Abstract
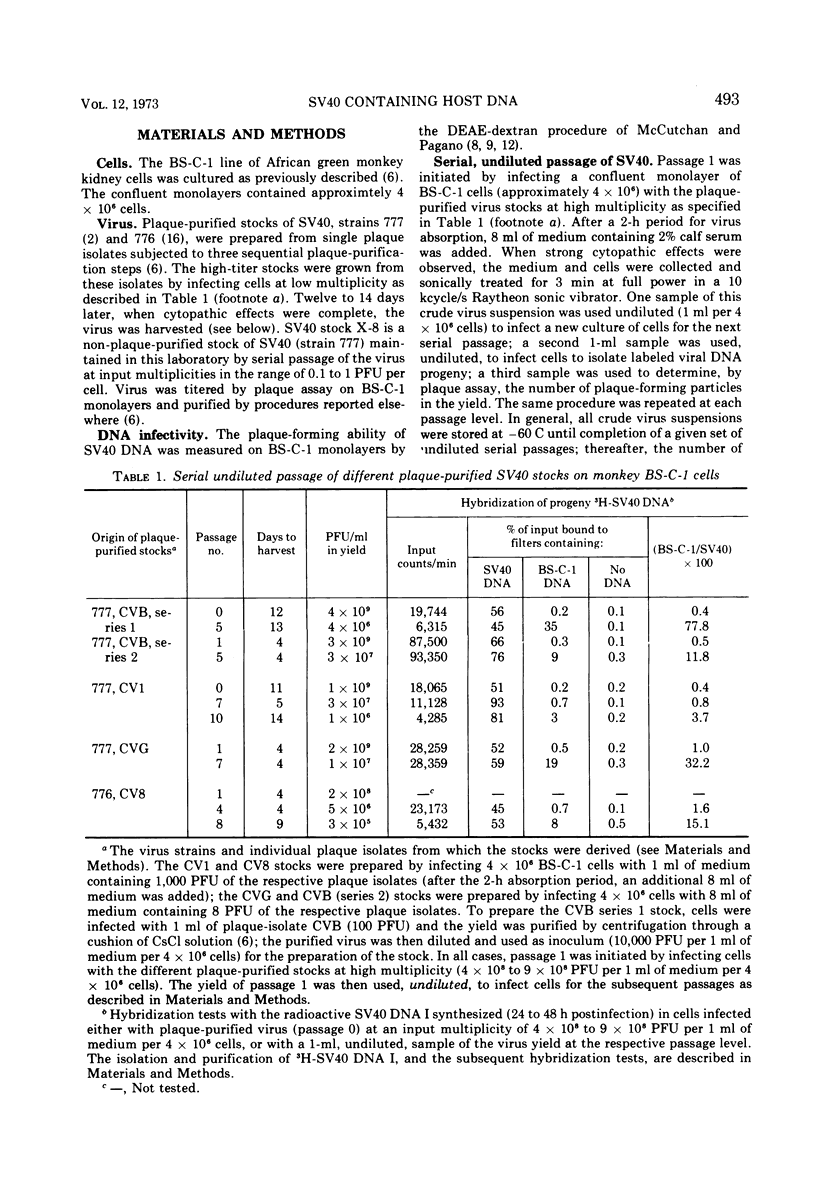
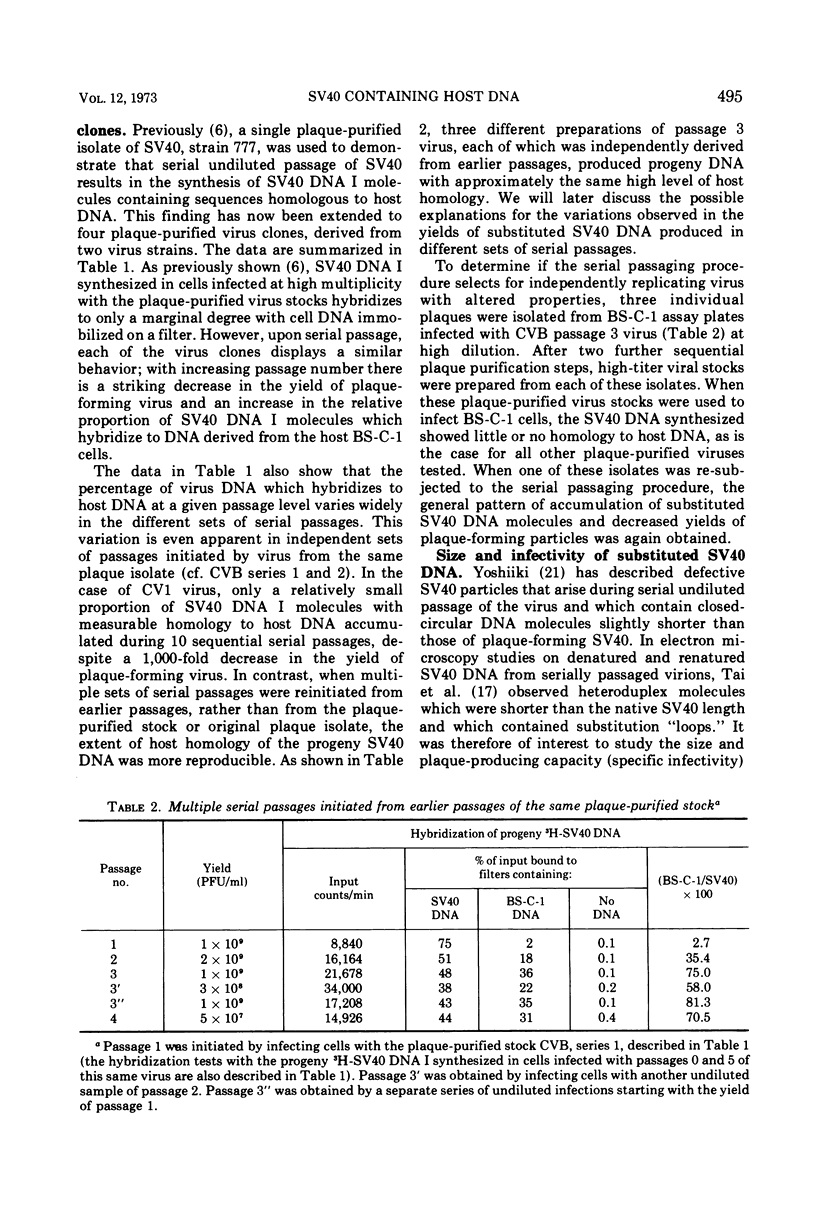
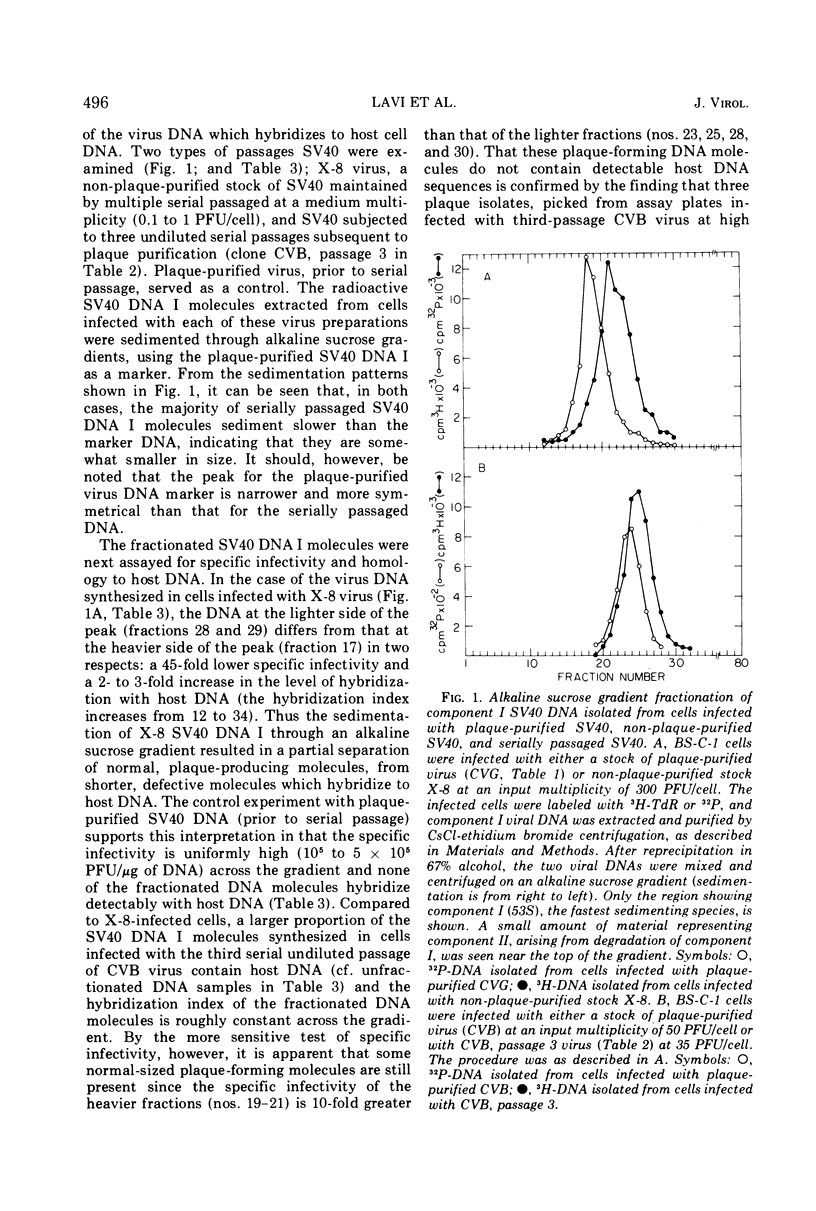
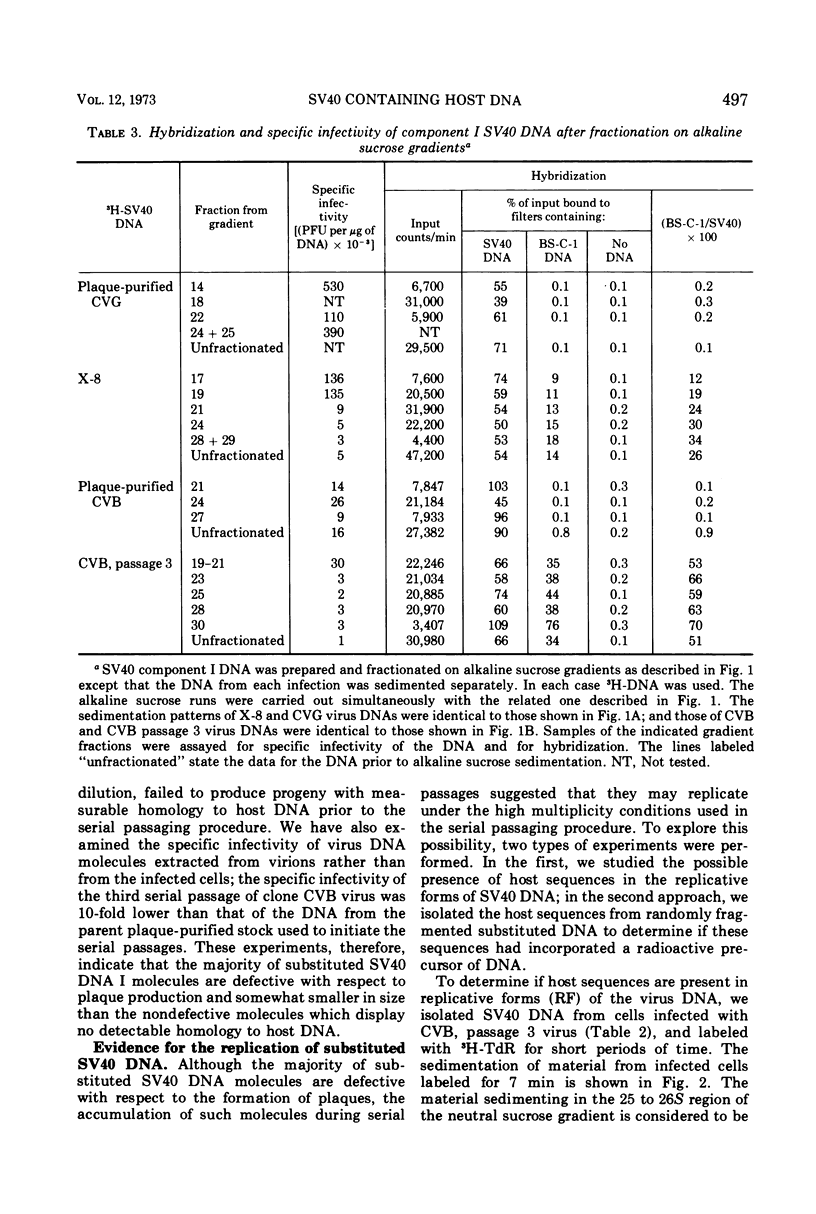
Three plaque isolates of SV40 strain 777 and 1 plaque isolate of strain 776 were grown to high-titer stocks and serially passaged, undiluted, in monkey BS-C-1 cells. In each case, the serial passaging procedure resulted in the accumulation of closed-circular SV40 DNA molecules containing covalently linked sequences homologous to reiterated host cell DNA (called substituted virus DNA). The relative yields, at a given passage level, of SV40 DNA with measurable homology to host DNA varied in different sets of serial passages, including passages of the same virus clone. More reproducible yields of substituted viral DNA progeny were obtained when the serial passaging procedure was initiated from earlier passages rather than from the original plaque-purified stock. Fractionation of closed-circular SV40 DNA molecules on alkaline sucrose gadients indicated that the majority of substituted virus DNA molecules are not plaque producers and are slightly smaller in size than plaque-forming DNA molecules which display no detectable homology to host DNA. Evidence that substituted SV40 DNA molecules replicate during serial undiluted passage was obtained from experiments which demonstrated (i) the presence of host sequences in replicative forms of the viral DNA and (ii) the incorporation of 3H-thymidine into host sequences isolated from the mature substituted virus DNA molecule.
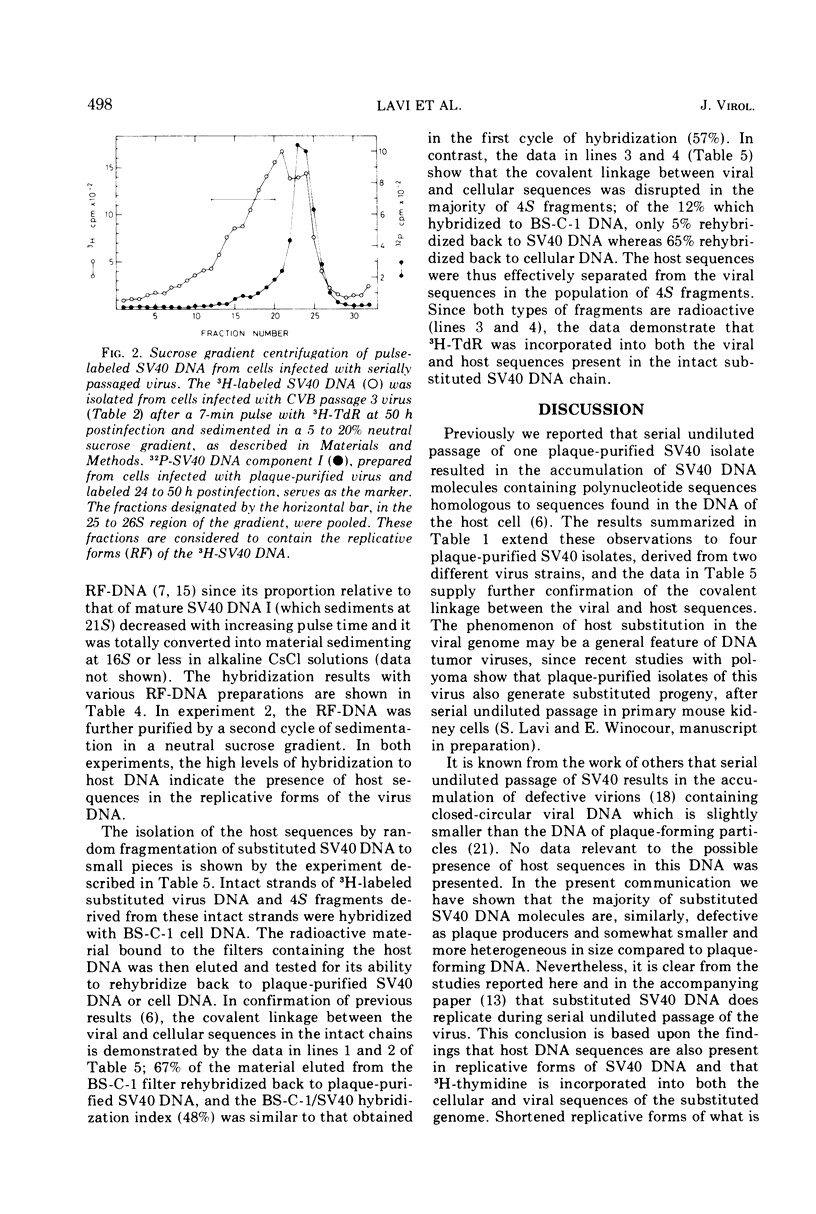
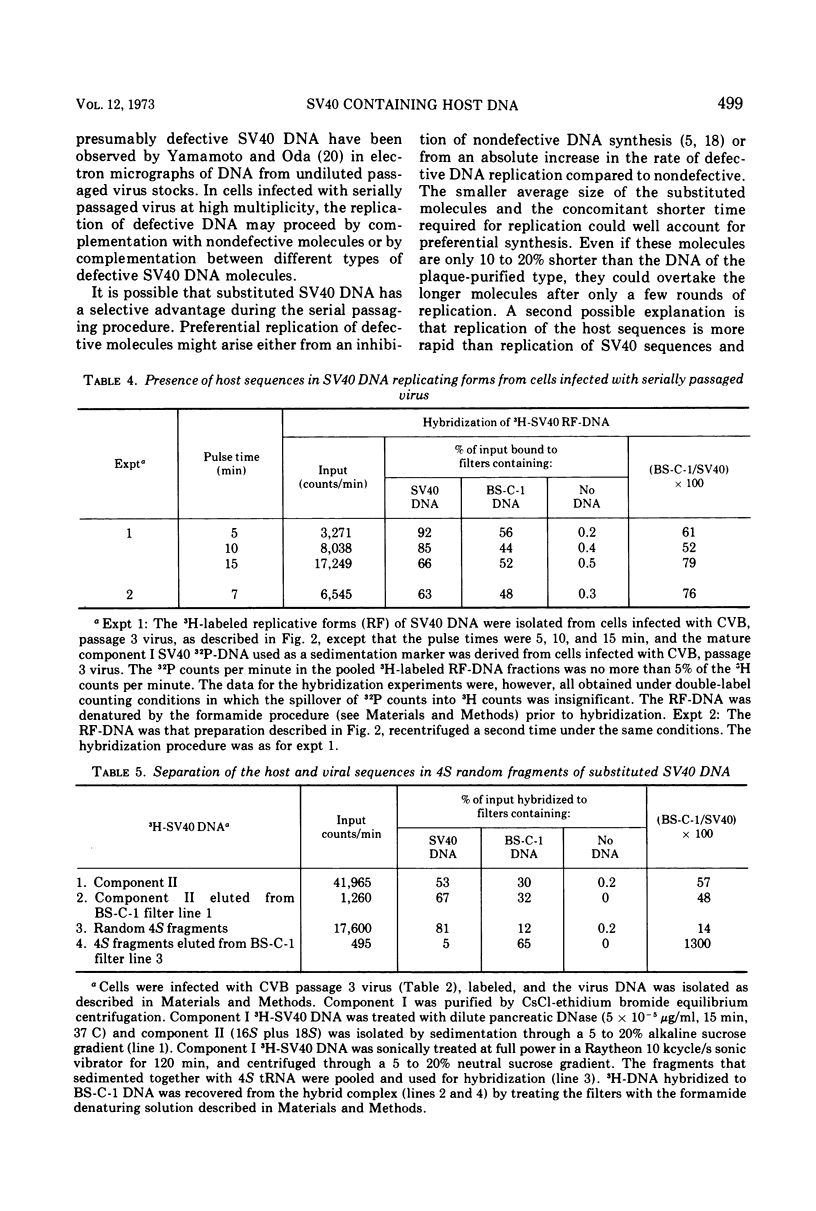
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Winocour E., Sachs L., Torten J. Hybridization between SV40 DNA and cellular DNA's. J Mol Biol. 1969 Sep 14;44(2):333–345. doi: 10.1016/0022-2836(69)90179-x. [DOI] [PubMed] [Google Scholar]
- GERBER P. An infectious deoxyribonucleic acid derived from vacuolating virus (SV40). Virology. 1962 Jan;16:96–97. doi: 10.1016/0042-6822(62)90209-x. [DOI] [PubMed] [Google Scholar]
- Hirai K., Defendi V. Integration of simian virus 40 deoxyribonucleic acid into the deoxyribonucleic acid of permissive monkey kidney cells. J Virol. 1972 Apr;9(4):705–707. doi: 10.1128/jvi.9.4.705-707.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt S., Lavi S., Singer M. F., Winocour E. Acquisition of sequences homologous to host DNA by closed circular simian virus 40 DNA. 3. Host sequences. J Virol. 1973 Sep;12(3):501–510. doi: 10.1128/jvi.12.3.501-510.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenblatt S., Winocour E. Covalently linked cell and SV40-specific sequences in an RNA from productively infected cells. Virology. 1972 Nov;50(2):558–566. doi: 10.1016/0042-6822(72)90407-2. [DOI] [PubMed] [Google Scholar]
- Rozenblatt S., Winocour E. Induction of cellular DNA synthesis by supercoiled SV40 DNA in x-irradiated mouse 3T3 cells. Virology. 1971 Jan;43(1):300–303. doi: 10.1016/0042-6822(71)90247-9. [DOI] [PubMed] [Google Scholar]
- SWEET B. H., HILLEMAN M. R. The vacuolating virus, S.V. 40. Proc Soc Exp Biol Med. 1960 Nov;105:420–427. doi: 10.3181/00379727-105-26128. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M., Burge B. W. Defective virus particles from Sindbis virus. Virology. 1972 May;48(2):615–617. doi: 10.1016/0042-6822(72)90076-1. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai H. T., Smith C. A., Sharp P. A., Vinograd J. Sequence heterogeneity in closed simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):317–325. doi: 10.1128/jvi.9.2.317-325.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S., Watanabe S., Kato M. Incomplete growth of simian virus 40 in African green monkey kidney culture induced by serial undiluted passages. Virology. 1966 Jan;28(1):135–141. doi: 10.1016/0042-6822(66)90314-x. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Oda T. Electron microscpic studies on replicating form of defective SV40 virus DNA. Arch Gesamte Virusforsch. 1972;38(1):29–37. doi: 10.1007/BF01241353. [DOI] [PubMed] [Google Scholar]
- Yoshiike K. Studies on DNA from low-density particles of SV40. I. Heterogeneous defective virions produced by successive undiluted passages. Virology. 1968 Mar;34(3):391–401. doi: 10.1016/0042-6822(68)90059-7. [DOI] [PubMed] [Google Scholar]



