Abstract
G protein-coupled receptors (GPCRs) are the largest class of membrane proteins that play key roles in transducing extracellular signals to intracellular proteins to generate cellular responses. The kinin GPCRs, named B1 (B1R) and B2 (B2R) are responsible for mediating the biological responses to kinin peptides released from the precursor kininogens. Bradykinin or kallidin are agonists for B2Rs whereas their carboxypeptidase-generated metabolites, des-Arg9-bradykinin or des-Arg10-kallidin are specific agonists for B1Rs. Here we review the evidence for a critical role of membrane-bound carboxypeptidase M in facilitating B1R signaling by its ability to directly activate the receptor via conformational crosstalk as well as generate its specific agonist. In endothelial cells, the carboxypeptidase M/B1R interaction facilitates B1R-dependent high output nitric oxide under inflammatory conditions.
Keywords: glycosylphosphatidylinositol anchor, G protein-coupled receptor, inducible nitric oxide synthase, inflammation, peroxynitrite, protein-protein interaction
Regulation of G protein-coupled receptor function
Cellular responses to extracellular stimuli are typically transduced by transmembrane proteins whose conformational changes in response to the stimulus is transmitted to intracellullar domains, which then interact with and activate signaling cascades. The largest class of such transmembrane proteins is G protein-coupled receptors (GPCRs), which comprise the largest human gene family and are targets for ~ 30% or more of all prescription drugs. GPCRs have unique structural flexibility and multiple dynamic conformational states that can be altered or stabilized by ligand binding, leading to intracellular signal generation. More recent studies have shown that binding to allosteric sites, typically on extracelluar domains and distinct from the normal orthosteric agonist binding site, can trigger or alter receptor signaling or lead to biased agonism (Kenakin, 2009).
Agonists for GPCRs are commonly small molecules, peptides or sensory stimuli that interact with receptors’ extracellular or transmembrane domains. Protein-protein interactions also play crucial roles in mediating and regulating receptor function. The best studied examples are the canonical G proteins, GPCR kinases and arrestins (Ritter and Hall, 2009). However, numerous other proteins can interact with GPCRs to facilitate signaling, localize them to specific subcellular regions, scaffold them with other molecules to enhance signaling efficiency, regulate receptor trafficking or alter receptor pharmacology. Most of these proteins interact with the intracellular domains of GPCRs, but some membrane proteins can also interact with GPCRs and regulate their function. Notable examples include GPCRs themselves, which can form homo- and hetero-dimers, and single transmembrane receptor activity-modifying proteins (or RAMPs) I – III, which bind to GPCRs and alter their agonist selectivity (Ritter and Hall, 2009). However, very few other membrane proteins have been described to regulate GPCR signaling.
Peptidase regulation of kinin signaling
Early interest in enzymes that cleave kinins (broadly termed “kininases”) was inspired by studies of acetylcholine degradation by cholinesterases, where it became evident that enhancing acetylcholine levels by inhibiting cholinesterases is a better therapeutic strategy than administering the agonist itself (Erdös, 2002). Initial investigations by Erdös and colleagues uncovered a prominent carboxypeptidase (CP) in plasma that cleaved the C-terminal Arg from kinins (named kininase I and later CPN) and another enzyme in kidney that cleaved the C-terminal Phe-Arg dipeptide from kinins called kininase II (Erdös, 2002). They later showed kininase II to be identical with the angiotensin I converting enzyme (ACE) (Yang et al., 1970; Erdös, 2002). The nonapeptide bradykinin (BK) or decapeptide kallidin (KD), released from kininogen precursors by plasma or tissue kallikrein, are both natural agonists for the kinin G protein-coupled receptor (GPCR) named B2 (B2R), which is constitutively expressed. However, a second kinin GPCR called B1 (B1R), whose expression is induced by inflammatory mediators, is instead activated by metabolites generated by carboxypeptidase-mediated removal of the C-terminal Arg from BK or KD to produce des-Arg9-BK (DABK) or des-Arg10-KD (DAKD), which are not agonists of B2Rs. In this regard, kininase I-type cleavage is required to generate B1R agonists but inactivates them as B2R agonists whereas kininase II-type cleavage inactivates kinins for both receptors. Numerous additional peptidases were found to cleave kinins (Erdös and Skidgel, 1997; Erdös, 2002) and can have significant roles in regulating kinin function depending on the tissue and pathological conditions, but ACE (kininase II) and kininase I-type carboxypeptidases remain key regulators of B2R and B1R function.
The renin-angiotensin and kallikrein-kinin systems were initially considered to be endocrine systems, and the systemic balance between vasopressor and vasodilator peptides were thought to play a key role in regulating blood pressure. However, it later became clear that these systems have primary functions as local hormone systems with autocrine/paracrine effects (Carretero and Scicli, 1991). Thus, peptides in the circulation likely represent “spillover” of those generated at local sites and although blood-borne enzymes may prevent the toxic buildup of these hormones in the blood, they have less to do with the normal regulation of peptide activity and signaling. This function is more likely carried out by membrane-bound peptidases with extracellular active sites, where local cleavage of peptide agonists regulates their levels at or near their cognate receptors.
Carboxypeptidase Generation of B1R Agonist
Mammalian CPs can be divided into two groups based on substrate specificity; CPA-type enzymes preferentially cleave C-terminal hydrophobic residues and CPB-type enzymes hydrolyze only peptides containing C-terminal Arg or Lys (Skidgel, 1996). Thus, CPB-type are good candidates to generate des-Arg-kinin B1R agonists to stimulate B1R signaling. The first discovered enzyme, pancreatic CPB, rapidly removes the C-terminal Arg from kinins (Erdös et al., 1963), but its localization and primary digestive function in the pancreas and intestine means it has no significant role in regulating kinin function. The two CPB-type enzymes in blood, CPN (the original kininase I) and CPU (also called TAFI) cleave the C-terminal Arg of BK (Erdös and Skidgel, 1997; Skidgel and Erdös, 2004). However, CPU is an inactive pro-enzyme that is activated during coagulation and CPN preferentially cleaves C-terminal Lys and has relatively poor kinetic constants with BK (Skidgel et al., 1984). In addition, generated des-Arg-kinins would be diluted into in the large blood volume before reaching the B1R, reducing efficacy. This process would more likely be efficiently carried out by cellular B-type CPs. Of the known cellular enzymes, CPE has an acidic pH optimum and is localized in secretory granules whereas CPZ is localized in the extracellular matrix (Reznik and Fricker, 2001), making these enzymes unlikely candidates. This function would be better served by a membrane-bound CP in close proximity to the receptor. CPD is membrane-bound and readily cleaves bradykinin (McGwire, G., Tan, F. and Skidgel, R.A., unpublished), however it is primarily localized in the trans-Golgi network and has an acidic pH optimum of 6.2 (Hadkar and Skidgel, 2001; Reznik and Fricker, 2001). Thus, CPD is unlikely to play a major role in generating B1R agonists.
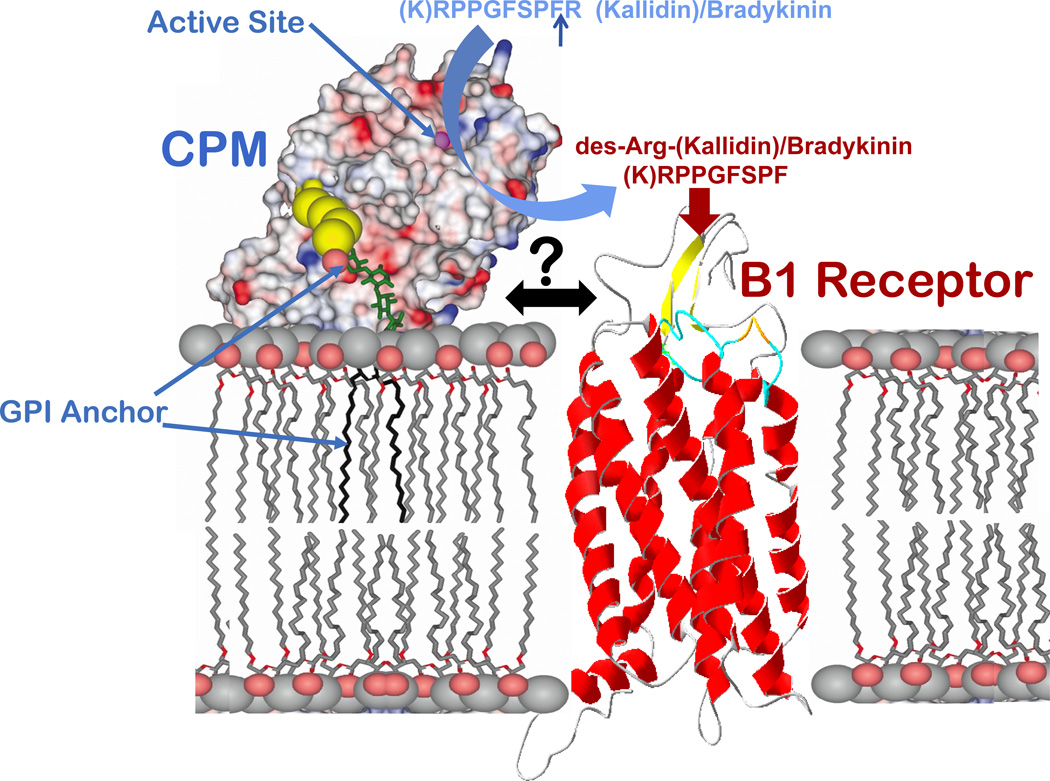
CPM has ideal properties as a processing enzyme to generate des-Arg kinin agonists in close proximity to the B1R. CPM is a glycosylphosphatidylinositol (GPI)-anchored ectoenzyme on plasma membranes with a neutral pH optimum, preferentially cleaves C-terminal Arg and has the lowest Km (16 µM) for BK of the physiological substrates tested, (Skidgel et al., 1984; Skidgel et al., 1989; Deddish et al., 1990). Furthermore, although CPM is constitutively expressed in a wide variety of cell types, endotoxin or cytokines, agents known to induce B1R expression (Leeb-Lundberg et al., 2005), also increase CPM expression(Sangsree et al., 2003; Hadkar et al., 2004). Thus, cells expressing B1Rs will likely also express CPM. The crystal structure of CPM revealed it to be comprised of a 295 residue N-terminal catalytic domain and an 86 residue, C-terminal β-sandwich (transthyretin-like) domain with a unique 25 residue extension to which the GPI anchor is post-translationally attached (Reverter et al., 2004). Molecular modeling indicated that CPM may adopt a favorable orientation on the membrane for cleaving juxtamembrane substrates via tethering by the C-terminal extension and interaction of positively charged residues in the C-terminal β sandwich domain with the phospholipid head groups (Reverter et al., 2004) (Fig. 1).
Figure 1.
Schematic diagram of the possible orientation and interaction of CPM and B1R on the membrane. The CPM model shows its electrostatic surface potential based on the crystal structure and probable membrane association via the GPI anchor and electrostatic interactions (Reverter et al., 2004). The active-site groove is indicated by the zinc ion (magenta sphere). A ribbon model of the B1R is shown at right. CPM cleaves the C-terminal Arg from kinins and its close association with the B1R facilitates delivery of des-Arg-kinin agonists to the receptor.
CPM interaction with B1R facilitates efficient signaling in response to B2R agonists
Because the native kinin peptides BK and KD are initially released from kininogen precursors, we investigated the role of CPM in the B1R response to these typical B2R agonists. The delivery of des-Arg-kinins to the B1R would likely be more efficient if there were close physical proximity between CPM and B1R on the membrane, more so if it involved direct protein-protein interaction (Fig. 1). To investigate this, we utilized HEK293 cells stably co-expressing CPM and B1R. We found that CPM and B1R were co-localized in lipid raft/caveolin-enriched membrane fractions as determined by gradient centrifugation (Zhang et al., 2008). Furthermore, CPM and the B1R do interact on the cell membrane as shown by co-immunoprecipitation, crosslinking and FRET analysis (Zhang et al., 2008).
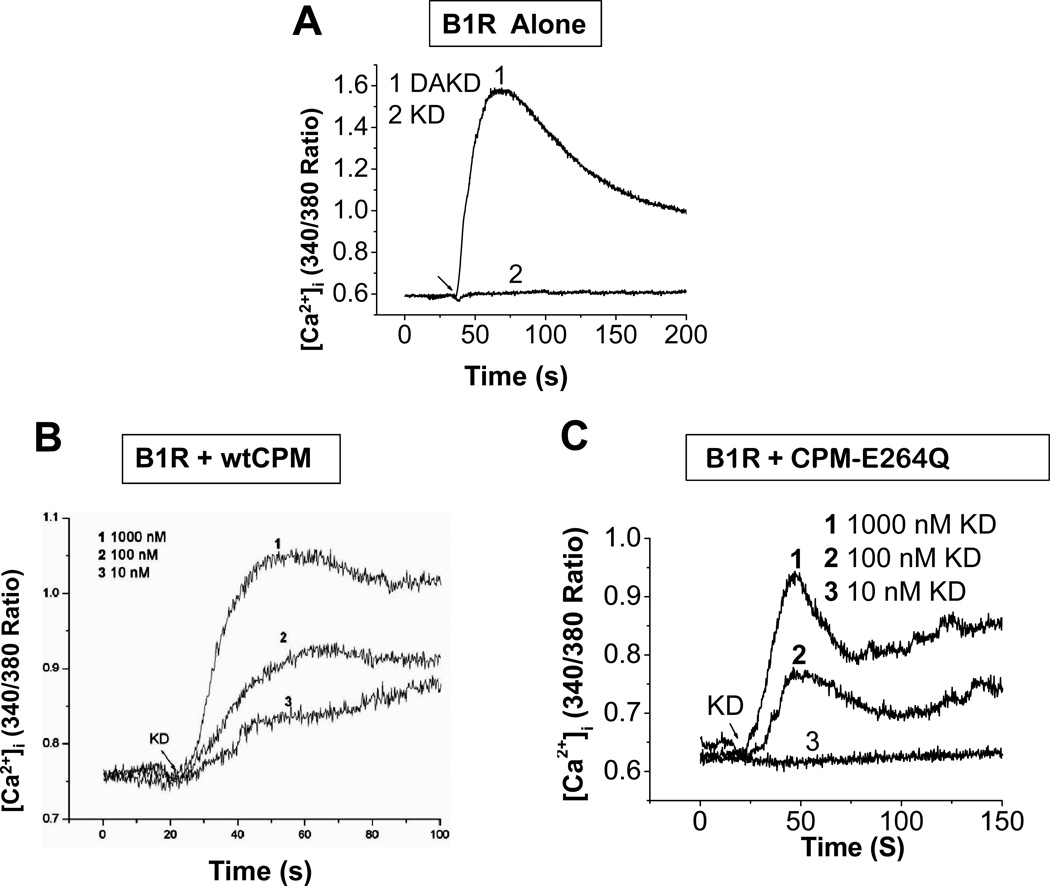
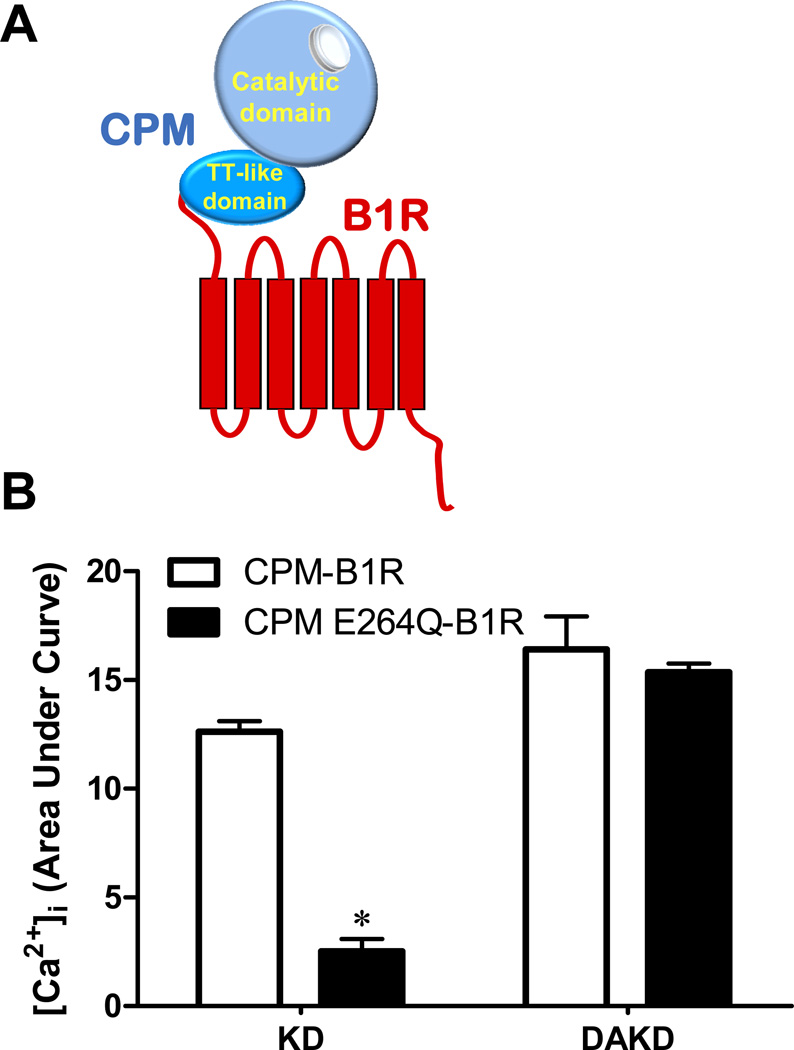
To investigate the role of CPM in regulating B1R signaling, we measured the increase in intracellular calcium ([Ca2+]i) in response to BK or KD, the natural B2R agonists. HEK cells stably expressing B1Rs alone did not respond to 1 µM KD, but exhibited a robust increase in [Ca2+]i in response to 1 µM des-Arg10-KD (DAKD), the B1R agonist (Fig. 2A). In contrast, cells co-expressing the B1R and CPM gave a dose-dependent increase in [Ca2+]i in response to 10 nM – 1 µM KD (Fig. 2B) that was blocked by a B1R antagonist or CPM inhibitor (Zhang et al., 2008). These data indicate that CPM can generate B1R agonist more efficiently than might be predicted by its Km of 16 µM with BK, determined for the purified protein (Skidgel et al., 1989). To further investigate the role of B1R/CPM interaction in this response, we generated a covalently linked fusion protein with the N-terminus of the B1R fused to the C-terminus of CPM (lacking the GPI anchor sequence) (Fig. 3A). This construct was a functional B1R as DAKD stimulated an increase in [Ca2+]i (Fig. 3B). The covalently linked CPM was able to generate and deliver agonist to the B1R as the B2R agonist KD also stimulated an increase in [Ca2+]i (Fig. 3B).
Figure 2.
Co-expression of the B1R with either CPM or CPM-E264Q generates a B1R-dependent calcium response to B2R agonist kallidin. Tracings showing the increase in [Ca2+]i induced by KD or DAKD in HEK cells stably expressing B1R (A), B1R and wtCPM (B), or B1R and CPM-E264Q (C). The concentrations of agonists in (A) were 1 µM. The results are representative of three independent experiments. This research was originally published in the Journal of Biological Chemistry. Zhang, X., Tan, F., Zhang, Y., and Skidgel, R. A. Carboxypeptidase M and kinin B1 receptors interact to facilitate efficient B1 signaling from B2 agonists. J Biol Chem 2008; 283: 7994–8004 (B). Zhang X, Tan F, Brovkovych V, Zhang Y, Skidgel RA. Cross-talk between carboxypeptidase M and the kinin B1 receptor mediates a new mode of G protein-coupled receptor signaling. J Biol Chem 2011; 286:18547 – 18561 (A and C). © the American Society for Biochemistry and Molecular Biology.
Figure 3.
The calcium response to kinin peptides in HEK cells stably expressing CPM-B1R or CPM-E264Q-B1R fusion proteins. (A) Schematic diagram of the chimera generated by fusing the C-terminus of CPM to the N-terminus of the B1R. (B) HEK cells stably expressing the B1R chimera with either wtCPM (CPM-B1R) or CPM-E264Q (CPM E264Q-B1R) were stimulated with 1 µM KD or DAKD and the increase in [Ca2+]i was quantified by integrating the area under the curve (expressed as mean ± SE (n=3). * = p<0.05 vs. CPM-B1R). This research was originally published in the Journal of Biological Chemistry. Zhang X, Tan F, Brovkovych V, Zhang Y, Skidgel RA. Cross-talk between carboxypeptidase M and the kinin B1 receptor mediates a new mode of G protein-coupled receptor signaling. J Biol Chem 2011; 286:18547 – 18561. © the American Society for Biochemistry and Molecular Biology.
To further investigate the role of the CPM/B1R interaction, we utilized a specific monoclonal antibody to CPM and identified its epitope to be residues 302–311 in the C-terminal transthyretin-like β sandwich domain, between β9 and β10. In cells co-expressing CPM and B1R we found that this antibody disrupted the CPM/B1R interaction and it inhibited the increase in [Ca2+]i in response to B2R agonist, but did not inhibit CPM activity or B1R activation by des-Arg-kinin agonists (Zhang et al., 2008). In addition, it did not block the increase in [Ca2+]i in cells expressing the covalently linked CPM-B1R fusion protein. Similarly, a peptide (“CT peptide”) containing this epitope (Ac-KGQVFDQNGNPLPN-NH2) also disrupted the CPM/B1R interaction and inhibited the response to B2R agonists whereas a scrambled peptide with the same amino acids had no effect (Zhang et al., 2011). Thus, the CPM/B1R interaction is important in enhancing the efficiency of B1R signaling in response to B2R kinin agonists and the C-terminal domain of CPM is important in mediating this interaction.
Kinin binding to CPM activates B1R signaling
We previously showed that Glu264 is the essential catalytic glutamic acid in CPM and that mutation to Gln (CPM-E264Q) yields a catalytically inactive enzyme that is still able to bind substrate (Tan et al., 2003). We initially planned to use this mutant as a negative control, but were surprised to find that HEK cells stably expressing CPM-E264Q and B1R also gave a dose-dependent increase in [Ca2+]i to B2R agonist KD (Fig. 2C) that was blocked by a B1R antagonist or CPM inhibitor (Zhang et al., 2011). To explore the role of substrate binding affinity on this non-catalytic response, we utilized cells co-expressing B1R and the catalytically inactive CPM mutant with an additional mutation (CPM-E264Q/ S180N) that reduces CPM’s affinity for C-terminal Arg and increases affinity for C-terminal Lys. These cells lost the B1R response to BK (with C-terminal Arg) but acquired a response to kinins in which the C-terminal Arg was replaced with Lys (K9-BK or K10-KD) (Zhang et al., 2011), indicating the importance of substrate binding.
To rule out the possibility that the interaction of CPM and B1R on the membrane somehow restored the catalytic activity of CPM to generate des-Arg-kinin B1R agonist, we measured the hydrolysis of a synthetic CPM substrate, dansyl-Ala-Arg, and BK in live cells stably co-expressing CPM-E264Q and B1R and found no activity with either substrate (Zhang et al., 2011).
We reasoned that the response mediated by CPM-E264Q likely requires it to be co-expressed on the same cell as the B1R in contrast to wild type (wt) CPM, which generates des-Arg-kinins that can diffuse to more distant B1Rs. To investigate this, HEK cells stably expressing only wtCPM or CPM-E264Q were mixed with cells stably expressing only B1Rs in a 1:1 ratio. Whereas 1 µM BK did induce a significant increase in [Ca2+]i in the mixed culture of cells expressing wtCPM and B1R, it did not increase [Ca2+]i in mixed cells expressing CPM-E264Q and B1R (Zhang et al., 2011). This response also required interaction between CPM and B1R as it was inhibited by disruption of the interaction with the CPM monoclonal antibody (Zhang et al., 2011).
To determine whether physical interaction between CPM-E264Q and B1R was sufficient to generate a response to BK or KD, we made a fusion protein as above with CPM-E264Q linked to the N-terminus of the B1R. Although this chimeric protein responded to direct application of B1R agonist DAKD, it gave only a minimal calcium response to 1 µM KD in contrast to the B1R fusion protein with wtCPM (Fig. 3B). Thus, tethering wtCPM to the N-terminus of the B1R still allows efficient delivery of enzymatically generated agonist to the B1R, but does not allow substrate binding to CPM-E264Q to generate a response to KD. We interpret this to mean that the CPM-E264Q-mediated response requires proper orientation/binding of CPM-E264Q and B1R on the membrane, which is not reproduced by covalent linkage.
CPM mediates B1R activation via conformational crosstalk
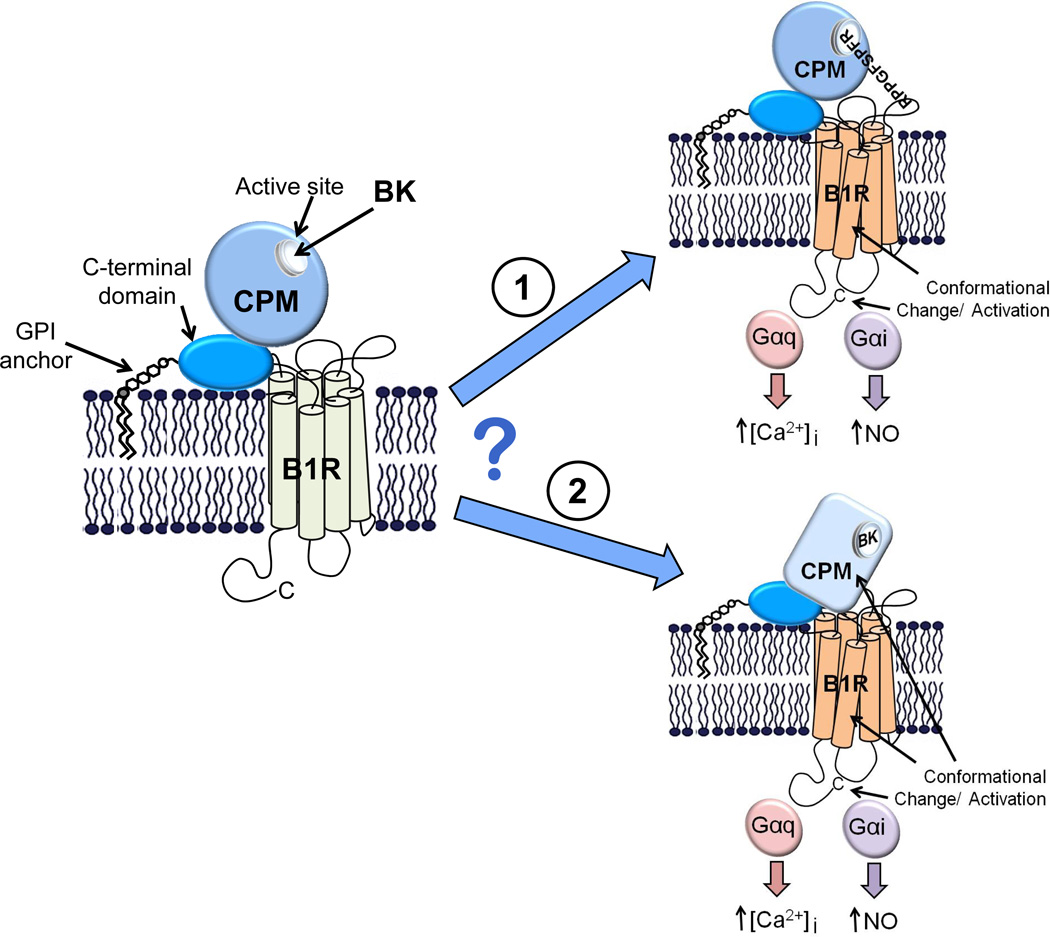
The presence or absence of the C-terminal Arg determines the specificity of kinin peptides for the B2R or B1R due to repulsion of Lys118 in transmembrane domain 3 of the B1R, which corresponds to Ser111 in the B2R (Leeb-Lundberg et al., 2005). One mechanism by which the CPM-E264Q/B1R interaction could cause B1R signaling in response to BK or KD is by participating in the binding of KD or BK simultaneously with the B1R (Fig.4). Thus, CPM binding of the C-terminal Arg might shield the positive charge from Lys118, allowing the kinin N-terminus to bind the B1R (Fig. 4; top). However, because the CPM active site interacts with three C-terminal residues of its substrates (Reverter et al., 2004), it would likely prevent productive binding of remaining peptide to the receptor. To address this, we assessed the ability of a B1R agonist (which would not bind CPM) or a CPM inhibitor (which would not bind the B1R) to displace [3H]-BK binding on cells co-expressing CPM-E264Q and B1Rs. Whereas the CPM inhibitor MGTA almost completely displaced bound 3[H]-BK, the B1R agonist DABK had little effect (Zhang et al., 2011). These data indicate that BK binds to CPM and does not bind simultaneously to the active site of CPM and orthosteric binding site of the B1R.
Figure 4.
Potential mechanisms by which BK binding to CPM could activate the B1R. A model of CPM and its potential membrane orientation and basal interaction with the B1R is shown in the left panel. BK (or KD) released from the kininogen precursor could stimulate B1R signaling in two ways via CPM. 1. CPM binding of the C-terminal Arg might shield the positive charge, which is typically repelled by Lys118, allowing the kinin N-terminus to bind and activate the B1R. 2. Binding as a substrate could cause a conformational change in CPM that is transmitted via protein-protein interaction to the B1R, resulting in activation. Our data are consistent with the second (bottom) mechanism, but not the first (top). For further details, see text.
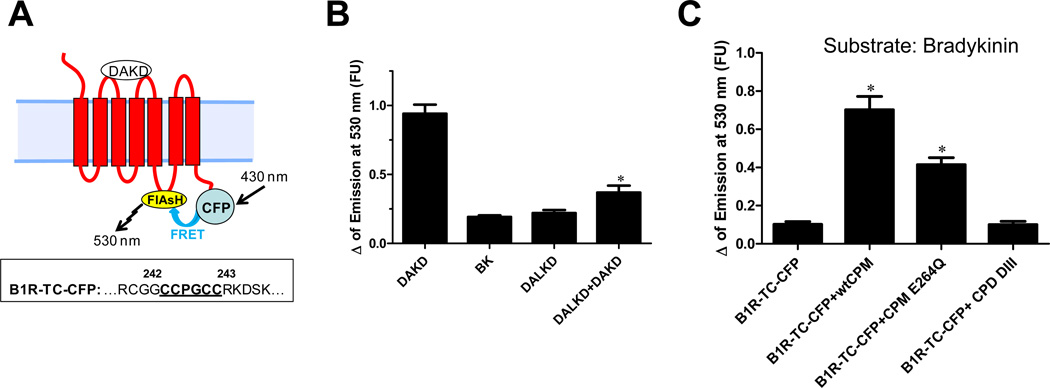
Taken together, our results are consistent with the possibility that binding of BK or KD to CPM-E264Q causes a conformational change that is transmitted to the B1R by protein-protein interaction, leading to receptor activation (Fig. 4; bottom). To address this possibility, we used an intramolecular FRET approach (Hoffmann et al., 2005), in which a tetracysteine motif (CCPGCC; binds the FRET acceptor, a small molecule called FlAsH,) is inserted into the third intracellular loop of the B1R and CFP FRET donor is fused to the C-terminus to generate B1R-TC-CFP (Fig. 5A). This receptor was fully functional, as shown by [3H]DAKD binding and a robust increase in [Ca2+]i and ERK activation in response to DAKD (Zhang et al., 2011). There was basal FRET between CFP and FlAsH in B1R-TC-CFP (Zhang et al., 2011) and FRET increased upon agonist stimulation (Fig. 5B). B1R antagonist des-Arg10-Leu8-KD (DALKD) or BK gave no response and pretreatment of cells with DALKD inhibited the increased FRET in response to DAKD (Fig. 5B). These data show that the fluorophores are close enough to exhibit FRET and the increase in FRET upon agonist stimulation is consistent with a conformational change that reduces the distance between the CFP and FlAsH fluorophores.
Figure 5.
Effect of kinin peptides on B1R conformational change. (A) Schematic representation of the intramolecular FRET reporter, B1R-TC-CFP. An agonist -induced conformational change that brings FlAsH and CFP closer together would result in an increase in the efficiency of FRET as detected by an increase in emission at 530 nm. Lower box: Sequence of the 3rd intracellular loop of the B1R showing the insertion of the tetracysteine biarsenical binding tag in between Gly242 and Arg243. (B) An increase in FRET of B1R-TC-CFP is induced by B1R agonist and is inhibited by B1R antagonist DALKD. The data are expressed as mean ± SE (n=3). * = p<0.05 vs. DAKD. (C) The change in intramolecular FRET of B1R-TC-CFP in response to BK. HEK cells stably expressing B1R-TC-CFP alone or with wtCPM, CPM-E264Q or CPD DIII were stimulated with 1 µM BK and the FlAsH emission at 530 nm was recorded in real-time while exciting CFP at 430 nm. The data are expressed as mean ± SE (n=4). * = p<0.05 vs. B1R-TC-CFP. This research was originally published in the Journal of Biological Chemistry. Zhang X, Tan F, Brovkovych V, Zhang Y, Skidgel RA. Cross-talk between carboxypeptidase M and the kinin B1 receptor mediates a new mode of G protein-coupled receptor signaling. J Biol Chem 2011; 286:18547 – 18561. © the American Society for Biochemistry and Molecular Biology.
To determine whether BK binding to CPM can cause a conformational change in the B1R consistent with activation, the change in intramolecular FRET of B1R-TC-CFP was examined. BK induced a significant increase in FRET in cells stably co-expressing B1R-TC-CFP and wtCPM or CPM-E264Q, but not in cells expressing only B1R-TC-CFP or co-expressing negative control CPD-DIII (the inactive membrane-bound domain of CPD) (Fig. 5C). These data show that BK binding to CPM causes a conformational change in the B1R that reduces the distance between the 3rd intracellular loop and the C-terminal tail, similar to that induced by B1R agonist. The increase in FRET was greater in cells co-expressing B1Rs and wtCPM compared with those co-expressing CPM-E264Q (Fig. 5C), likely due to the ability of wtCPM to also convert BK to B1R agonist.
CPM - B1R crosstalk: Why does it have to be so complicated?
The classical notion that peptidases regulate peptide activity by enzymatic hydrolysis to generate active or inactive fragments is true as far as it goes, but it is not the whole story. The idea that peptidases and peptide receptors have more subtle and complex relationships than originally thought may strike some as unnecessarily complicated. However, it has become clear in recent years that cells are highly complex, organized structures that can carry out their signaling, metabolic and synthetic functions much more efficiently and with higher fidelity than proteins and enzymes floating freely in solution. For example, protein signaling complexes form functional units that are critical to transmission of extracellular signals across the cell membrane and their efficient propagation intracellularly (Ritter and Hall, 2009). Although the location of both membrane peptidases and receptors on the plasma membrane undoubtedly enhances the efficiency of local control of peptide hormones, peptidase functions can also include protein-protein interactions as shown above for CPM and B1R. In addition to the present findings, there is significant evidence for functional interactions between ACE and B2Rs on their extracellular domains that goes beyond cleavage of kinin peptides by ACE (Erdös et al., 2010). Thus, ACE inhibitors potentiate the B2R effects of BK analogs that are not cleaved by ACE and can reactivate B2Rs that have been desensitized. ACE and B2Rs also form a heterodimeric complex on the cell membrane (Marcic et al., 2000; Chen et al., 2006), indicating that a conformational change in ACE upon inhibitor binding is likely transmitted to the receptor to mediate these actions (Erdös et al., 2010).
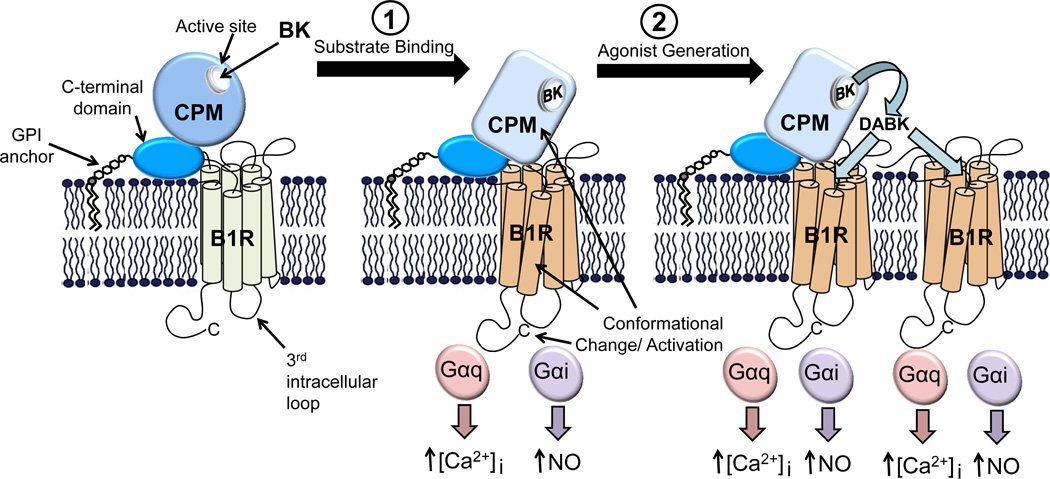
Because CPM is a GPI-anchored protein, it would only be capable of interacting with the extracellular domains of the B1R (Fig. 1). In fact, recent evidence shows that the extracellular loops of GPCRs contain potential ligand binding sites for allosteric control of receptor functions (Kenakin, 2009). Thus, a membrane peptidase with an extracellular domain(s) could regulate receptor function by interacting with such a site. Taken together, the above data indicate that CPM promotes B1R signaling in at least two ways; first, it causes B1R activation upon binding of BK or KD and second, it generates B1R agonist which can further activate the bound receptor or diffuse and activate adjacent receptors (Fig. 6).
Figure 6.
Model of how CPM/B1R interactions mediate signaling in response to BK. CPM and its potential membrane orientation and basal interaction with the B1R is shown in the left panel. Based on our results, BK (or KD) released from the kininogen precursor can stimulate B1R signaling in two ways via CPM. 1. Binding as a substrate causes a conformational change in CPM that is transmitted via protein-protein interaction to the B1R, resulting in G protein coupling and activation of calcium signaling. 2. Catalytic conversion of BK (or KD) to B1R agonist that can further activate the associated receptor or additional B1Rs. For the catalytically inactive CPM-E264Q mutant, only the first mechanism of activation is possible. This research was originally published in the Journal of Biological Chemistry. Zhang X, Tan F, Brovkovych V, Zhang Y, Skidgel RA. Cross-talk between carboxypeptidase M and the kinin B1 receptor mediates a new mode of G protein-coupled receptor signaling. J Biol Chem 2011; 286:18547 – 18561. © the American Society for Biochemistry and Molecular Biology.
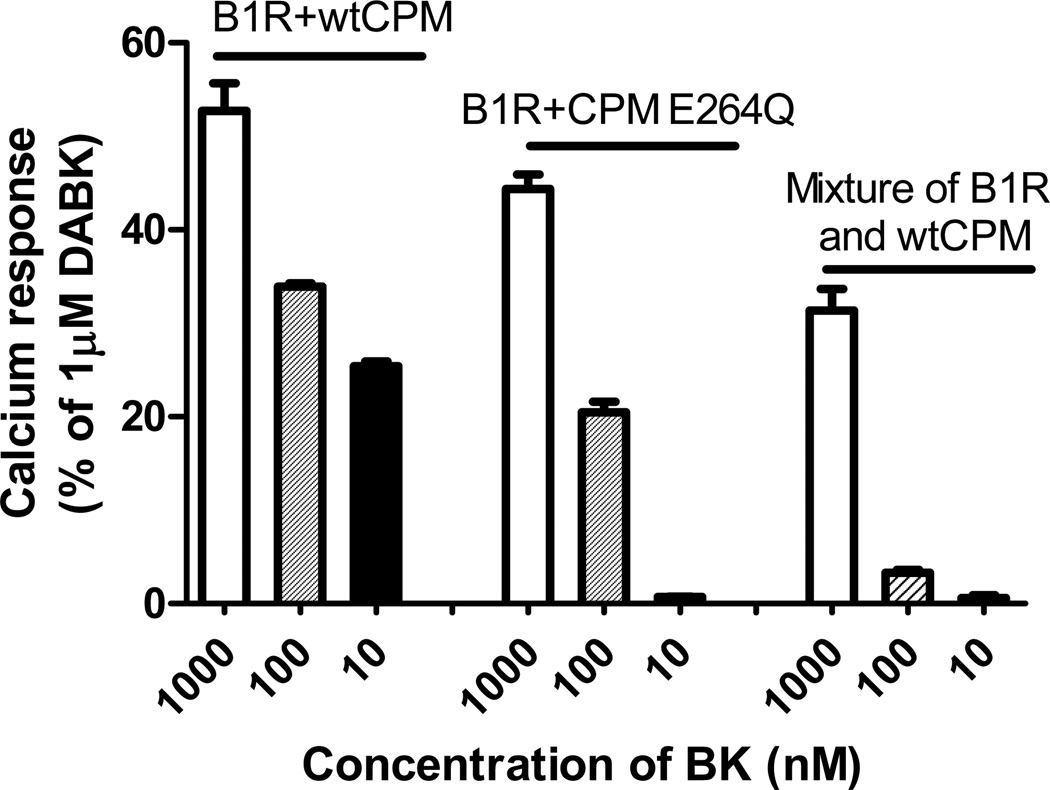
What would be the advantage to a cell of having this type of signaling complex that allows conformational cross-talk to activate a receptor? First, it would potentially boost the receptor signal at relatively lower concentrations of kinins. Second, it would allow localized signaling to occur without the drawback of unwanted distant effects that would likely occur if the agonist was generated at a distant site and had to diffuse to the receptor on a different cell. This can be seen in our studies by comparing the relative dose-responses to BK in the various CPM/B1R systems we investigated. Thus, in mixed co-cultures of cells singly expressing either wtCPM or B1R, receptor activation would require diffusion of CPM-generated DABK from cells expressing CPM to those expressing B1R. In this case, a significant increase in [Ca2+]i was only achieved with 1 µM BK (Fig. 7). Cells co-expressing CPM-E264Q and B1Rs, in which only interaction-dependent receptor activation was possible, were more sensitive to BK with both 1 µM and 100 nM (but not 10 nM) BK generating a calcium response (Fig. 7). The most potent response was obtained in cells co-expressing wtCPM and B1Rs, where both CPM/B1R interaction and agonist generation would occur. In this case, 10 nM BK generated a calcium response similar to that achieved with 100 nM BK in cells co-expressing CPM-E264Q and B1R (Fig. 7).
Figure 7.
The relative efficiency of the BK-stimulated increase in [Ca2+]i in three experimental models. In the first two groups (the first 6 bars) the increase in [Ca2+]i was recorded and quantified in HEK cells stably co-expressing B1Rs and either wtCPM or CPM-E264Q and B1R in response to treatment with various concentrations of B2R agonist BK. In the last group (last 3 bars), the calcium response was quantified in mixed co-cultures of cells singly expressing either wtCPM or B1R after stimulation with the indicated concentration of BK. The results were normalized by defining the calcium response of cells stimulated by 1000 nM B1R agonist DABK as 100% in the corresponding experimental system. The data are expressed as mean ± SE (n=3). This research was originally published in the Journal of Biological Chemistry. Zhang X, Tan F, Brovkovych V, Zhang Y, Skidgel RA. Cross-talk between carboxypeptidase M and the kinin B1 receptor mediates a new mode of G protein-coupled receptor signaling. J Biol Chem 2011; 286:18547 – 18561. © the American Society for Biochemistry and Molecular Biology.
Kinin receptor-dependent nitric oxide production in endothelial cells
Stimulation of the kinin B2R in endothelial cells is well known to generate nitric oxide (NO) as a mediator of its vasodilator effects (Kuhr et al., 2010). Signaling pathways mediating B2R-dependent NO production have been extensively characterized in cultured bovine aortic endothelial cells and human umbilical vein endothelial cells and found to result in a calcium-mediated and phosphorylation-dependent activation of eNOS (Venema, 2002). However, activation of the kallikrein-kinin system and generation of B2R agonist kinins is typically associated with inflammation and B2R-stimulated NO production has not been studied in endothelial cells under these conditions. We recently found that human lung microvascular endothelial cells (HLMVEC) pretreated with cytokines (to mimic inflammatory conditions) produced a higher and more prolonged output of NO (~80 min) when stimulated with BK than the transient response (~10 min) found in control HLMVEC. Furthermore, the BK-stimulated NO generated by cytokine-treated HLMVEC was produced by eNOS via a novel Gαi-mediated signaling pathway that was independent of increased [Ca2+]i (J.L. Lowry, V. Brovkovych, Y. Zhang and R.A. Skidgel, submitted). Thus, B2R-stimulated NO production in both control and inflamed endothelium is due to activation of eNOS, although the signaling pathways are distinct and the resulting NO output differs both in quantity and time course.
We found that B1R stimulation of cytokine-treated HLMVEC led to even higher and more prolonged NO output than B2R, but in contrast to the B2R response, it was mediated through acute activation of iNOS and not eNOS (Ignjatovic et al., 2004; Zhang et al., 2007). This was confirmed by the lack of B1R-mediated NO production in control HLMVEC (expressing eNOS) that were transfected with B1Rs or in HEK293 co-transfected with B1Rs and eNOS (Zhang et al., 2007; Brovkovych et al., 2011). It was surprising that iNOS activity could be acutely activated in a receptor-dependent process as iNOS is considered to be regulated only by expression. We found the mechanism of this novel iNOS-activation pathway to be Gαi-mediated and β-arrestin 2-dependent ERK1/2 activation, resulting in ERK phosphorylation of iNOS at Ser745 (Zhang et al., 2007; Kuhr et al., 2010). Taken together, the data show that kinin-mediated NO production in human endothelial cells is more profound and prolonged under inflammatory conditions, and that B2Rs activate eNOS whereas B1Rs activate iNOS.
CPM interaction with the B1R enhances signaling in primary endothelial cells
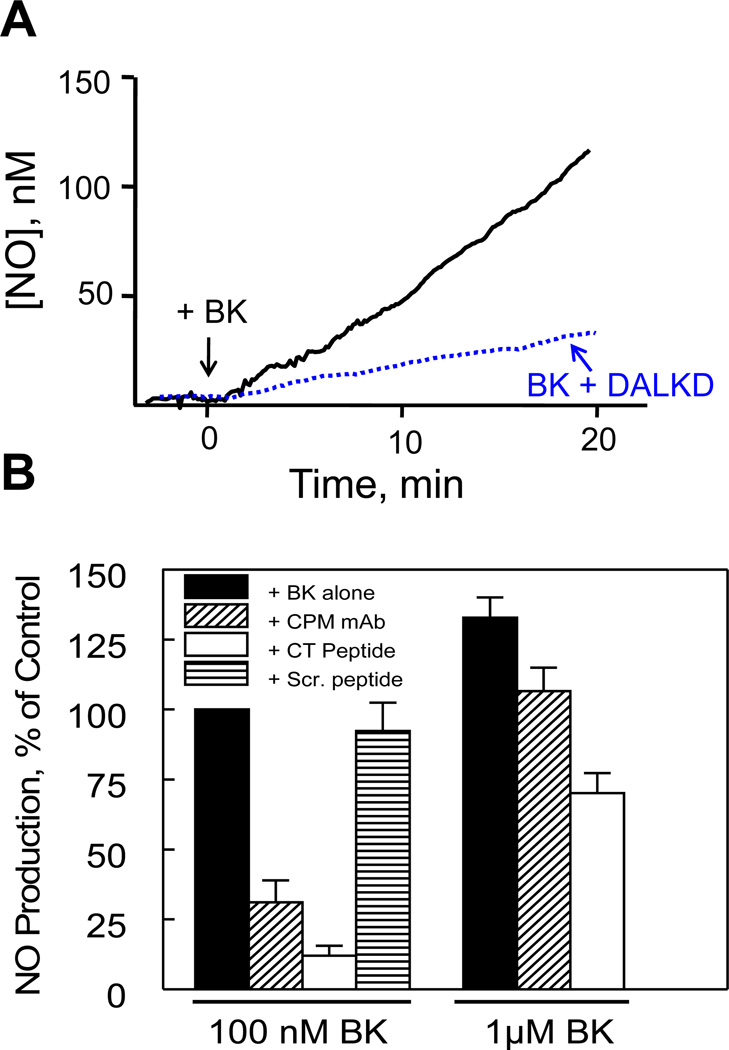
We investigated the role of the CPM/B1R interaction by measuring B1R-dependent NO production in response to B2R agonist BK in primary HLMVEC pretreated with cytokines (Zhang et al., 2011). To disrupt the CPM/B1R interaction without inhibiting CPM, we utilized the “CT peptide” and CPM monoclonal antibody described above. Cytokine-treated HLMVEC were preincubated with B2R antagonist HOE140 to block direct B2R responses. BK (100 nM) stimulated prolonged NO output that was blocked by B1R antagonist DALKD (Fig. 8). Pre-incubation with either the CPM antibody or CT peptide, blocked B1R-mediated NO production in response to 100 nM BK but the scrambled peptide had no effect (Fig. 8). However, the CPM antibody and CT peptide were less effective inhibitors when cells were stimulated with 1 µM BK (Fig.8). Thus, the CPM/B1R interaction plays a more critical role at lower BK concentration, indicating that at high BK concentrations, the concentration of CPM-generated B1R agonist is high enough to diffuse and activate dissociated B1Rs to generate a partial response.
Figure 8.
Disruption of the CPM/B1R interaction inhibits B1R-mediated NO production in response to BK in human endothelial cells. (A) Cytokine-treated HLMVEC were pre-incubated for 30 min with 1 µM HOE140 (B2R antagonist) without (solid line) or with (dotted line) 1 µM DALKD (B1R antagonist). At time zero, 100 nM BK was added and NO production was measured in real time for 20 min with a porphyrinic microsensor. B, Cells were pretreated with 1 µM HOE140 without or with 500 ng/ml CPM monoclonal antibody, 50 µM CT peptide or scrambled CT peptide (Scr. peptide) for 30 min. Cells were stimulated with 100 nM or 1 µM BK and NO production measured for 20 min. Shown are mean values as % control (100 nM BK alone = 100%) ± SE (n = 3). This research was originally published in the Journal of Biological Chemistry. Zhang X, Tan F, Brovkovych V, Zhang Y, Skidgel RA. Cross-talk between carboxypeptidase M and the kinin B1 receptor mediates a new mode of G protein-coupled receptor signaling. J Biol Chem 2011; 286:18547 – 18561. © the American Society for Biochemistry and Molecular Biology.
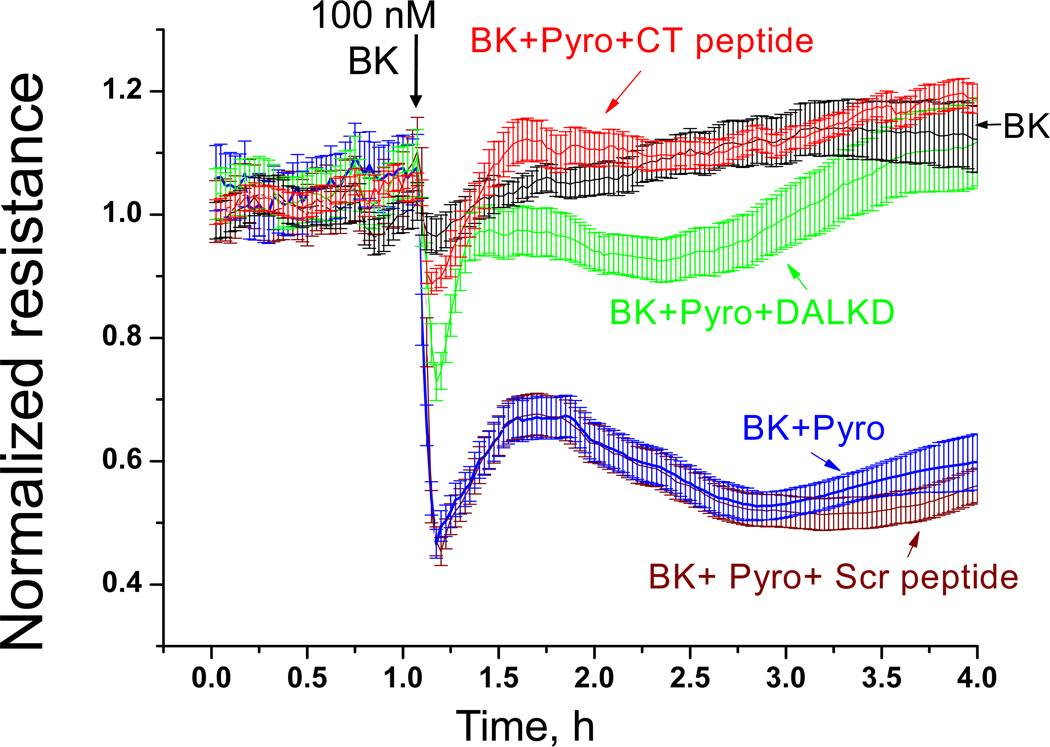
Peroxynitrite (ONOO−), generated by the rapid reaction of NO with O2−, is a potent oxidant that can mediate endothelial barrier disruption (Pacher et al., 2007). We used transendothelial electrical resistance (TER) to investigate the role of CPM/B1R interaction in ONOO− -mediated endothelial barrier disruption (Zhang et al., 2011). As above, cytokine-pretreated HLMVEC were preincubated with HOE140 to block B2Rs. Addition of 100 nM BK to stimulate B1R-mediated NO production (as above) resulted in a modest increase in TER (Fig. 9). However, when combined with pyrogallol (which auto-oxidizes to produce O2·−), BK caused a large drop in resistance, consistent with a ONOO− -mediated increase in endothelial permeability, and this was reversed by B1R antagonist DALKD (Fig. 9). Interestingly, the CPM CT peptide almost completely reversed decrease in resistance caused by BK + pyrogallol whereas the scrambled peptide had no effect (Fig. 9). Thus, the CPM/B1R interaction is important in mediating B1R-iNOS signaling and its effects on barrier function in primary endothelial cells that express both proteins at native levels.
Figure 9.
B1R-dependent increase in endothelial permeability caused by BK combined with superoxide depends on CPM/B1R interaction. HLMVEC, grown to confluence on gold electrodes coated with 10 µg/ml fibronectin, were cytokine pretreated (10ng/ml IL-1β + 100u/ml IFN-γ, 16 h). Medium was changed, cells were allowed to stabilize, and all samples were pretreated with 10 µM HOE140 to block B2R responses. Cells were then treated with 1 µM BK (added at the black arrow) alone (black line) or combined with 200 µM pyrogallol (Pyro; superoxide generator) without (blue line) or with 1 µM B1R antagonist DALKD (green line), 50 µM CT peptide (red line) or 50 µM scrambled (Scr) peptide (brown line) and transendothelial electrical resistance was measured. Results show mean values ± S.E. for n = 4. This research was originally published in the Journal of Biological Chemistry. Zhang X, Tan F, Brovkovych V, Zhang Y, Skidgel RA. Cross-talk between carboxypeptidase M and the kinin B1 receptor mediates a new mode of G protein-coupled receptor signaling. J Biol Chem 2011; 286:18547 – 18561. © the American Society for Biochemistry and Molecular Biology.
Conclusions
We found that CPM functions as an important mediator of B1R signaling, acting as a kind of “co-receptor”. In addition to its important role in generating B1R agonists at the cell surface in the vicinity of the B1R, CPM also mediates BK or KD stimulation of B1R signaling by a novel conformational crosstalk that depends on kinin binding to the active site of CPM, and is independent of its enzymatic activity (Fig. 6). This effect of CPM is critically dependent on the proper orientation of CPM on the membrane and the integrity of its complex with the B1R. This interaction facilitates the ability of CPM to potentiate B1R signaling at low concentrations of native kinins as we showed in both transfected HEK cells and cytokine-treated HLMVEC expressing native levels of B1R and CPM. Inflammatory cytokines upregulate B1R, iNOS and CPM expression in human endothelial cells and activation of B1R signaling stimulates iNOS-dependent high output NO in endothelial cells under inflammatory conditions that could alter endothelial barrier function. Thus, this novel mechanism of regulating GPCR signaling might be exploited to develop drugs to alter CPM/B1R interaction and thereby regulate kinin signaling.
Acknowledgments
This work was supported by National Institutes of Health Grants DK41431 and HL60678. Xianming Zhang was the recipient of an American Heart Association Postdoctoral Fellowship and Jessica L. Lowry was the recipient of an American Heart Association Predoctoral Fellowship
References
- Brovkovych V, Zhang Y, Brovkovych S, Minshall RD, Skidgel RA. A novel pathway for receptor-mediated post-translational activation of inducible nitric oxide synthase. J. Cell. Mol. Med. 2011;15:258–269. doi: 10.1111/j.1582-4934.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero OA, Scicli AG. Local hormonal factors (intracrine, autocrine, and paracrine) in hypertension. Hypertension. 1991;18:I58–I69. doi: 10.1161/01.hyp.18.3_suppl.i58. [DOI] [PubMed] [Google Scholar]
- Chen Z, Deddish PA, Minshall RD, Becker RP, Erdös EG, Tan F. Human ACE and bradykinin B2 receptors form a complex at the plasma membrane. FASEB J. 2006;20:2261–2270. doi: 10.1096/fj.06-6113com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deddish PA, Skidgel RA, Kriho VB, Li XY, Becker RP, Erdös EG. Carboxypeptidase M in Madin-Darby canine kidney cells. Evidence that carboxypeptidase M has a phosphatidylinositol glycan anchor. J. Biol. Chem. 1990;265:15083–15089. [PubMed] [Google Scholar]
- Erdös EG. Kinins, the long march--a personal view. Cardiovasc. Res. 2002;54:485–491. doi: 10.1016/s0008-6363(02)00284-5. [DOI] [PubMed] [Google Scholar]
- Erdös EG, Renfrew AG, Sloane EM, Wohler JR. Enzymatic studies on bradykinin and similar peptides. Ann. N.Y. Acad. Sci. 1963;104:222–234. [Google Scholar]
- Erdös EG, Skidgel RA. Metabolism of bradykinin by peptidases in health and disease. In: Farmer SG, editor. The Kinin System. London: Academic Press; 1997. pp. 111–141. [Google Scholar]
- Erdös EG, Tan F, Skidgel RA. Angiotensin I-converting enzyme inhibitors are allosteric enhancers of kinin B1 and B2 receptor function. Hypertension. 2010;55:214–220. doi: 10.1161/HYPERTENSIONAHA.109.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadkar V, Sangsree S, Vogel SM, Brovkovych V, Skidgel RA. Carboxypeptidase-Mediated Enhancement of Nitric Oxide Production in Rat Lungs and Microvascular Endothelial Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L35–L45. doi: 10.1152/ajplung.00346.2003. [DOI] [PubMed] [Google Scholar]
- Hadkar V, Skidgel RA. Carboxypeptidase D is up-regulated in RAW 264.7 macrophages and stimulates nitric oxide synthesis by cells in arginine-free medium. Mol. Pharmacol. 2001;59:1324–1332. doi: 10.1124/mol.59.5.1324. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Gaietta G, Bunemann M, Adams SR, Oberdorff-Maass S, Behr B, Vilardaga JP, Tsien RY, Ellisman MH, Lohse MJ. A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells. Nat. Methods. 2005;2:171–176. doi: 10.1038/nmeth742. [DOI] [PubMed] [Google Scholar]
- Ignjatovic T, Stanisavljevic S, Brovkovych V, Skidgel RA, Erdös EG. Kinin B1 receptors stimulate nitric oxide production in endothelial cells: Signaling pathways activated by angiotensin I-converting enzyme inhibitors and peptide ligands. Mol. Pharmacol. 2004;66:1310–1316. doi: 10.1124/mol.104.001990. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. '7TM receptor allostery: putting numbers to shapeshifting proteins. Trends Pharmacol. Sci. 2009;30:460–469. doi: 10.1016/j.tips.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Kuhr F, Lowry J, Zhang Y, Brovkovych V, Skidgel RA. Differential regulation of inducible and endothelial nitric oxide synthase by kinin B1 and B2 receptors. Neuropeptides. 2010;44:145–154. doi: 10.1016/j.npep.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhr FK, Zhang Y, Brovkovych V, Skidgel RA. β-Arrestin 2 is required for B1 receptor-dependent post-translational activation of inducible nitric oxide synthase. FASEB J. 2010;24:2475–2483. doi: 10.1096/fj.09-148783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol. Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Marcic B, Deddish PA, Skidgel RA, Erdös EG, Minshall RD, Tan F. Replacement of the transmembrane anchor in angiotensin I-converting enzyme (ACE) with a glycosylphosphatidylinositol tail affects activation of the B2 bradykinin receptor by ACE inhibitors. J. Biol. Chem. 2000;275:16110–16118. doi: 10.1074/jbc.M909490199. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverter D, Maskos K, Tan F, Skidgel RA, Bode W. Crystal structure of human carboxypeptidase M, a membrane-bound enzyme that regulates peptide hormone activity. J. Mol. Biol. 2004;338:257–269. doi: 10.1016/j.jmb.2004.02.058. [DOI] [PubMed] [Google Scholar]
- Reznik SE, Fricker LD. Carboxypeptidases from A to Z: implications in embryonic development and Wnt binding. Cell. Mol. Life Sci. 2001;58:1790–1804. doi: 10.1007/PL00000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat. Rev. Mol. Cell Biol. 2009;10:819–830. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangsree S, Brovkovych V, Minshall RD, Skidgel RA. Kininase I-type carboxypeptidases enhance nitric oxide production in endothelial cells by generating bradykinin B1 receptor agonists. Am J Physiol Heart Circ Physiol. 2003;284:H1959–H1968. doi: 10.1152/ajpheart.00036.2003. [DOI] [PubMed] [Google Scholar]
- Skidgel RA. Structure and function of mammalian zinc carboxypeptidases. In: Hooper NM, editor. Zinc Metalloproteases in Health and Disease. London: Taylor and Francis Ltd.; 1996. pp. 241–283. [Google Scholar]
- Skidgel RA, Davis RM, Tan F. Human carboxypeptidase M. Purification and characterization of a membrane-bound carboxypeptidase that cleaves peptide hormones. J. Biol. Chem. 1989;264:2236–2241. [PubMed] [Google Scholar]
- Skidgel RA, Erdös EG. Lysine carboxypeptidase. In: Barrett AJ, Rawlings ND, Woessner JF, editors. Handbook of Proteolytic Enzymes. 2nd Ed. San Diego: Elsevier Academic Press; 2004. pp. 837–840. 1. [Google Scholar]
- Skidgel RA, Johnson AR, Erdös EG. Hydrolysis of opioid hexapeptides by carboxypeptidase N. Presence of carboxypeptidase in cell membranes. Biochem. Pharmacol. 1984;33:3471–3478. doi: 10.1016/0006-2952(84)90122-9. [DOI] [PubMed] [Google Scholar]
- Tan F, Balsitis S, Black JK, Blochl A, Mao JF, Becker RP, Schacht D, Skidgel RA. Effect of mutation of two critical glutamic acid residues on the activity and stability of human carboxypeptidase M and characterization of its signal for glycosylphosphatidylinositol anchoring. Biochem. J. 2003;370:567–578. doi: 10.1042/BJ20021495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema RC. Post-translational mechanisms of endothelial nitric oxide synthase regulation by bradykinin. Int Immunopharmacol. 2002;2:1755–1762. doi: 10.1016/s1567-5769(02)00185-6. [DOI] [PubMed] [Google Scholar]
- Yang HYT, Erdös EG, Levin Y. A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim. Biophys. Acta. 1970;214:374–376. doi: 10.1016/0005-2795(70)90017-6. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tan F, Brovkovych V, Zhang Y, Skidgel RA. Cross-talk between carboxypeptidase M and the kinin B1 receptor mediates a new mode of G protein-coupled receptor signaling. J. Biol. Chem. 2011;286:18547–18561. doi: 10.1074/jbc.M110.214940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Tan F, Zhang Y, Skidgel RA. Carboxypeptidase M and kinin B1 receptors interact to facilitate efficient B1 signaling from B2 agonists. J. Biol. Chem. 2008;283:7994–8004. doi: 10.1074/jbc.M709837200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brovkovych V, Brovkovych S, Tan F, Lee B-S, Sharma T, Skidgel RA. Dynamic receptor-dependent activation of inducible nitric-oxide synthase by ERK-mediated phosphorylation of Ser745. J. Biol. Chem. 2007;282:32453–32461. doi: 10.1074/jbc.M706242200. [DOI] [PubMed] [Google Scholar]