Abstract
Objectives
Ascertainment of adrenal function assessing free (FC) rather that total (TC) cortisol may be beneficial for the diagnosis of critical illness related cortisol insufficiency (CIRCI). We hypothesized that centrifugal ultrafiltration (CUF) would provide timely FC data that highly correlated with the gold standard, but logistically cumbersome, equilibrium dialysis (EQD) technique when the FC fractions were identically quantified by chemiluminescence immunoassay. We also hypothesized that FC would correlate with illness severity in a large cohort of critically ill children.
Design
Prospective, multi-institutional, observational cohort investigation.
Setting
Seven pediatric intensive care units (PICUs) within the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network.
Patients
165 critically ill children across the spectrum of illness severity.
Interventions
Blood sampling.
Measurements and main results
Time to derive plasma FC concentrations following CUF or EQD fractionation with chemiluminescence immunoassay was ~2 versus ~24 hours, respectively. Utilizing CUF, mean plasma FC was 4.1 ± 6.7 ug/dL (median 1.6, range 0.2-43.6), representing an average of 15.2 ± 9.4% of total cortisol. Nearly 60% of subjects exhibited FC < 2 and 30% < 0.8 ug/dL, previously suggested threshold concentrations for defining CIRCI. Plasma FC concentrations comparing CUF versus EQD fractionation demonstrated a strong correlation (R2 = 0.97). For FC < 2 ug/dL Bland-Altman analysis revealed minimal negative bias for the CUF technique. Illness severity assessed by PRISM III correlated moderately with FC and percent TC as FC.
Conclusions
Determination of CUF fractionated FC was fast and results correlated highly with EQD fractionated FC. Many children exhibited FC < 2 and < 0.8 ug/dL, but did not demonstrate clinical evidence of CIRCI. This study ascertains that real time FC quantification is feasible to potentially help guide clinical decision making for cortisol replacement therapy in the PICU.
Keywords: cortisol, total cortisol, free cortisol, hydrocortisone, children, equilibrium dialysis, centrifugal ultrafiltration, radioimmunoassay, chemiluminescence immunoassay, adrenal insufficiency, relative adrenal insufficiency, critical illness, critical illness related cortisol insufficiency (CIRCI)
INTRODUCTION
Stress from critical illness activates a neurogenic-endocrine-inflammatory response, including activation of the hypothalamic-pituitary-adrenal axis resulting in increased cortisol production (1, 2). Cortisol plays a key role in the compensatory anti-inflammatory response syndrome (CARS) in the setting of an evolving systemic inflammatory response syndrome (SIRS). Critical illness related cortisol insufficiency (CIRCI) defines a seemingly inadequate hypothalamic-pituitary-adrenal-cortisol response relative to the intensity of stress. This may involve insufficient de novo adrenal production of cortisol, altered cortisol transport or peripheral tissue resistance to cortisol (3-5). Defining appropriate adrenocortical status in the setting of critical illness remains crucial but confusing (6). Despite significant clinical research in this area, there is no consensus on what constitutes an adequate adrenal response to severe stress such as septic shock (7).
Historically adrenal function has been assessed with serum total cortisol (TC) concentrations, typically at baseline and/or following adrenal stimulation with exogenous corticotropin. The CORTICUS (Corticosteroid Therapy of Septic Shock) interventional trial examined the potential benefits and risks of adjunctive cortisol for adult septic shock. An important conclusion of this trial was that corticotropin adrenal stimulation testing that measures serum TC did not reliably identify a subgroup of critically ill septic adults who might benefit from exogenous cortisol replacement (8).
An evolving consensus suggests that free cortisol (FC) rather than protein-bound cortisol is responsible for the protean actions of this hormone (9, 10). Normally cortisol binding globulin (transcortin) and albumin bind over 90% of circulating cortisol. During critical illness the concentrations of these proteins may plummet by 50% or more, but individual variation is significant. Among critically ill adults with sepsis and septic shock, FC concentrations may correspond more closely to illness severity than total cortisol (9, 11).
For the measurement of FC, two methodological considerations are important: 1) FC fractionation from protein-bound cortisol; and 2) actual cortisol assay. Traditionally isolation of the FC fraction has been accomplished utilizing equilibrium dialysis (EQD). This technique is typically a “send out” test for most clinical laboratories, requires relatively large blood volumes, and involves prolonged incubation times to generate the FC fraction. More recently, temperature-controlled centrifugal ultrafiltration (CUF) (12) was suggested as an alternative method for FC fractionation. This approach requires only small blood volumes, and has a short turn around time. These test characteristics would make real time FC measurements feasible among critically ill children.
While designing an interventional trial of adjunctive cortisol replacement for children with severe sepsis/septic shock, the Collaborative Pediatric Critical Care Research Network (CPCCRN) investigators realized the importance of identifying the population critically ill children who would most likely benefit from such an intervention. We sought to generate TC and FC data on a cohort of critically ill children across a spectrum of ages and illness severity. We hypothesized that plasma FC fractionated by temperature-controlled CUF is equivalent to plasma FC fractionated by the gold standard EQD technique when a common assay method (chemiluminescence immunoassay) is utilized. We further hypothesized that FC would correlate with severity of illness.
MATERIALS AND METHODS
Institutional Review Board
This project was reviewed and approved by the Institutional Review Boards of Seattle Children’s Hospital, each CPCCRN clinical performance site, and the CPCCRN Data Coordinating Center.
Cortisol Quantification Investigation (CQI) Protocol
Within CPCCRN PICUs, critically ill children with a wide spectrum of illness severity were screened for enrollment by CPCCRN research coordinators and investigators during their first day following admission to pediatric intensive care units (PICUs). Enrolled subjects met all inclusion criteria and no exclusion criteria.
Inclusion criteria included: admission to a PICU; age greater than 40 weeks gestation and less than 18 years; weight greater than 5 kg; blood sample obtainable within 24 hours of PICU admission; and an indwelling vascular catheter capable of providing blood samples.
Exclusion criteria included: administration of systemic steroid within the previous month; lack of commitment to aggressive intensive care therapy (children with limited resuscitation orders); subject status post cardiac surgery requiring cardiopulmonary bypass, extracorporeal membrane oxygenation (ECMO), leukopheresis, plasmapheresis, ultrafiltration, any dialysis therapy or massive transfusion (> 50% of total blood volume); subject was not expected to survive PICU admission; subject was previously enrolled in the same study; or subject was administered etomidate or ketoconazole within the previous month.
Research Procedures
Following screening identification of appropriate CQI subjects, parental permission was obtained for data collection and a single blood sample from an indwelling vascular catheter. Demographic data were recorded. Illness severity was assessed for each child utilizing the Pediatric Risk of Mortality version III score (PRISM III) utilizing data collected during the first 12 hours of PICU admission (13). Each subject was also assessed for the systemic inflammatory response syndrome (SIRS, presence of tachypnea, tachycardia/ bradycardia, hyper-/hypothermia, and leukocytosis/leukopenia with left shift on differential) utilizing published consensus criteria (14). A single 4 mL blood sample was drawn from each subject utilizing existing intravascular access. Plasma samples were isolated by centrifugation immediately following blood collection, frozen and subsequently shipped on dry ice to Seattle Children’s Hospital. Integrity of each frozen sample at arrival to Seattle was ascertained. Each sample underwent concurrent fractionation by EQD and CUF. For comparison, existing plasma samples from 21 healthy, unstressed adults were identically processed and assayed.
Free Cortisol Fractionation
Temperature-controlled CUF was conducted according to the method of Lentjes, et al (12) employing Millipore YM-30 membrane filters (molecular weight cutoff 30,000) (Millipore, Billerica, MA). Plasma samples (0.40 mL) were centrifuged at 1700 × g for 30 minutes at 37°C in a pre-warmed centrifuge to yield a protein-poor FC fraction. Equilibrium dialysis was performed utilizing the Rapid Equilibrium Dialysis (RED) Device, Pierce Protein Research Products (Thermo Fisher Scientific Inc., Rockford, IL). .
Cortisol Assay
Both TC and FC (following CUF or EQD) were measured at the Clinical Laboratory of Seattle Children’s Hospital utilizing the Ortho Clinical Diagnostics ECi® Immunodiagnostic System, employing enhanced chemiluminescence detection. Total assay time on the analyzer was 38 minutes.
Data and Statistical Analyses
Conversion of cortisol units was nM × 0.0362 = ug/dL; ug/dL × 27.6 = nM. PRISM III scores were categorized as follows to reflect different levels of illness severity: 0 to 7, 8 to 15, 16 to 23, 24 or greater. Continuous variable data were summarized using the mean ± 1 standard deviation (S.D.), median and 25th and 75th percentiles. Dichotomous variables were summarized as percentages. We performed linear regression to examine correlation between the different measures. Agreement between EQD FC and CUF FC was also evaluated using the Bland-Altman approach (15, 16) for all FC values and for FC values restricted to < 2 ug/dL.
RESULTS
Study Enrollment
Parents provided permission for enrollment for 174 of 201 eligible subjects (87%). Blood samples were obtained from 165 enrolled subjects (95%) within the first 24 hours of PICU admission in all but one subject, with an average time from admission to blood sampling of 16.1 ± 4.9 hours (range 1.6 to 25.1 hours). One atypical outlier observation was overly influential in the results due to TC and FC values of 281 and 150 ug/dL, respectively. These values were excluded from all analyses.
Characteristics of the Study Population
Demographics for the study population are summarized in Table 1, while primary PICU admission diagnoses are summarized in Table 2 and the most commonly encountered chronic diagnoses summarized in Table 3.
Table 1.
Demographics of the study population
| Characteristic | Number | % | Mean | S.D. | Median | Q1, Q3 |
|---|---|---|---|---|---|---|
| Age | 165 | --- | 9.7 | 5.7 | 11.3 | 3.9, 14.7 |
| PRISM III Score | 165 | --- | 8.0 | 6.9 | 8.0 | 3.0, 11.0 |
| PRISM III Score Category | ||||||
| 0-7 | 82 | 49.7 | ||||
| 8-15 | 63 | 38.2 | ||||
| 16-23 | 15 | 9.1 | ||||
| ≥ 24 | 5 | 3.0 | ||||
| Gender | ||||||
| Male | 86 | 52.1 | ||||
| Female | 79 | 47.9 | ||||
| SIRS Diagnosis | ||||||
| Yes | 127 | 77.0 | ||||
| No | 38 | 23.0 | ||||
| Chronic Diagnoses | ||||||
| Yes | 88 | 53.3 | ||||
| No | 77 | 46.7 | ||||
PRISM, Pediatric Risk of Mortality Score, version III; SIRS, systemic inflammatory response syndrome; Q1 and Q3 represent 25th and 75th percentiles.
Table 2.
Acute primary PICU admission diagnoses for the study population
| Diagnosis | Number | Percent |
|---|---|---|
| Sepsis/meningitis/pneumonia | 26 | 15.1 |
| DKA, other metabolic disease | 22 | 12.8 |
| Trauma | 20 | 11.6 |
| Post-op orthopedic surgery | 20 | 11.6 |
| Post-op neurosurgery | 13 | 7.6 |
| Post-op CHD surgery | 8 | 4.7 |
| Seizures, other neurological | 8 | 4.7 |
| Hypoxia-ischemia-reperfusion | 7 | 4.1 |
| Cancer | 7 | 4.1 |
| Acute abdomen | 6 | 3.5 |
| Acquired cardiovascular disease | 5 | 2.9 |
| Renal failure | 5 | 2.9 |
| Hematologic disease | 5 | 2.9 |
| Hepatic failure | 4 | 2.3 |
| Other diagnoses | 16 | 9.3 |
DKA, diabetic ketoacidosis; post-op, post-operative; CHD, congenital heart disease
Table 3.
Chronic primary diagnoses for the study population.
| Diagnosis | Number | Percent |
|---|---|---|
| Motor/mental developmental delay | 34 | 26.2 |
| Diabetes, other metabolic disease | 17 | 13.1 |
| Cardiovascular disease | 10 | 7.7 |
| Cancer | 9 | 6.9 |
| Other chronic neurological disease | 9 | 6.9 |
| Hydrocephalus, MMC, Chiari | 8 | 6.2 |
| Chromosomal abnormalities | 8 | 6.2 |
| Chronic seizure disorder | 7 | 3.4 |
| ADHD, autism | 5 | 3.8 |
| Chronic gastrointestinal disease | 5 | 3.8 |
| Defined syndromes | 5 | 3.8 |
| Chronic orthopedic disease | 5 | 3.1 |
| Bronchopulmonary dysplasia | 3 | 2.3 |
| Asthma | 3 | 2.3 |
| Psychosocial disorders | 3 | 2.3 |
Specific diagnoses were combined to yield MMC, meningomyelocele; Chiari, Chiari malformation; ADHD, attention deficit hyperactivity disorder.Categories are not mutually exclusive; subjects may be included in more than one category.
Healthy Adult Controls
Table 4 summarizes TC, FC and FC as a % of TC using the CUF and chemiluminescence immunoassay, for 21 healthy, unstressed adults. Morning TC averaged about 10 ug/dL and FC about 1 ug/dL, with FC comprising about 10% of TC. Afternoon TC and FC concentrations were approximately 60% of morning values with FC again comprising about 10% of TC.
Table 4.
Total cortisol, free cortisol, and free cortisol as percent of total cortisol concentrations among 21 healthy, non-stressed adults assessed with paired serum samples obtained at ~0800 and ~1600 utilizing temperature-controlled centrifugal ultrafiltration and chemiluminescence immunoassay methodology. All concentrations are expressed as ug/dL. S.D., standard deviation; n = 21.
| 0800 Blood Sample | 1600 Blood Sample | |||||
|---|---|---|---|---|---|---|
| Measure | Total Cortisol |
Free Cortisol |
Free as % of Total |
Total cortisol |
Free Cortisol |
Free as % of Total |
| Mean ± S.D. | 10.5 ± 5.1 | 1.0 ± 0.4 | 9.9 ± 2.6 | 6.6 ± 2.7 | 0.6 ± 0.2 | 9.6 ± 2.7 |
| Median | 9.0 | 0.9 | 10.0 | 6.0 | 0.6 | 9.7 |
| Min, Max | 5.0, 27.8 | 0.6, 2.1 | 4.6, 15.0 | 2.3, 9.9 | 0.3, 1.1 | 4.5, 16.7 |
Critically Ill Children
Table 5 summarizes TC, FC and FC as a % of TC derived using both EQD and CUF plasma fractionation methodologies and chemiluminescence immunoassay for the 164 critically ill children.
Table 5.
Total cortisol, free cortisol, and free cortisol as a percent of total cortisol fractionated by two methods. All concentrations expressed as ug/dL. S.D., standard deviation; n = 164.
| Free Cortisol Fractionation Method | |||||
|---|---|---|---|---|---|
| Equilibrium Dialysis (EQD) | Centrifugal Ultrafiltration (CUF) | ||||
| Measure | Total Cortisol |
Free Cortisol | Free as % of Total Cortisol |
Free Cortisol | Free as % of Total Cortisol |
| Mean ± S.D. | 20.1 ± 18.3 | 3.8 ± 5.7 | 16.6 ± 9.9 | 4.1 ± 6.7 | 15.2 ± 9.4 |
| Median | 14.3 | 1.9 | 14.1 | 1.6 | 12.8 |
| Min, Max | 0.6, 109 | 0.2, 37.2 | 4.5, 67.9 | 0.2, 43.6 | 3.6, 48.3 |
These PICU patients exhibited roughly a 100% higher TC and a 100-400% higher FC compared to the adult controls. Free cortisol as percent of TC in this pediatric population was approximately 60% greater than adult controls (i.e . ~16% in the critically ill children versus ~ 10% in the healthy adults). However for a few children the FC fraction comprised 50-60% of the TC.
Equilibrium Dialysis Versus Centrifugal Ultrafiltration Free Cortisol Fractionation
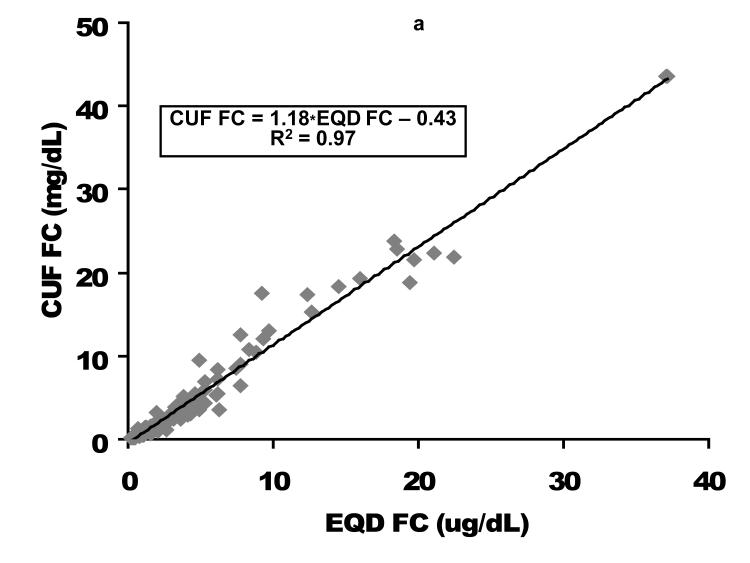
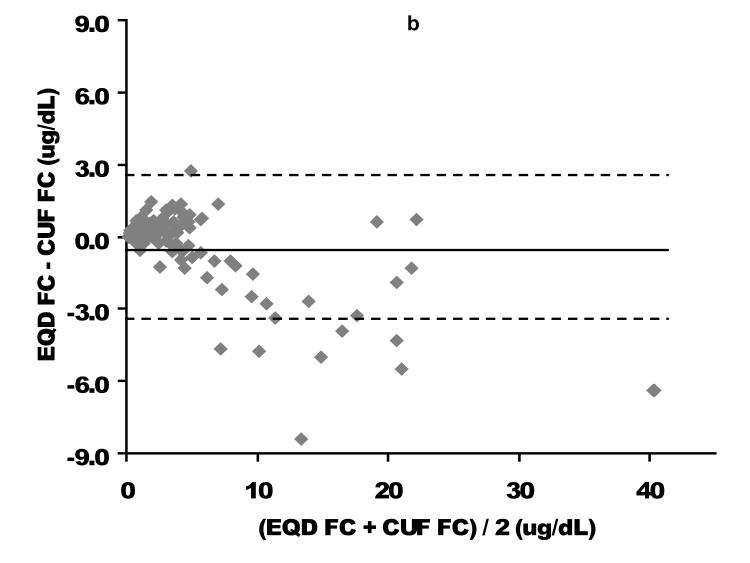
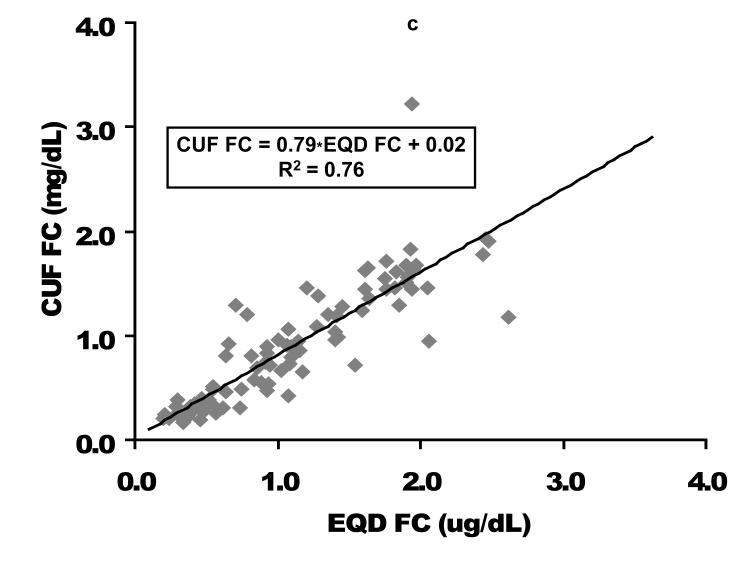
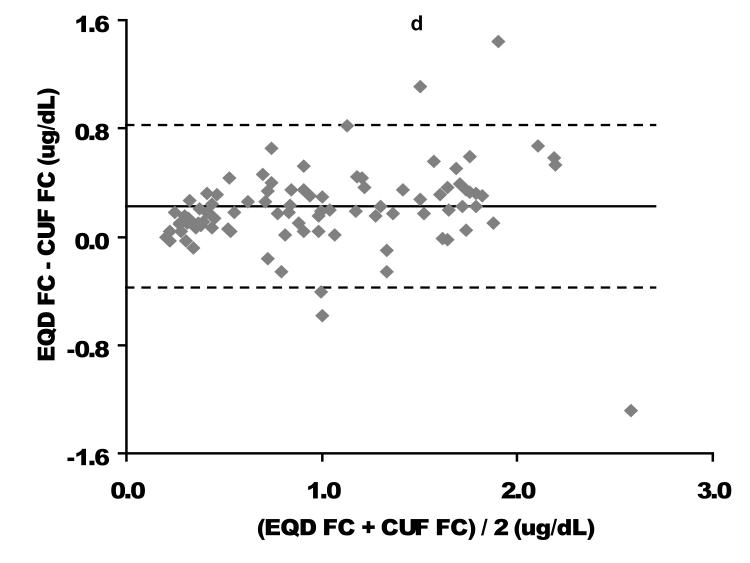
Direct comparison based on linear regression as well as Bland-Altman analysis of the relationship between EQD and CUF plasma fractionated FC is displayed in Figure 1. Among the 94 children with free cortisol < 2 ug/dL by either fractionation technique (where providers might be considering cortisol replacement therapy), a slight negative bias for the CUF compared to the EQD fractionation technique was apparent. The mean bias (EQD FC – CUF FC) was 0.20 ± 0.31 ug/dL with lower and upper limits of agreement of −0.41 and 0.81, respectively. Process time for CUF vs EQD fractionation/analysis was approximately 2 versus 24 hours. This difference was due to the prolonged time needed for dialysis versus ultracentrifugation to isolate the FC fraction.
Figure 1. Free cortisol per centrifugal ultrafiltration (CUF) plasma fractionation as a function of free cortisol per equilibrium dialysis (EQD) plasma fractionation.
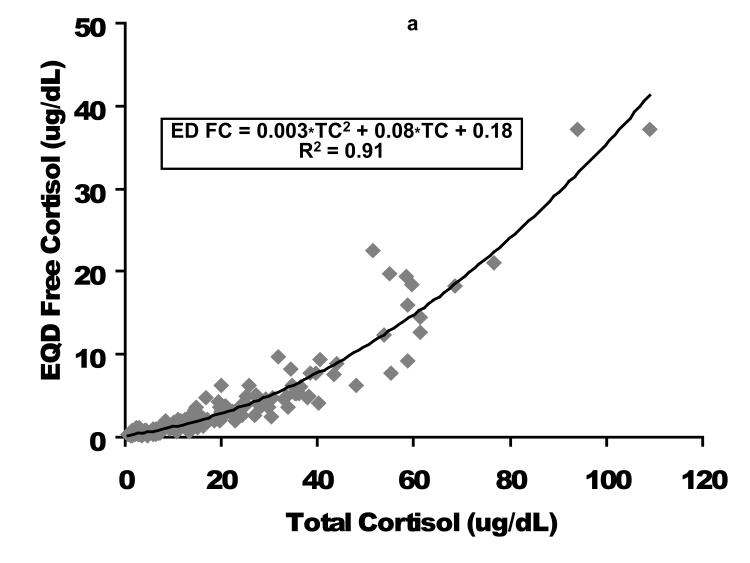
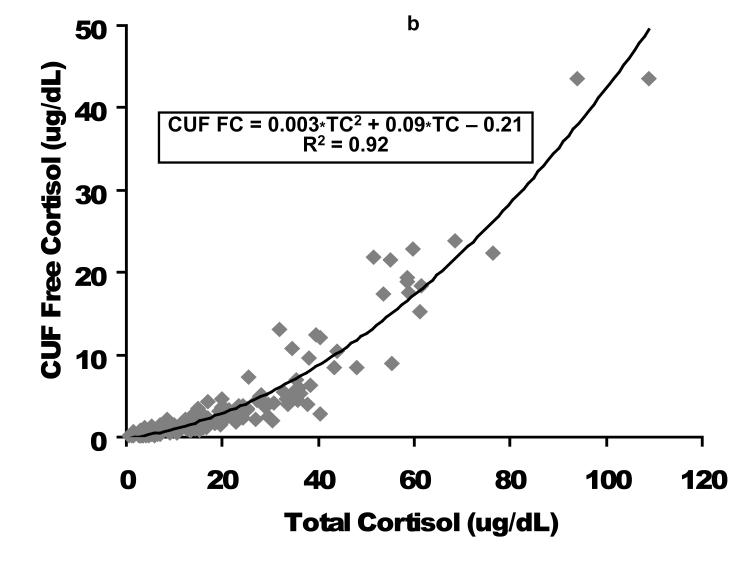
A comparison of the CUF versus EQD techniques is displayed for the entire population (n = 164) in the upper graphs and for the population of children with a free cortisol level < 2 ug/dL (n=94) in the lower graphs. Cortisol concentrations in ug/dL. CUF FC, centrifugal ultrafiltration free cortisol; EQD FC, equilibrium dialysis free cortisol. As displayed in Figure 2, the relationship between FC and TC was quadratic. These graphs demonstrate the non-linear binding characteristics between cortisol and its binding proteins (17).
As displayed in Figure 2, the relationship between FC and TC was quadratic. These graphs demonstrate the non-linear binding characteristics between cortisol and its binding proteins (17).
Figure 2. Free cortisol as a function of total cortisol following plasma fractionation per equilibrium dialysis (left figure, EQD) or temperature-controlled centrifugal ultrafiltration (right figure, CUF).
Cortisol concentrations are expressed as ug/dL. EQD, equilibrium dialysis; CUF, centrifugal ultrafiltration; TC, total cortisol; FC, free cortisol.
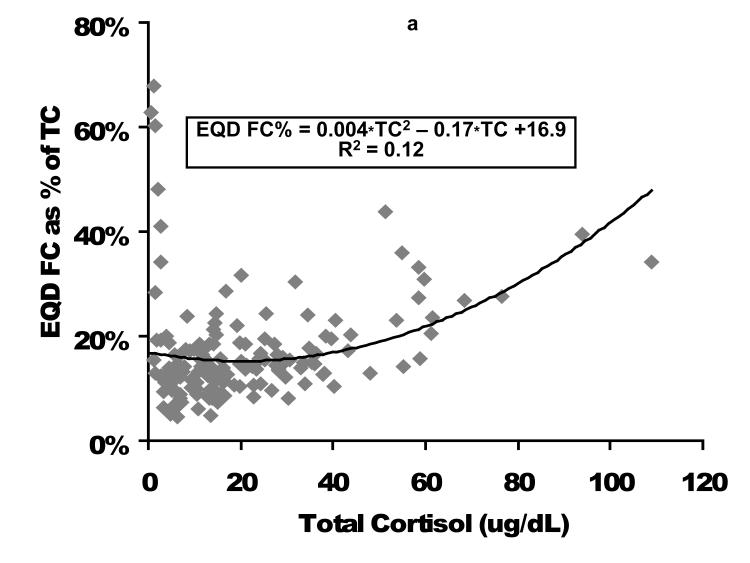
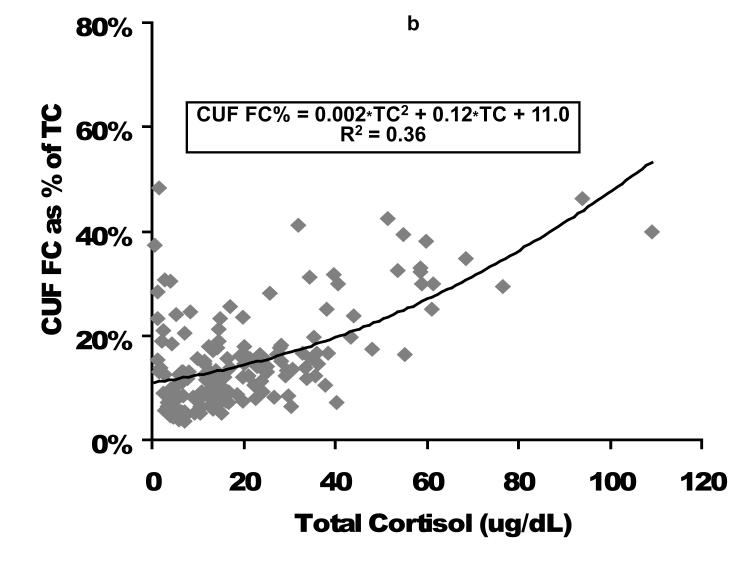
Relationship of FC as expressed as a percentage of TC is displayed in Figure 3. In general as TC increases, the percent of cortisol as the FC fraction increases, but there is considerable variation, particularly at low TC and FC concentrations.
Figure 3. Percent of total cortisol as free cortisol following plasma fractionation per equilibrium dialysis (left figure, EQD) or temperature-controlled centrifugal ultrafiltration (right figure, CUF).
Cortisol concentrations are expressed as ug/dL. EQD, equilibrium dialysis; CUF, centrifugal ultrafiltration; TC, total cortisol; FC, free cortisol.
Free Cortisol Relation to Illness Severity
For this cohort, 33% of children exhibited TC < 10; 57% exhibited FC < 2 ug/dL by either EQD or CUF and 30% exhibited FC < 0.8 ug/dL by either EQD or CUF. These reference TC and FC concentrations were previously suggested as potential thresholds for defining adrenal insufficiency in critically ill adults based on direct total and free cortisol assessment and metyrapone testing (9, 18). No subjects in any of these subgroups developed clinical symptoms suggestive of adrenal insufficiency such as recalcitrant shock, severe hyponatremia, or hypoglycemia. Seventy-seven percent of subjects (126/164) fulfilled criteria for SIRS. As summarized in Table 6, and in contrast to our expectations, no statistically significant differences occurred in TC and FC concentrations among subjects with and without SIRS.
Table 6.
Total cortisol and free cortisol fractionated by equilibrium dialysis (EQD) or centrifugal ultrafiltration (CUF) among subjects without (SIRS−, n=38) or with (SIRS+, n=126) systemic inflammatory response syndrome (SIRS).
| Total Cortisol | EQD Free Cortisol | CUF Free Cortisol | ||||
|---|---|---|---|---|---|---|
| Measure | SIRS− | SIRS+ | SIRS− | SIRS+ | SIRS− | SIRS+ |
| Mean ± S.D. | 18.8 ± 16.1 | 20.5 ± 19.0 | 3.1 ± 3.5 | 4.0 ± 6.2 | 3.1 ± 4.4 | 4.3 ± 7.3 |
| Median | 14.2 | 14.3 | 1.9 | 1.9 | 1.6 | 1.6 |
| Min, Max | 1.4, 61.2 | 0.6, 109 | 0.3, 16 | 0.2, 37.2 | 0.2, 19.3 | 0.2, 43.6 |
Pearson correlation coefficients for PRISM score versus cortisol concentrations were: TC, 0.36; EQD FC, 0.33; and CUF FC, 0.38. Pearson correlation coefficients for PRISM score versus EQD FC as % TC and CUF FC as % TC were 0.12 and 0.34 respectively. As shown in Table 6 median TC, CUF FC, and CUF FC as % TC values increased with increasing PRISM III categories. Whereas TC increased 4.6 fold over the PRISM illness severity categories, CUF FC increased 13.4 fold. Free cortisol as % TC increased 2.6 fold over the PRISM illness severity categories.
TC, total cortisol; FC, free cortisol per centrifugal ultrafiltration fractionation
DISCUSSION
In this study, we have demonstrated that plasma cortisol may be fractionated using clinically relevant, real time, temperature-controlled CUF rather than a prolonged EQD technique, preparatory to measurement of FC by chemiluminescence immunoassay. Both TC and FC concentrations as well as FC as a percent of TC were markedly higher among critically ill children compared to unstressed adult volunteers. Illness severity per PRISM III correlated moderately with TC, FC, and FC as % TC. While nearly 60% of the children exhibited FC < 2 and 30% < 0.8 ug/dL, none were suspected of clinical CIRCI. These data suggest that FC measurement is feasible in real time and should be further investigated among specific populations of critically ill children with outcome data to potentially clarify the diagnosis of pediatric CIRCI. Our data also suggest that FC < 2 or perhaps even <0.8 ug/dL is probably not an appropriate definition for CIRCI in children.
Most circulating cortisol is protein bound to albumin and transcortin. However the FC fraction is responsible for the biological effects of the hormone. In general, measurement of total serum/plasma concentrations of thyroid and steroid hormones has limited biological validity, because the bioavailability of these hormones is substantially determined by variable concentrations and binding characteristics of their respective hormone binding proteins (19). It is recognized that concentrations of the cortisol binding proteins may change considerably with critical illness.
Variously derived cortisol concentrations have been suggested in an attempt to identify CIRCI employing a random baseline TC or incremental increase in TC following high or low dose corticotropin (6). However a key conclusion of the largest trial examining adjunctive hydrocortisone for adult septic shock, CORTICUS, was that that the short, high-dose corticotropin adrenal stimulation test, as it is currently being utilized, did not appear to be useful for identifying a population with adrenal insufficiency who would most likely benefit from hydrocortisone supplementation (8). Accordingly it has been suggested that FC rather than TC may be the preferred measure of adrenal sufficiency particularly for critically ill patients (9, 11). It is acknowledged that a recent prospective multicenter investigation of adrenal function among 381 critically ill children employing low dose corticotropin adrenal stimulation reported a significant increase requirement for fluid boluses and vasoactive-inotropic infusions among children with stimulated minus baseline TC < 9 ug/dL (20).
Equilibrium dialysis or ultrafiltration/ligand binding methods have traditionally been employed to isolate FC, but these methodologies have been hampered by expense, technical difficulties, large volume serum requirement, long analysis times, or need for a radionuclide tracer (21). Equilibrium dialysis additionally requires correction for the dilution-induced shift of binding equilibria. Furthermore, for many clinical laboratories FC assessment by EQD or liquid chromatography/mass spectroscopy (22) represents a “send out” study that requires days for reporting results. On the other hand, CUF with chemiluminescence immunoassay requires about two hours from receipt of blood specimen to reporting results.
Results reported here comparing healthy adult controls with critically ill children are very similar to those previously reported comparing healthy adult controls with a general population of critically ill adults utilizing EQD fractionation and immunoradiometric assay (9); comparing healthy adult controls with stressed medical and surgical patients utilizing EQD fractionation and chemiluminescence immunoassay (23); and comparing healthy adult controls with septic adults utilizing a ligand binding/ultrafiltration methodology (11).
More recently FC was measured directly following temperature-controlled CUF of serum/plasma samples (12, 19). This alternative approach avoids radioactivity, is faster, and accommodates smaller serum/plasma volumes. A recent comparison of CUF and EQD fractionation methodologies concluded that both produce acceptable reproducibility with very similar results when the filtrate or dialysate is assessed by the same automated immunoanalyzer system (19). Utilizing CUF methodology essentially identical to that used in the current study, investigators reported CUF FC concentrations for 115 healthy adults with age ranging 18-60 years with a median value of 0.86 ug/dL (23.8 nM), range 0.43-1.56 ug/dL (12-43 nM) representing 4.0-9.5% of TC (12).
Results reported here for FC values among critically ill children are similar to those reported for critically ill adults (also utilizing CUF with a YM-30 filter with 30k Da cut-off for serum fractionation and an immunoanalyzer) (19). In that investigation FC for the critically ill adults averaged 6.55 ug/dL (181 nM) representing 25.0% of TC as compared to healthy controls with FC 0.63 ug/dL (17.4 nM) representing 4.0% of TC. In the same investigation EQD was compared to CUF and demonstrated a slight positive bias with, EQD FC = 1.2 * CUF FC+ 3.9 (nM).
Free cortisol may also be measured directly from saliva samples, eliminating the need for serum or plasma fractionation (24). Salivary cortisol is in equilibrium and correlates with the free (unbound) fraction of the hormone in the circulation. Gender-specific morning salivary cortisol reference values have been published (25). However, reduced flow of saliva may be encountered among critically ill patients (26). Moreover, any oropharyngeal blood (instrumentation from suctioning, tracheal intubation) will contaminate a salivary sample with blood-derived cortisol.
Free cortisol may also be calculated knowing the concentrations of TC and the cortisol binding proteins, albumin and cortisol binding protein (transcortin) (27). For example, den Brinker M, et al. (28) calculated ‘bioavailable cortisol’ in children with meningococcal sepsis or septic shock. Unlike previous adult studies, these investigators found no difference in cortisol binding protein and albumin concentrations between controls and adults surmising that this reflected considerable use of plasma proteins for volume resuscitation. This may reflect why calculated ‘bioavailable cortisol’ concentrations were not more informative than total cortisol in informing adrenal sufficiency. Similarly Bendel S, et al. (29) compared total cortisol and calculated free cortisol in septic adults utilizing the Coolens’ method and again discerned no difference in terms of predicting outcomes. Although this study demonstrated good correlation between total cortisol and calculated free cortisol, the authors noted that other investigators have concluded that despite good correlation of measured and calculated serum free cortisol, there may be a significant mean percentage difference between both methods and significant individual differences (19). These investigators further pointed out that they used Coolens’ calculated free cortisol instead of direct free cortisol measurement because of the time-consuming, expensive equilibrium dialysis methodology, precisely the focus of the current investigation.
In fact the concentrations of both of the cortisol binding proteins may vary considerably, particularly among critically ill patients (9, 30). Other investigators have concluded that FC calculation using the Coolens’ equation significantly underestimates FC as compared to direct measurements (19). Mathematically calculating FC using three independent variables (total cortisol, cortisol binding globulin, albumin) each with inherent variability, seems inferior to directly quantifying free cortisol with a real time, reasonable expense methodology.
Potential advantages of the CUF method include smaller plasma volumes (~0.40 mL) as well as rapid turn around time (~2 hours) with chemiluminescence immunoanalyzers commonly available in most clinical laboratories. In this investigation the cassette EQD methodology employed utilizes smaller plasma volumes than traditional EQD, but is not widely available in clinical laboratories and still would not provide data for real time clinical decision making for critically ill patients (31).
An important limitation of the current study relates to the fact that TC and FC concentrations were not analyzed with respect to patient outcomes. Although effort was made to enroll children across the entire spectrum of illness severity, children with lower PRISM scores are more heavily represented. When standard, accepted methodologies emerge for both FC fractionation and FC analysis, FC concentration ranges among normal children of various ages will also need to be determined, since TC varies not only with illness severity but also with age (32). However, given that nearly 60% and 30% of the current cohort exhibited FC < 2 and < 0.8 ug/dL respectively without evidence of clinical manifestations of CIRCI, this investigation calls into question the use of such thresholds for assigning a diagnosis of CIRCI among critically ill children.
With respect to cortisol kinetics in pediatric sepsis, the next logical investigation should be designed to quantify both TC and FC concentrations at presentation among children with a spectrum of sepsis illness severity. Long term follow-up of clinically meaningful outcome measures should be included with an eventual goal of identifying the population with the highest benefit/risk ratio of pharmacologic corticosteroid intervention for CIRCI, however it may be defined. Utilization of the CUF methodology for FC analysis appears to be a valid, usable tool to facilitate such studies. Current data suggest that real time FC quantification is possible to ultimately facilitate clinical decision making regarding cortisol replacement therapy for children with critical illness.
Table 7.
Total and free cortisol values in relation to PRISM III categories
| PRISM III Score Category |
Median TC |
Median CUF FC |
Median FC as % TC |
|---|---|---|---|
| 0-7 | 11.7 | 1.3 | 12.4 |
| 8-15 | 19.3 | 2.3 | 12.3 |
| 16-23 | 17.0 | 2.3 | 15.5 |
| ≥24 | 53.7 | 17.4 | 32.4 |
Acknowledgments
This work was supported, in part, by cooperative agreements (U10HD050096, U10HD049981, U10HD500009, U10HD049945, U10HD049983, U10HD050012 and U01HD049934) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Department of Health and Human Services.
Footnotes
Presented in part in abstract form at the Society of Critical Care Medicine, 38th Critical Care Congress, Nashville, TN 1/31/2009-2/3/2008. Crit Care Med 2008; 36 (Suppl): A163 (Abstract 636); and at the Pediatric Academic Societies’ Annual Meeting, Vancouver, Canada, 5/1/2010-5/4/2010 (Abstract 3711.125) http://www.abstracts2view.com/pas/view.php?nu=PAS10L1_477
REFERENCES
- 1.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 2.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332(20):1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 3.Marik PE. Critical illness-related corticosteroid insufficiency. Chest. 2009;135(1):181–193. doi: 10.1378/chest.08-1149. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesh B, Cohen J, Hickman I, et al. Evidence of altered cortisol metabolism in critically ill patients: a prospective study. Intensive Care Med. 2007;33(10):1746–1753. doi: 10.1007/s00134-007-0727-7. [DOI] [PubMed] [Google Scholar]
- 5.Dimopoulou I, Alevizopoulou P, Dafni U, et al. Pituitary-adrenal responses to human corticotropin-releasing hormone in critically ill patients. Intensive Care Med. 2007;33(3):454–459. doi: 10.1007/s00134-006-0491-0. [DOI] [PubMed] [Google Scholar]
- 6.Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36(6):1937–1949. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 7.Annane D. Time for a consensus definition of corticosteroid insufficiency in critically ill patients. Crit Care Med. 2003;31(6):1868–1869. doi: 10.1097/01.CCM.0000063445.91548.20. [DOI] [PubMed] [Google Scholar]
- 8.Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 9.Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350(16):1629–1638. doi: 10.1056/NEJMoa020266. [DOI] [PubMed] [Google Scholar]
- 10.Loriax L. Glucocorticoid therapy in the intensive care unit. N Engl J Med. 2004;350:1601–1602. doi: 10.1056/NEJMp048052. [DOI] [PubMed] [Google Scholar]
- 11.Ho JT, Al-Musalhi H, Chapman MJ, et al. Septic shock and sepsis: a comparison of total and free plasma cortisol levels. J Clin Endocrinol Metab. 2006;91(1):105–114. doi: 10.1210/jc.2005-0265. [DOI] [PubMed] [Google Scholar]
- 12.Lentjes EG, Romijn F, Maassen RJ, et al. Free cortisol in serum assayed by temperature-controlled ultrafiltration before fluorescence polarization immunoassay. Clin Chem. 1993;39(12):2518–2521. [PubMed] [Google Scholar]
- 13.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 16.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 17.Coolens JL, Van Baelen H, Heyns W. Clinical use of unbound plasma cortisol as calculated from total cortisol and corticosteroid-binding globulin. J Steroid Biochem. 1987;26(2):197–202. doi: 10.1016/0022-4731(87)90071-9. [DOI] [PubMed] [Google Scholar]
- 18.Annane D, Maxime V, Ibrahim F, et al. Diagnosis of adrenal insufficiency in severe sepsis and septic shock. Am J Respir Crit Care Med. 2006;174(12):1319–1326. doi: 10.1164/rccm.200509-1369OC. [DOI] [PubMed] [Google Scholar]
- 19.Vogeser M, Mohnle P, Briegel J. Free serum cortisol: quantification applying equilibrium dialysis or ultrafiltration and an automated immunoassay system. Clin Chem Lab Med. 2007;45(4):521–525. doi: 10.1515/CCLM.2007.104. [DOI] [PubMed] [Google Scholar]
- 20.Menon K, Ward RE, Lawson ML, et al. A Prospective Multicenter Study of Adrenal Function in Critically Ill Children. Am J Respir Crit Care Med. 182(2):246–251. doi: 10.1164/rccm.200911-1738OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geiseler D, Ritter M. On the validity of free hormone measurements. Anal Biochem. 1983;132(1):174–182. doi: 10.1016/0003-2697(83)90444-x. [DOI] [PubMed] [Google Scholar]
- 22.Janzen N, Sander S, Terhardt M, et al. Fast and direct quantification of adrenal steroids by tandem mass spectrometry in serum and dried blood spots. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;861(1):117–122. doi: 10.1016/j.jchromb.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Christ-Crain M, Jutla S, Widmer I, et al. Measurement of serum free cortisol shows discordant responsivity to stress and dynamic evaluation. J Clin Endocrinol Metab. 2007;92(5):1729–1735. doi: 10.1210/jc.2006-2361. [DOI] [PubMed] [Google Scholar]
- 24.Arafah BM, Nishiyama FJ, Tlaygeh H, et al. Measurement of salivary cortisol concentration in the assessment of adrenal function in critically ill subjects: a surrogate marker of the circulating free cortisol. J Clin Endocrinol Metab. 2007;92(8):2965–2971. doi: 10.1210/jc.2007-0181. [DOI] [PubMed] [Google Scholar]
- 25.Patel RS, Shaw SR, Macintyre H, et al. Production of gender-specific morning salivary cortisol reference intervals using internationally accepted procedures. Clin Chem Lab Med. 2004;42(12):1424–1429. doi: 10.1515/CCLM.2004.264. [DOI] [PubMed] [Google Scholar]
- 26.Dennesen P, van der Ven A, Vlasveld M, et al. Inadequate salivary flow and poor oral mucosal status in intubated intensive care unit patients. Crit Care Med. 2003;31(3):781–786. doi: 10.1097/01.CCM.0000053646.04085.29. [DOI] [PubMed] [Google Scholar]
- 27.Poomthavorn P, Lertbunrian R, Preutthipan A, et al. Serum free cortisol index, free cortisol, and total cortisol in critically ill children. Intensive Care Med. 2009;35(7):1281–1285. doi: 10.1007/s00134-009-1480-x. [DOI] [PubMed] [Google Scholar]
- 28.den Brinker M, Joosten KF, Liem O, et al. Adrenal insufficiency in meningococcal sepsis: bioavailable cortisol levels and impact of interleukin-6 levels and intubation with etomidate on adrenal function and mortality. J Clin Endocrinol Metab. 2005;90(9):5110–5117. doi: 10.1210/jc.2005-1107. [DOI] [PubMed] [Google Scholar]
- 29.Bendel S, Karlsson S, Pettila V, et al. Free cortisol in sepsis and septic shock. Anesth Analg. 2008;106(6):1813–1819. doi: 10.1213/ane.0b013e318172fdba. [DOI] [PubMed] [Google Scholar]
- 30.Rosner W. Plasma steroid-binding proteins. Endocrinol Metab Clin North Am. 1991;20(4):697–720. [PubMed] [Google Scholar]
- 31.Waters NJ, Jones R, Williams G, et al. Validation of a rapid equilibrium dialysis approach for the measurement of plasma protein binding. J Pharm Sci. 2008;97(10):4586–4595. doi: 10.1002/jps.21317. [DOI] [PubMed] [Google Scholar]
- 32.Sippell WG, Dorr HG, Bidlingmaier F, et al. Plasma levels of aldosterone, corticosterone, 11-deoxycorticosterone, progesterone, 17-hydroxyprogesterone, cortisol, and cortisone during infancy and childhood. Pediatr Res. 1980;14(1):39–46. doi: 10.1203/00006450-198001000-00010. [DOI] [PubMed] [Google Scholar]