was also evident in these cells. Compared with the parental cells, TGF-β-treated hES-MCs sustained the endothelial tube formation for a longer time due to the sustained SMC phenotype. Mechanistically, TGF-β-induced differentiation was both Smad- and serum response factor/myocardin dependent. TGF-β regulated myocardin expression via multiple signaling pathways including Smad2/3, p38 MAPK, and PI3K. Importantly, we found that a low level of myocardin was present in mesoderm prior to SMC lineage determination, and a high level of myocardin was not induced until the differentiation process was initiated. Taken together, our study characterized a novel SMC differentiation model that can be used for studying human SMC differentiation from mesoderm during vascular development.
Keywords: vascular smooth muscle cells, human embryonic stem cell-derived mesenchymal cells, transforming growth factor-β, differentiation
vascular smooth muscle cells (SMCs) play a pivotal role in angiogenesis and vasculogenesis during embryonic development (2). SMC functional impairments lead to the development of several prominent cardiovascular diseases including congenital heart disease, atherosclerosis, hypertension, and restenosis after angioplasty (13, 33, 36). The molecular mechanisms governing SMC differentiation, especially the progenitor-specific regulation, however, remain largely unknown. This is due to, at least in part, the lack of in vitro model systems from different progenitors.
Lineage-tracing studies have shown that during embryonic development, SMCs originate from at least eight progenitors including neural crest, secondary heart field, somites, mesoangioblasts, proepicardium, splanchnic mesoderm, mesothelium, and various stem cells (27). Interestingly, SMCs from different origins are regulated differently and have distinct functional properties. For example, neural crest-derived SMCs and mesoderm-derived SMCs display dramatically different responses to the stimulation of morphogenetic factors such as transforming growth factor-β (TGF-β) (27). As a matter of fact, TGF-β stimulates cell growth, extracellular protein expression, as well as gene promoter activation in almost opposite ways in these two SMC subtypes (39). Functionally, mesoderm-derived SMCs cannot rescue the outflow tract defects observed in neural crest-ablated chicken embryos (37). Additional studies from different laboratories demonstrate that embryonically distinct subpopulations of SMCs are not functionally equivalent and that SMCs within different vascular beds utilize distinct cis-elements and control regions to regulate SMC marker gene activation (20, 23, 24, 28, 30). These studies suggest that SMC differentiation is controlled by different intracellular mechanisms among distinct SMC subtypes. Therefore, independent model systems using different progenitors, especially the natural progenitors, are essential for understanding the molecular mechanisms controlling SMC differentiation from different origins.
Previously, we have developed an in vitro model using neural crest cell Monc-1 because neural crest cells are the natural progenitors for neural crest-derived SMCs. This model has enabled us to dissect the molecular mechanisms governing SMC differentiation from neural crest progenitor cells (5, 7, 18, 21). To explore the molecular regulation of SMC differentiation from mesoderm, the present study was aimed to develop another model system using human embryonic stem cell-derived mesenchymal cells (hES-MCs). hES-MCs are natural SMC progenitors for mesoderm-derived SMCs. hES-MCs were derived from H9 human embryonic stem cells (1). hES-MCs have the capacity to produce the three lineages associated with mesenchymal stem cells, including osteogenic, chondrogenic, and SMC lineages (1). We found that hES-MCs can robustly differentiate to SMC phenotype upon TGF-β induction. hES-MC-derived SMCs expressed SMC-specific marker genes and exhibited functional SMC morphology. The cells contracted and displayed intracellular calcium transient after stimulation with muscarinic agonist or KCl. Most importantly, these cells were recruited to endothelial tubes and sustained the endothelial tube formation. We also found that myocardin, the master regulator of SMC differentiation, was present at a low level in mesoderm prior to SMC lineage determination. The high level of myocardin expression was not induced until the SMC differentiation process was initiated. In hES-MCs, TGF-β appeared to induce myocardin expression via multiple signaling pathways.1
METHODS
Cell culture.
hES-MCs were obtained from ArunA Biomedical (Athens, GA) and are characterized by the expression of mesenchymal stem cell surface markers (1). The cells were maintained in α-minimal essential medium (αMEM, Cellgro, Fisher Scientific, Pittsburgh, PA) containing 10% mesenchymal stem cell-qualified fetal bovine serum (Hyclone) and 2 mM l-glutamine (Hyclone). For TGF-β-induced SMC differentiation, the cells were grown for 12 h in serum-free medium followed by incubation with TGF-β for various times. Endothelial cells (ECs) C166, and rat aortic smooth muscle cells (RASMCs) were grown in Dulbecco's modification of Eagle's medium (Invitrogen) supplemented with 10% FBS. Human umbilical vein ECs (HUVECs) were cultured in endothelial basal medium (EGM-2 Bullet Kit, Lonza).
Semiquantitative and quantitative reverse transcription-polymerase chain reaction.
Total RNA from cultured cells was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. cDNA was synthesized from 1 μg of total RNA by iScript cDNA Synthesis kit (Bio-Rad). Semiquantitative RT-PCR was performed as described (6). Quantitative PCR analyses were performed with the Stratagene Mx3005P using SYBR Green (Agilent Technologies). The sequences of the forward and reverse primers were described previously (5, 7, 18, 21).
Immunofluorescent staining.
hES-MCs were cultured on sterile coverslips in 24-well plates at a density of 103 cells/cm2. Immunostaining was performed as described previously (7). The primary antibodies for smooth muscle α-actin (α-SMA, ACTA2, Abcam), calponin (CNN1, Santa Cruz), smooth muscle myosin heavy chain (SMMHC, MYH11, Biomedical Technologies), Smad2 (Cell Signaling), or Smad3 (Santa Cruz) were used at 1:100. The slides were analyzed using a Nikon microscope.
Western blot analysis.
hES-MCs were washed twice with PBS followed by protein extraction using RIPA buffer. Western blot analysis was performed as described previously (7). Antibodies against ACTA2, CNN1, SM22α (TAGLN, Abcam), MYH11, and α-TUBA (Cell Signaling) were used for detecting SMC-specific protein expression. Phospho-Smad2 and phospho-Smad3 antibodies were from Cell Signaling.
Contractility assay.
hES-MCs were treated with TGF-β for 3 days. The cells were then washed once with PBS followed by stimulation with 1 mM carbachol or 75 mM KCl in Krebs solution. Contractility of the cells was observed with an inverted microscope for up to 30 min. Images of the same field before and after carbachol or KCl treatment were snapped and compared. Krebs solution contained 120 mM NaCl, 5.9 mM KCl, 25 mM NaHCO3, 1.2 mM NaH2PO4, 11.5 mM dextrose, 2.5 mM CaCl2, and 1.2 mM MgCl2. The solution was incubated for 30 min at 37°C and 5% (vol/vol) CO2 in air to adjust the pH to 7.4. The high-KCl depolarizing solution had the same composition as normal Krebs solution with equal molar substitution of NaCl with KCl.
Measurement of intracellular Ca2+ signaling.
hES-MCs were cultured on sterile coverslips in 24-well plates. hES-MCs were treated with TGF-β or vehicle for 3 days. The cells were incubated in Fluo-4 Direct calcium reagent solution with a probenecid concentration of 5 mM (Invitrogen) for 60 min at 37°C and 5% (vol/vol) CO2. The cells were then washed once with PBS and stimulated by 75 mM KCl for 1 min, followed by image capture using a Nikon microscope.
ECs and hES-MCs coculture on Matrigel.
Matrigel (BD Biosciences) was plated in 24-well plates and allowed to polymerize at 37°C and 5% (vol/vol) CO2 for at least 30 min before addition of cells. C166 cells were transduced with Ad-GFP (green) for 2 days. hES-MCs were treated with 1 ng/ml TGF-β or vehicle for 3 days. RASMCs, hES-MCs, and TGF-β-treated hES-MCs were labeled with PKH26 (Sigma), a red fluorescent dye used for cell membrane labeling. C166 cells (6 × 104 cells/well) were mixed with PKH26-labeled RASMCs, hES-MCs, or TGF-β-treated hES-MCs (1.2 × 104 cells/well). The mixed cells were added to different wells in a 24-well plate coated with Matrigel and incubated at 37°C for 24 h. Photographs were taken using a Nikon microscope. To detect the SMC differentiation of hES-MCs in coculture with ECs, hES-MCs were treated with 1 ng/ml TGF-β for 3 days. hES-MCs treated with vehicle or TGF-β were cocultured with HUVECs for 12 h and 24 h. mRNA expression of SMC markers was examined by qPCR.
Transfection and luciferase assay.
Acta2 and Tagln promoter constructs were transfected into hES-MCs as described previously (6, 21). Cells were starved in serum-free medium for 12 h followed by 1 ng/ml TGF-β treatment for 24 h. Luciferase assay was performed 36 h after transfection. Experiments were repeated at least three times, and the results from a representative experiment are shown with standard deviations.
Statistical analysis.
The data are expressed as means ± SE. Statistical analysis was performed by ANOVA with pairwise comparisons between groups. A value of P < 0.05 was considered as statistically significant.
RESULTS
TGF-β induced hES-MCs to express SMC-specific genes.
SMCs are characterized by a large number of SMC-specific genes including ACTA2, TAGLN, CNN1, MYH11, α-actin, smoothelin B, etc. To test whether TGF-β induced hES-MCs to become a SMC phenotype, we detected the expression of Acta2, Cnn1, and Myh11 because ACTA2 is one of the early differentiation markers, MYH11 is a late marker, and CNN1 is activated in the middle stage of the SMC differentiation (36). Dose-dependent studies showed that SMC marker mRNAs were induced by as little as 0.1 ng/ml of TGF-β. Administration of 1 or 5 ng/ml of TGF-β dramatically induced the marker gene expression (Fig. 1A). Since 1 ng/ml of TGF-β induced a high level expression of most SMC markers, we chose 1 ng/ml of TGF-β for all of the subsequent experiments. As shown in Fig. 1B, TGF-β induced Acta2, Cnn1, and Myh11 as early as 2 h, 4 h, and 8 h after TGF-β treatment, respectively, suggesting that hES-MCs can be robustly induced to SMC phenotype by TGF-β. Western blot analysis confirmed that SMC marker proteins were rapidly induced by TGF-β. As shown in Fig. 1C, ACTA2 and CNN1 were induced as early as 8 h while MYH11 proteins were induced 24 h after TGF-β treatment. Immunostaining of ACTA2, CNN1, and MYH11 showed that, at the first day of TGF-β induction, a small portion of hES-MCs were converted to SMC phenotype. However, after 3 days of the induction, more than 90% of the cells expressed SMC marker genes (Fig. 1, D and E). Since SMCs display phenotypic plasticity in culture, we sought to determine whether hES-MC-derived SMCs are able to maintain their phenotype after passaging. We found that hES-MC-derived SMCs continued to express ACTA2, CNN1, and TAGLN even after two passages (Fig. 1F). The marker gene expression in passaged SMCs was independent of TGF-β because these cells were cultured in medium without addition of TGF-β. Therefore, TGF-β appears to induce a long-lasting and perhaps heritable differentiation of hES-MCs into a smooth muscle phenotype.
Fig. 1.
Transforming growth factor-β (TGF-β) induced smooth muscle cell (SMC)-specific gene expression in human embryonic stem cell-derived mesenchymal cells (hES-MCs). A: TGF-β stimulates SMC marker mRNA expression in a dose-dependent manner. hES-MCs were treated with various dosages of TGF-β as indicated for 24 h. mRNA expression of SMC markers was examined by qPCR. *P < 0.05 compared with the untreated group for each individual marker. B: TGF-β stimulates SMC marker mRNA expression in a time-dependent manner. hES-MCs were treated with 1 ng/ml TGF-β for various time points as indicated. qPCR was performed as in A. *P < 0.05 compared with the untreated group for each individual marker. C: TGF-β induces SMC marker protein expression. hES-MCs were treated with 1 ng/ml TGF-β for various time points as indicated, and Western blot analysis was performed. α-TUBA was an internal control. D: global expression of SMC-specific genes in TGF-β-treated hES-MCs. hES-MCs were treated with 1 ng/ml TGF-β for 0 (D0), 1 (D1), 2 (D2), and 3 days (D3) to detect SMC marker expression by immunostaining. E: percentage of smooth muscle α-actin (α-SMA)-positive cells before and after 3 days of TGF-β treatment. *P < 0.05 compared with untreated cells (D0). F: stability of SMC phenotype after trypsinization and reculture. hES-MCs were treated with vehicle (-β) or 1 ng/ml TGF-β (+β) for 3 days. Cells were then trypsinized and recultured without TGF-β (+β-β). After 2 days of incubation, cells were trypsinized again and recultured without TGF-β for an additional 2 days (+β-β-β). Marker expression was examined by Western blotting.
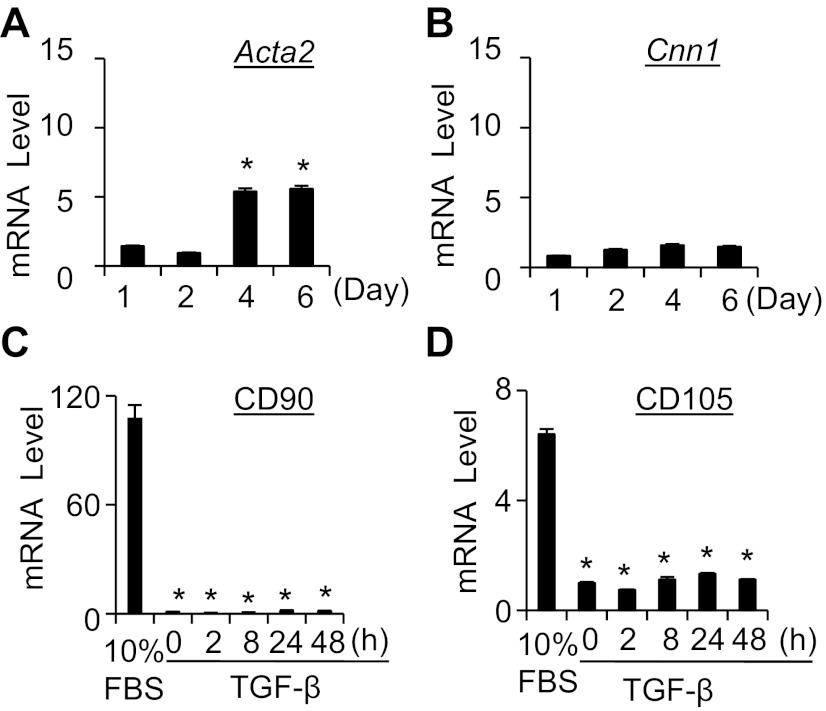
Considering that the complete culture medium containing FBS may influence the cell phenotype and lead to the spontaneous differentiation of hES-MCs, we detected the spontaneous differentiation of hES-MCs under normal growing conditions and found that early marker Acta2 was slightly induced after cultured for 4 days (Fig. 2A). However, the more SMC-specific marker Cnn1 was not significantly induced even after cultured for up to 6 days (Fig. 2B). Phenotypically, hES-MCs express a number of mesenchymal cell markers including CD90 and CD105 (3). High levels of CD90 and CD105 were observed in hES-MCs under normal growing conditions (Fig. 2, C and D). However, serum starvation decreased the expression of CD90 and CD105 but did not induce SMC differentiation (Fig. 2, C and D). SMC differentiation was only induced after TGF-β treatment (Fig. 1).
Fig. 2.
Characterization of hES-MC differentiation under normal growing conditions. A and B: Acta2 (A) and Cnn1 (B) mRNA expression in hES-MCs under normal growing conditions (10% FBS) for the times indicated. *P < 0.05 compared with cells cultured for 1 day. C: CD90 mRNA expression in hES-MCs under normal growing conditions or treated with TGF-β for various times as indicated. *P < 0.01 compared with the cells with 10% FBS. D: CD105 mRNA expression in hES-MCs under normal growing conditions or treated with TGF-β for various times as indicated. *P < 0.01 compared with the cells with 10% FBS.
hES-MC-derived SMCs exhibited functional SMC morphology and contractility.
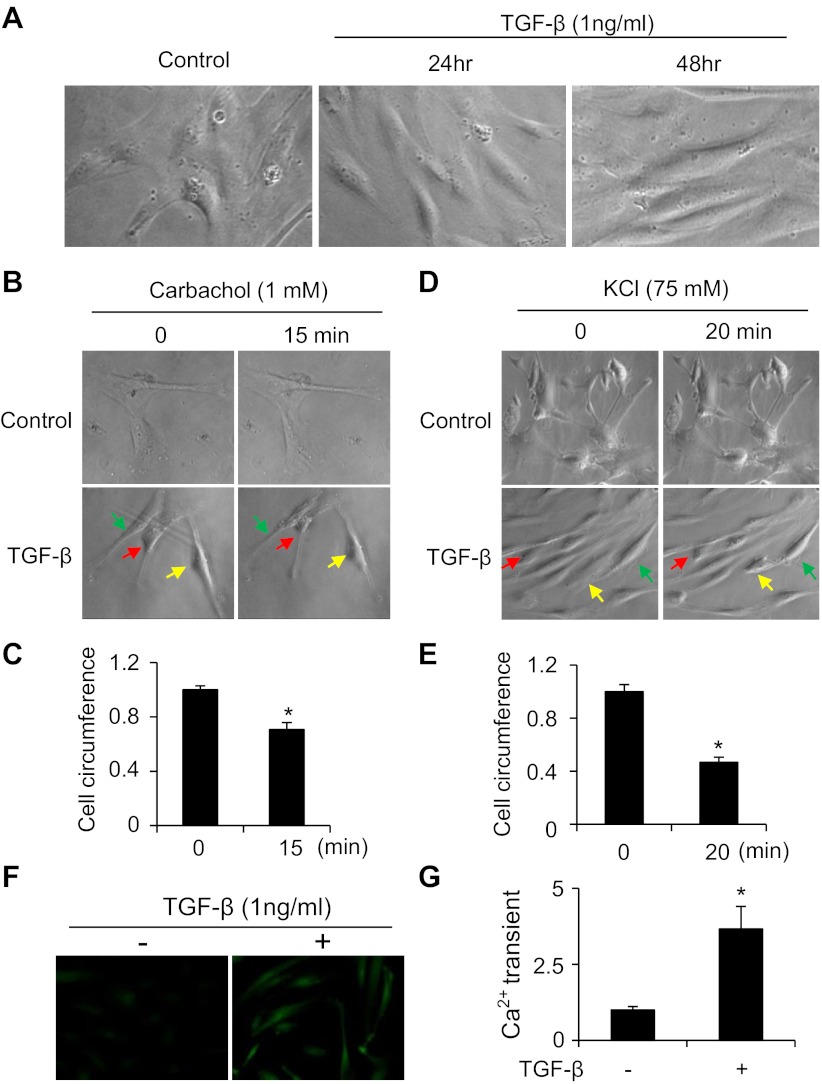
SMCs display two phenotypes in human or animal bodies, i.e., contractile and synthetic (proliferating) phenotype depending on the physiological or pathological conditions (36). SMC phenotypic modulation from contractile to synthetic phenotype contributes to a number of vascular diseases including atherosclerosis, hypertension, and restenosis after angioplasty. To determine whether TGF-β-treated hES-MCs differentiate to contractile or synthetic SMC phenotype, we observed the morphological alteration after TGF-β induction. As shown in Fig. 3A, TGF-β induced hES-MCs to become an elongated and spindle-shaped morphology resembling the contractile SMCs observed in vivo in the physiological condition. To test whether hES-MC-derived SMCs are functional, we performed contractility assays using both the muscarinic agonist carbachol and KCl to stimulate vehicle- or TGF-β-treated hES-MCs. As shown in Fig. 3, B–E, vehicle-treated cells failed to contract with either carbachol or KCl stimulation. However, both carbachol and KCl induced TGF-β-treated cells to contract, as indicated by the shortening of the cells. Moreover, TGF-β-treated cells, but not the control cells, exhibited strong intracellular calcium transient after KCl stimulation (Fig. 3, F and G). These data suggest that TGF-β converted hES-MCs to a contractile functional SMC phenotype.
Fig. 3.
TGF-β converted hES-MCs to a contractile SMC phenotype. A: TGF-β converted hES-MCs to an elongated and spindle-shaped functional SMC morphology. B: TGF-β-treated hES-MCs contracted in response to carbachol induction. hES-MCs were cultured on silicon-coated coverslips and treated with vehicle (control) or 1 ng/ml TGF-β for 3 days. Photographs were taken before and after 1 mM carbachol induction for 15 min. Arrows indicate representative contracted cells in the TGF-β-treated group. C: quantification of the circumference of TGF-β-treated hES-MCs before and after carbachol induction. *P < 0.05 compared with carbachol-untreated cells (0 min). D: TGF-β-treated hES-MCs contracted in response to KCl stimulation. hES-MCs were treated with vehicle (control) or 1 ng/ml TGF-β for 3 days. Photographs were taken before and after 75 mM KCl stimulation for 20 min. Arrows indicate representative contracted cells in the TGF-β-treated group. E: quantification of the circumference of TGF-β-treated hES-MCs before (0 min) and after KCl stimulation (20 min). *P < 0.05 compared with KCl-untreated cells (0 min). F: TGF-β-treated hES-MCs exhibited intracellular Ca2+ transient in response to KCl induction. hES-MCs were treated with vehicle (−) or 1 ng/ml TGF-β (+) for 3 days. Cells were preloaded in Fluo-4 Direct calcium reagent with a probenecid concentration of 5 mM (Invitrogen) for 60 min and then stimulated with 75 mM KCl for 1 min before images were taken. G: quantification of intracellular Ca2+ transient after KCl stimulation. *P < 0.05 compared with vehicle-treated cells (−).
TGF-β-treated hES-MCs stabilized endothelial tubes.
The primary function of SMCs during embryonic development is to participate in vasculogenesis and angiogenesis by attaching to endothelial tubes and regulating growth and function of endothelial cells. To further characterize TGF-β-induced SMC differentiation of hES-MCs, we tested whether TGF-β-treated hES-MCs interact with endothelial cells by observing whether these cells participate in endothelial tube formation in vitro. As shown in Fig. 4A, 12 h after coculture of actual SMCs, TGF-β-treated, or untreated hES-MCs with C166 cells on Matrigel, endothelial tube networks were developed in all cocultures. SMCs, hES-MCs, and TGF-β-treated hES-MCs were recruited to endothelial tubes and closely attached to the tubes (Fig. 4A). After 24 h, endothelial tubes cocultured with SMCs or vehicle-treated hES-MCs were significantly disrupted (Fig. 4, A and B, 24 h). Most of the SMCs or vehicle-treated hES-MCs were disassociated with the endothelial tubes and kept with the endothelial aggregates (Fig. 4A). However, the tubes cocultured with TGF-β-treated hES-MCs were well maintained (Fig. 4, A and B, 24 h). TGF-β-treated hES-MCs were still tightly associated with the tubes at this time, similar to 12 h of coculture. These data indicate that hES-MC-derived SMCs play a critical role in stabilizing endothelial tubes.
Fig. 4.

Recruitment of hES-MCs to endothelial tubes. A: hES-MCs participated in endothelial tube formation. C166 cells were transduced with Ad-GFP (green) for 2 days. hES-MCs were treated with vehicle or 1 ng/ml TGF-β (hES-MC+Tβ) for 3 days. hES-MCs and rat aortic smooth muscle cells (SMC) were labeled with the red fluorescent dye PKH26. C166 cells were mixed with SMCs, hES-MCs, or hES-MC+Tβ and cocultured on two-dimensional Matrigels in individual wells in a 24-well plate. Images were obtained by fluorescent microscopy. Images of the PKH26-labeled cells were overlaid (merge) with GFP-labeled endothelial tubes after 12 h (left) and 24 h (right) of coculture. Bottom images in the 24-h group are higher magnifications of the endothelial tubes in the rectangular boxes in C166/hES-MC+Tβ coculture. B: endothelial tube formation was quantified by assessing tube length. *P < 0.05 compared with corresponding coculture for 12 h; #P < 0.05 compared with C166/SMC coculture for 24 h. C: transient differentiation of hES-MCs to SMC phenotype in coculture with endothelial cells. hES-MCs treated with vehicle or TGF-β (1 ng/ml) for 3 days were cocultured with human umbilical vein endothelial cells (HUVECs) for 12 and 24 h. mRNA expression of SMC markers was examined by qPCR. *P < 0.05 compared with hES-MCs without coculture; #P < 0.05 compared with the corresponding group and coculture for 12 h.
To determine the mechanism by which TGF-β-treated hES-MCs, but not the vehicle-treated cells, sustained EC tube formation, we examined the phenotype of these cells after cocultured with ECs. hES-MCs treated with vehicle or TGF-β were cocultured with HUVECs for 12 and 24 h, and SMC-specific markers Acta2, Cnn1, and Myh11 were detected. Interestingly, both vehicle- and TGF-β-treated hES-MCs expressed SMC markers after 12 h of coculture. although the expression levels in vehicle-treated cells were lower than in TGF-β-treated cells (Fig. 4C). The marker gene expression in vehicle-treated cells was likely attributed to EC signaling. However, after 24 h of coculture, SMC marker expression in vehicle-treated hES-MCs was significantly reduced to the basal level. The marker expression in TGF-β-treated cells was also reduced, but the expression levels were significantly higher than in the vehicle-treated cells (Fig. 4C), and appeared to be sufficient to support the endothelial tube formation. The transient expression of SMC marker genes in vehicle-treated cells is similar to the SMC recruitment and differentiation by ECs observed in vivo. However, the maintenance of the SMC phenotype may require additional endocrine support available in vivo but is not present in the in vitro system.
hES-MC differentiation to SMC was Smad2/3 dependent.
TGF-β signals through ligand binding to type II receptors (TβRII), recruitment and phosphorylation of type I receptors (TβRI), and activation downstream signal. To examine whether TGF-β signaling components are present in hES-MCs, we examined the expression of the TGF-β receptors. As shown in Fig. 5A, both type I and type II receptors were expressed in hES-MCs prior to TGF-β treatment. TGF-β did not significantly alter the receptor expression. TGF-β signaling is predominantly transduced by Smad proteins. Both Smad2 and Smad3 have been shown to play important roles in SMC differentiation from embryonic body, 10T1/2 cells, or neural crest cells (42). To determine whether Smad signaling plays a role in TGF-β-induced SMC differentiation of hES-MCs, we first detected whether Smad2 and Smad3 are activated in TGF-β-treated cells. As shown in Fig. 5B, both Smad2 and Smad3 were activated in hES-MCs as early as 30 min after TGF-β induction. TGF-β slightly altered Smad2 and Smad3 protein expression, but the difference was not significant (Fig. 5B). To test whether Smad2 and Smad3 are important for hES-MC differentiation, we knocked down their expression individually and tested whether hES-MCs can be induced to SMC phenotype in the absence of Smad2 or Smad3. As shown in Fig. 5, C and D, knockdown of Smad2 blocked TGF-β-induced ACTA2 expression. This effect is specific because in cells where Smad2 was not knocked down, ACTA2 expression was evident (Fig. 5D, arrowheads). Similar with Smad2, knockdown of Smad3 also blocked TGF-β-induced ACTA2 expression (Fig. 5, E and F). These results suggest that TGF-β-induced SMC differentiation from hES-MCs was Smad dependent.
Fig. 5.
TGF-β-induced SMC differentiation of hES-MCs was Smad dependent. A: TGF-β receptor expression in hES-MCs. hES-MCs were treated with vehicle (0) or 1 ng/ml TGF-β for various time points as indicated. TGF-β type I (TβRI) and type II (TβRII) receptors were detected by RT-PCR. Cyclophilin was an internal control. B: Smad2 and Smad3 activation in TGF-β-treated hES-MCs. hES-MCs were treated with vehicle (0) or 1 ng/ml TGF-β for the times indicated. Western blotting was performed to detect Smad2 and Smad3 expression and phosphorylation. C: Smad2 knockdown by Smad2 shRNA (shS2) was detected by Western blotting. Ctrl, control. D: Smad2 knockdown by shS2 blocked TGF-β-induced ACTA2 expression, as examined by immunostaining. GFP shRNA (shGFP) served as a control. Arrowheads indicate that the cells in which Smad2 was not knocked down expressed ACTA2. Photographs were taken at the same area for DAPI (blue), Smad2 (red), and ACTA2 (green). E: Smad3 knockdown by Smad3 shRNA (shS3) was detected by Western blotting. F: Smad3 knockdown by shS3 blocked TGF-β-induced ACTA2 expression, as examined by immunostaining. GFP shRNA served as a control. Photographs were taken at the same area for DAPI (blue), Smad3 (red), and ACTA2 (green).
hES-MC differentiation to SMC was SRF/CArG/myocardin dependent.
Expression of most SMC marker genes is regulated by serum response factor (SRF)/CArG/myocardin (46). To determine whether or not TGF-β-induced SMC differentiation from hES-MCs depends on SRF, we analyzed Acta2 and Tagln promoter activity in hES-MCs treated with or without TGF-β. We found that TGF-β activated both Acta2 and Tagln promoters in hES-MCs (Fig. 6, A and B). However, CArG box mutations significantly inhibited TGF-β-induced Acta2 promoter activity (Fig. 6A) and completely blocked Tagln promoter activity (Fig. 6B). Moreover, SRF knockdown by shRNA significantly blocked TGF-β-induced expression of Acta2, Cnn1, and Myh11 (Fig. 6, C–F), suggesting that TGF-β-induced hES-MC differentiation is SRF dependent. Myocardin is a SRF coactivator and is considered as a master regulator of SMC differentiation (4, 9, 25, 40, 47). In hES-MCs, TGF-β induced Myocd mRNA expression in a time-dependent manner (Fig. 7, A and B). Myocardin expression reached the highest level at 8 h after TGF-β induction. Previous studies have shown that p38 MAPK is involved in TGF-β-induced myocardin expression in human coronary artery SMCs (26). In hES-MCs, TGF-β appeared to induce myocardin expression through multiple signaling pathways including p38 MAPK, PI3K, and Smad2/3 pathways because pathway-specific inhibitors for p38 MAPK or PI3K blocked myocardin expression (Fig. 7C). This inhibitory effect was extended to the expression of the SMC marker calponin (Fig. 7D). In addition to p38 MAPK and PI3K, both Smad2 and Smad3 were also important to TGF-β-induced myocardin expression (Fig. 7, E and F).
Fig. 6.
TGF-β-induced SMC differentiation of hES-MCs was serum response factor (SRF) dependent. A: TGF-β-induced Acta2 promoter activity was CArG dependent. Acta2 promoter (from −2.6 to +2.8 kb) constructs with wild-type or mutant CArG box either in the promoter region [CArG(A+B)m] or in the first intron (CArGintm) were transfected into hES-MCs followed by vehicle (control) or 1 ng/ml TGF-β treatment for 24 h. Luciferase assay was performed. *P < 0.01 compared with wild-type promoter with vehicle treatment; #P < 0.01 compared with TGF-β-induced wild-type promoter. B: TGF-β-induced Tagln promoter activity was CArG dependent. Tagln promoter constructs with wild-type or mutant CArG box in the two SRF-binding sites (CArGnearm or CArGfarm) were transfected into hES-MCs followed by vehicle (control) or 1 ng/ml TGF-β treatment for 24 h. Luciferase assay was performed. *P < 0.01 compared with wild-type promoter with vehicle treatment; #P < 0.05 compared with TGF-β-induced wild-type promoter. C: SRF knockdown by SRF shRNA (shSRF) was detected by qPCR. *P < 0.01 compared with vehicle-treated group; #P < 0.01 compared with TGF-β-treated group with control shRNA (shCtrl) transfection. D–F: SRF knockdown blocked Acta2 (D), Cnn1 (E), and Myh11 (F) expression. *P < 0.01 compared with vehicle-treated group; #P < 0.05 compared with TGF-β-treated group with shCtrl transfection in individual gene.
Fig. 7.
Myocardin expression in SMC differentiation of hES-MCs. A: TGF-β-induced myocardin (Myocd) mRNA expression as detected by RT-PCR. B: TGF-β-induced Myocd expression as detected by qPCR. *P < 0.05 compared with vehicle-treated cells (0 h). C: TGF-β-induced Myocd expression via p38 MAPK and PI3K signaling pathways. hES-MCs were treated with pathway-specific inhibitors for p38 (SB203580), JNK (SP600125), RhoA (Y27632), PI3K (LY294002), or ERK1/2 (U0126) for 30 min prior to vehicle (Ctrl-β) or TGF-β (1 ng/ml) treatment for 8 h. Myocd expression was detected by qPCR. *P < 0.05 compared with vehicle-treated group; #P < 0.05 compared with TGF-β-treated group without inhibitor (Ctrl+β). D: TGF-β-induced Cnn1 expression was blocked by p38 MAPK and PI3K inhibitors. *P < 0.01 compared with vehicle-treated group (Ctrl-β); #P < 0.01 compared with TGF-β-treated group without inhibitor (Ctrl+β). E and F: both Smad2 and Smad3 were required for TGF-β-induced Myocd expression. Knockdown of Smad2 expression by shRNA (shS2; E) or blockade of Smad3 activity by Smad3 inhibitor (SIS3, Smad3 inhibitor; F) inhibited Myocd expression. *P < 0.01 compared with vehicle-treated group; #P < 0.05 compared with TGF-β-treated group with control shRNA (shCtrl; E) or SIS3 vehicle (Ctrl; F). G: Myocd expression in different developmental stages. Total RNA was extracted from hES cells (H9), mesoderm (J38), hES-MCs, and TGF-β-treated hES-MCs. Myocd expression was detected by RT-PCR. H: quantitative analysis of Myocd expression shown in G by normalization to cyclophilin. *P < 0.05 compared with H9 cells; #P < 0.05 compared with J38 mesoderm; $P < 0.001 compared with all other cells.
Since myocardin is very important to SMC differentiation, we sought to determine in what SMC developmental stage myocardin is induced. As aforementioned, hES-MCs were derived from mesoderm that was differentiated from H9 human embryonic stem cells (1). We detected myocardin expression in these three cells with different developmental stages and found that myocardin was barely detectable in H9 stem cells (Fig. 7G). However, a low level of myocardin was present in mesoderm, and a slightly higher expression was found in hES-MCs (Fig. 7, G and H). Of importance, TGF-β treatment robustly induced myocardin expression (Fig. 7, G and H). These data indicate that myocardin expression is gradually activated along the differentiation stages. A surge of myocardin expression may be needed for the effective SMC differentiation.
DISCUSSION
Since SMC differentiation is an important process in vascular development, much effort has been made to illustrate the molecular mechanisms underlying the differentiation process using various in vitro model system including C3H10T1/2 cells, neural crest cells, A404, embryonic body (EB), and embryonic stem cells (7, 8, 14, 16, 29, 43, 45). Although these models have significantly contributed to our understanding of SMC differentiation, each of these models has its limitations. For example, C3H10T1/2 cell is a very convenient model, but the definitive SMC marker MYH11 cannot be readily induced in these cells (42). Neural crest cells are the demonstrated SMC progenitors in vivo, but culture of neural crest cell line Monc-1 and Joma 1.3 requires a complex defined medium, making culture difficult (19, 31). A404 is a great model, but it is not a natural SMC progenitor found in vivo because it was developed by introducing Acta2 promoter into the cells (29). EB recapitulates the embryonic development, but the purity of SMC lineage in the induced EB cannot be clearly defined. SMC differentiation from embryonic stem cells reflects the normal process of the differentiation, but maintaining the pure undifferentiated embryonic stem cell clones takes a lot of effort and may be a challenge to many laboratories (38, 41). In addition, previous studies have shown that human embryonic stem cells can be differentiated to both EC and SMC populations in the same differentiation conditions (38). These cells are excellent for in vivo neoangiogenesis and regeneration of blood vessels (38, 45). However, they may not be ideal for studying the mechanism of SMC differentiation, especially the progenitor-specific regulation, because the SMCs differentiated from embryonic stem cells are heterogenic and thus a mixed population. Therefore, a new robust model system is essential for a better understanding of the molecular mechanisms governing SMC differentiation from mesoderm. Several studies have used bone marrow-derived mesenchymal stem cells (BM-MSC) to study SMC differentiation, but they are not well characterized, and no model system has been established using BM-MSCs (15, 34). It is also unclear whether or not BM-MSCs are natural SMC progenitors in vivo. The model system described in the present study is likely an ideal model for SMC differentiation due to the following facts: 1) hES-MCs are likely the natural SMC progenitors because most of the vascular SMCs are derived from mesoderm (27). Characterization of the mesenchymal cells used in this study indicates they are derived from mesoderm (1) and the mesenchymal cell markers are diminished during the SMC differentiation. Moreover, the spontaneous differentiation of hES-MCs is minimal due to the low expression of Acta2 and Cnn1 under normal growing conditions. 2) hES-MCs are easy to culture. It only needs αMEM and 10% MSC-qualified fetal bovine serum. The cells adhere to the culture dish and can be split up to 10 passages. 3) One single cytokine, TGF-β, is sufficient for the induction of SMC differentiation. Moreover, TGF-β can induce a rapid SMC differentiation. Three days of TGF-β treatment converts most of hES-MCs into SMC phenotype. 4) SMCs differentiated from this model are functional. They can contract in response to muscarinic agonist carbachol as well as KCl. Most importantly, hES-MC-derived SMCs can be recruited to endothelial tube network and help stabilize the tubes. 5) Since hES-MCs are derived from human embryonic stem cells, this model may be used to study SMC differentiation in humans.
During embryonic development, the SMC marker ACTA2 appears very early during the vascular development. CNN1, a later marker, expresses after the initiation of SMC differentiation. MYH11 appears at the late stage of vascular development (22, 32, 35). The TGF-β-induced SMC differentiation from hES-MCs recapitulates the normal SMC differentiation process. Acta2 is activated at 2 h and Cnn1 at 4 h, while Myh11 is activated at 8 h after TGF-β induction of hES-MCs, which mimics the SMC marker gene expression pattern observed during normal SMC development (35). TGF-β-induced expression of SMC markers in hES-MCs appears to depend on both Smad signaling and SRF/CArG/myocardin because knockdown of Smad2, Smad3, or SRF blocks SMC marker expression. Consistently, mutation of SRF-binding CArG box blocks SMC marker promoter activity. These results indicate that TGF-β induction of hES-MC may serve as a physiologically relevant model for SMC differentiation.
Interestingly, in addition to TGF-β-treated cells, untreated hES-MCs are also recruited to endothelial tubes in 12 h after coculture. A previous study suggests that notch signaling is important for pericyte/SMC recruitment to endothelial tubes (10, 11). It is likely that notch signaling is activated in these untreated cells, leading to their recruitment to endothelial tubes. Alternatively, hES-MCs may be stimulated by cytokines from ECs or presented in the Matrigel including TGF-β. Indeed, control hES-MCs cocultured with ECs transiently express SMC marker genes in the initial period of recruitment (12 h) but the differentiation is blocked in a prolonged coculture (24 h), resulting in disruption of endothelial tube network after 24 h of coculture. The unsustained expression of SMC marker genes in control hES-MCs is probably due to the lack of endocrine support required for a complete SMC differentiation that is only available in vivo. The endothelial tubes cocultured with actual SMCs also disrupted after 24 h of incubation, probably because these SMCs have undergone phenotypic modulation in culture, i.e., become noncontractile SMCs that have lost their function in maintaining endothelial integrity (36). hES-MC-derived SMCs are able to preserve the endothelial tubes probably because they have acquired the contractile property of functional SMCs.
TGF-β induces myocardin expression, which is important for the SRF/CArG-mediated SMC marker gene activation. It appears that TGF-β induces hES-MCs to express myocardin via mechanisms different from other cells because multiple signaling pathways, including Smads, p38 MAPK, and PI3K, are all required for TGF-β-induced myocardin expression in hES-MCs (26, 44). Although myocardin is considered as a master regulator of SMC differentiation, several reports indicate that its role in SMC development is not as critical as previously anticipated because the expression of early SMC marker genes such as Tagln and Acta2 emerges prior to detectable myocardin mRNA in the embryonic dorsal aorta; this may suggest that myocardin has a minor role in the initiation of SMC differentiation in some vascular tissues (12, 17, 22, 44, 48). To better understand myocardin function in SMC development, especially in human SMC differentiation, we have detected the dynamic expression of myocardin in different phases of the differentiation from mesoderm. We found that myocardin expression is not obvious in human embryonic stem cells. However, myocardin mRNA is detectable at a low level in mesoderm, and its expression was increased when mesoderm is differentiated to hES-MCs. These low levels of myocardin appear not to be able to activate SMC differentiation. However, when hES-MCs are treated with TGF-β, myocardin mRNA is dramatically induced. These results suggest that a threshold expression is required for myocardin to be functionally involved in SMC differentiation. This notion is also supported by a previous report showing that a low level of myocardin is expressed in A404 cells, but it is not converted to SMC phenotype until retinoic acid induction (47).
In addition to serving as a model for SMC differentiation, hES-MC-derived SMCs have potential to be used for tissue engineering because of their human origin. Since hES-MCs are derived from mesoderm, these cells are potentially important resources for regenerating human SMCs that are mesoderm-origin including most vascular SMCs.
GRANTS
This work was supported by grants from National Institutes of Health (HL-093429 and HL-107526 to S.-Y. Chen) and American Heart Association (Pre-doctoral Fellowship Award 12PRE12030180 to X. Guo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
X.G. performed the experiments; X.G. and S.-Y.C. analyzed the data; X.G. and S.-Y.C. interpreted the results of the experiments; X.G. prepared the figures; X.G. drafted the manuscript; X.G., S.L.S., N.L.B., and S.-Y.C. approved the final version of the manuscript; S.L.S., N.L.B., and S.-Y.C. edited and revised the manuscript; S.-Y.C. conception and design of the research.
Footnotes
This article is the topic of an Editorial Focus by Amy L. Firth and Jason X.-J. Yuan (12a).
REFERENCES
- 1. Boyd NL, Robbins KR, Dhara SK, West FD, Stice SL. Human embryonic stem cell-derived mesoderm-like epithelium transitions to mesenchymal progenitor cells. Tissue Eng Part A 15: 1897– 1907, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 6: 389– 395, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25: 2739– 2749, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol 34: 1345– 1356, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Chen S, Crawford M, Day RM, Briones VR, Leader JE, Jose PA, Lechleider RJ. RhoA modulates Smad signaling during transforming growth factor-beta-induced smooth muscle differentiation. J Biol Chem 281: 1765– 1770, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen S, Kulik M, Lechleider RJ. Smad proteins regulate transcriptional induction of the SM22alpha gene by TGF-beta. Nucleic Acids Res 31: 1302– 1310, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen S, Lechleider RJ. Transforming growth factor-beta-induced differentiation of smooth muscle from a neural crest stem cell line. Circ Res 94: 1195– 1202, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Compton LA, Potash DA, Mundell NA, Barnett JV. Transforming growth factor-beta induces loss of epithelial character and smooth muscle cell differentiation in epicardial cells. Dev Dyn 235: 82– 93, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem 284: 3728– 3738, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doi H, Iso T, Sato H, Yamazaki M, Matsui H, Tanaka T, Manabe I, Arai M, Nagai R, Kurabayashi M. Jagged1-selective notch signaling induces smooth muscle differentiation via a RBP-Jkappa-dependent pathway. J Biol Chem 281: 28555– 28564, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Doi H, Iso T, Shiba Y, Sato H, Yamazaki M, Oyama Y, Akiyama H, Tanaka T, Tomita T, Arai M, Takahashi M, Ikeda U, Kurabayashi M. Notch signaling regulates the differentiation of bone marrow-derived cells into smooth muscle-like cells during arterial lesion formation. Biochem Biophys Res Commun 381: 654– 659, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Du KL, Ip HS, Li J, Chen M, Dandre F, Yu W, Lu MM, Owens GK, Parmacek MS. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol 23: 2425– 2437, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a. Firth AL, Yuan JX. Human models for smooth muscle cell differentiation. Focus on “A novel in vitro model system for smooth muscle differentiation from human embryonic stem cell-derived mesenchymal cells.” Am J Physiol Cell Physiol (January 16, 2013). doi:10.1152/ajpcell.00010.2013 [DOI] [PubMed] [Google Scholar]
- 13. Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, Amor D, Ades L, McConnell V, Willoughby CE, Abuelo D, Willing M, Lewis RA, Kim DH, Scherer S, Tung PP, Ahn C, Buja LM, Raman CS, Shete SS, Milewicz DM. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 39: 1488– 1493, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Han Y, Li N, Tian X, Kang J, Yan C, Qi Y. Endogenous transforming growth factor (TGF) beta1 promotes differentiation of smooth muscle cells from embryonic stem cells: stable plasmid-based siRNA silencing of TGF beta1 gene expression. J Physiol Sci 60: 35– 41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hegner B, Weber M, Dragun D, Schulze-Lohoff E. Differential regulation of smooth muscle markers in human bone marrow-derived mesenchymal stem cells. J Hypertens 23: 1191– 1202, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Hirschi KK, Rohovsky SA, D'Amore PA. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 141: 805– 814, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoofnagle MH, Neppl RL, Berzin EL, Teg Pipes GC, Olson EN, Wamhoff BW, Somlyo AV, Owens GK. Myocardin is differentially required for the development of smooth muscle cells and cardiomyocytes. Am J Physiol Heart Circ Physiol 300: H1707– H1721, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang WY, Xie W, Guo X, Li F, Jose PA, Chen SY. Smad2 and PEA3 cooperatively regulate transcription of response gene to complement 32 in TGF-β-induced smooth muscle cell differentiation of neural crest cells. Am J Physiol Cell Physiol 301: C499– C506, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jain MK, Layne MD, Watanabe M, Chin MT, Feinberg MW, Sibinga NE, Hsieh CM, Yet SF, Stemple DL, Lee ME. In vitro system for differentiating pluripotent neural crest cells into smooth muscle cells. J Biol Chem 273: 5993– 5996, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Kim S, Ip HS, Lu MM, Clendenin C, Parmacek MS. A serum response factor-dependent transcriptional regulatory program identifies distinct smooth muscle cell sublineages. Mol Cell Biol 17: 2266– 2278, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li F, Luo Z, Huang W, Lu Q, Wilcox CS, Jose PA, Chen S. Response gene to complement 32, a novel regulator for transforming growth factor-beta-induced smooth muscle differentiation of neural crest cells. J Biol Chem 282: 10133– 10137, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Li L, Miano JM, Cserjesi P, Olson EN. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res 78: 188– 195, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Li L, Miano JM, Mercer B, Olson EN. Expression of the SM22alpha promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J Cell Biol 132: 849– 859, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lilly B, Olson EN, Beckerle MC. Identification of a CArG box-dependent enhancer within the cysteine-rich protein 1 gene that directs expression in arterial but not venous or visceral smooth muscle cells. Dev Biol 240: 531– 547, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Long X, Bell RD, Gerthoffer WT, Zlokovic BV, Miano JM. Myocardin is sufficient for a smooth muscle-like contractile phenotype. Arterioscler Thromb Vasc Biol 28: 1505– 1510, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Long X, Miano JM. Transforming growth factor-beta1 (TGF-beta1) utilizes distinct pathways for the transcriptional activation of microRNA 143/145 in human coronary artery smooth muscle cells. J Biol Chem 286: 30119– 30129, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 27: 1248– 1258, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Manabe I, Owens GK. CArG elements control smooth muscle subtype-specific expression of smooth muscle myosin in vivo. J Clin Invest 107: 823– 834, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manabe I, Owens GK. Recruitment of serum response factor and hyperacetylation of histones at smooth muscle-specific regulatory regions during differentiation of a novel P19-derived in vitro smooth muscle differentiation system. Circ Res 88: 1127– 1134, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Manabe I, Owens GK. The smooth muscle myosin heavy chain gene exhibits smooth muscle subtype-selective modular regulation in vivo. J Biol Chem 276: 39076– 39087, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Maurer J, Fuchs S, Jager R, Kurz B, Sommer L, Schorle H. Establishment and controlled differentiation of neural crest stem cell lines using conditional transgenesis. Differentiation 75: 580– 591, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Miano JM, Cserjesi P, Ligon KL, Periasamy M, Olson EN. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res 75: 803– 812, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Milewicz DM, Guo DC, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Kwartler CS, Pannu H. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet 9: 283– 302, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Narita Y, Yamawaki A, Kagami H, Ueda M, Ueda Y. Effects of transforming growth factor-beta 1 and ascorbic acid on differentiation of human bone-marrow-derived mesenchymal stem cells into smooth muscle cell lineage. Cell Tissue Res 333: 449– 459, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75: 487– 517, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767– 801, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Rosenquist TH, Beall AC. Elastogenic cells in the developing cardiovascular system. Smooth muscle, nonmuscle, and cardiac neural crest. Ann NY Acad Sci 588: 106– 119, 1990 [DOI] [PubMed] [Google Scholar]
- 38. Sone M, Itoh H, Yamahara K, Yamashita JK, Yurugi-Kobayashi T, Nonoguchi A, Suzuki Y, Chao TH, Sawada N, Fukunaga Y, Miyashita K, Park K, Oyamada N, Taura D, Tamura N, Kondo Y, Nito S, Suemori H, Nakatsuji N, Nishikawa S, Nakao K. Pathway for differentiation of human embryonic stem cells to vascular cell components and their potential for vascular regeneration. Arterioscler Thromb Vasc Biol 27: 2127– 2134, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Topouzis S, Majesky MW. Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-beta. Dev Biol 178: 430– 445, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105: 851– 862, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Wobus AM, Kaomei G, Shan J, Wellner MC, Rohwedel J, Ji G, Fleischmann B, Katus HA, Hescheler J, Franz WM. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol Cell Cardiol 29: 1525– 1539, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Xie C, Ritchie RP, Huang H, Zhang J, Chen YE. Smooth muscle cell differentiation in vitro: models and underlying molecular mechanisms. Arterioscler Thromb Vasc Biol 31: 1485– 1494, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xie CQ, Zhang J, Villacorta L, Cui T, Huang H, Chen YE. A highly efficient method to differentiate smooth muscle cells from human embryonic stem cells. Arterioscler Thromb Vasc Biol 27: e311– 312, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Xie WB, Li Z, Miano JM, Long X, Chen SY. Smad3-mediated myocardin silencing: a novel mechanism governing the initiation of smooth muscle differentiation. J Biol Chem 286: 15050– 15057, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamaguchi S, Yamahara K, Homma K, Suzuki S, Fujii S, Morizane R, Monkawa T, Matsuzaki Y, Kangawa K, Itoh H. The role of microRNA-145 in human embryonic stem cell differentiation into vascular cells. Atherosclerosis 219: 468– 474, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res 96: 280– 291, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Yoshida T, Sinha S, Dandre F, Wamhoff BR, Hoofnagle MH, Kremer BE, Wang DZ, Olson EN, Owens GK. Myocardin is a key regulator of CArG-dependent transcription of multiple smooth muscle marker genes. Circ Res 92: 856– 864, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Zhang JC, Kim S, Helmke BP, Yu WW, Du KL, Lu MM, Strobeck M, Yu Q, Parmacek MS. Analysis of SM22alpha-deficient mice reveals unanticipated insights into smooth muscle cell differentiation and function. Mol Cell Biol 21: 1336– 1344, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]