Abstract
Studies investigating differences in regional brain volumes in children born preterm and term during early childhood are limited. Neuroimaging could help understand patterns of deficit in children born preterm and target areas of development associated with these regions. The goal of this study was to identify differences in regional brain volume at two different ages using magnetic resonance imaging in preterm and term children. Magnetic resonance imaging and developmental testing occurred in children 18-22 month olds (16 preterm and 10 term children) and 36-47 month olds (12 preterm and 10 term children). There were significant differences between the four groups in the parietal region, cerebral white matter, third ventricle and lateral ventricle. Correlations between regional cerebral volume and developmental testing were explored for the third and lateral ventricles. Our findings indicate that in young toddlers differences in regional cerebral volume are due to both maturation and prematurity.
The past decade has provided us with magnetic resonance imaging techniques that allow detailed regional volumetric assessment (1). While it is known that children born prematurely have smaller regional volumes including white matter, hippocampus, thalamus, prefrontal cortex, orbital frontal cortex, and temporal lobes, most studies were performed in adolescents and young adults (2). Technical difficulties involved in acquiring high quality imaging of toddlers resulted in a limited number of studies investigating differences in regional brain volumes in children born preterm and term during their first years of life. Comparing a group of 18 to 22 month old children born preterm and full term we previously found significantly larger third ventricle volumes in the preterm group and smaller cerebral white matter, thalamus, hippocampus, cerebellum white matter volumes and anterior cingulated volume (3). An understanding of the patterns of deficit in children born preterm compared to children born term in the early years of life could help target areas of development that may be associated with these regions.
This study is an extension of our previous works and looked at a different group of preterm and full-term 36-47 month old children and compared these findings to those found at 18 to 22 months. Specifically high resolution structural magnetic resonance imaging was performed in 4 groups: children born very low birth weight (<1500 grams, preterm) at 18-22 months adjusted age, children born term at 18-22 months, children born preterm at 36-47 months, and children born term at 36-47 months. Our goal was to identify the regional structural differences associated with both prematurity and age. We hypothesized that both age groups of children born preterm would have smaller regional brain volumes and larger ventricles compared to children born at term. We further hypothesized that, compared to the three other groups; 18-22 month old children born preterm would have the smallest volumes in selected brain regions (when normalized to total brain volume) and larger ventricles.
METHODS
Participants
The current investigation was part of a larger developmental follow up study involving children born preterm at the University of New Mexico Hospital. Newborn intensive care unit (NICU) graduates followed by the Special Baby Clinic at the University of New Mexico Hospital were invited to participate. Children born term were recruited from general pediatric clinics and community advertisements. The study was approved by the University of New Mexico Institutional Review Board, and informed consent was obtained from the parents of participating children.
Children born preterm weighed less than 1,500 grams and were born prior to 32 weeks gestation. Exclusionary criteria included genetic disorders, visual or hearing impairment, grade IV intraventricular hemorrhage, or contraindication to magnetic resonance imaging. All scans were read by a radiologist and none of the children included in this study had cerebral malformations, although one of the preterm children had asymmetric periventricular leukomalacia. Age was adjusted for gestational age only in the group of children born preterm and only at 18-22 months. Term children were born by uncomplicated delivery and were without known medical or developmental disorders. Thirty-nine children born preterm attempted an MRI scan of which 28 successfully provided scans (16 were between 18 – 22 months and 12 were between 36-47 months). Thirty children born term attempted a MRI scan of which 20 successfully provided scans (10 were 18 months and 10 were 36-47 months old). This is not a longitudinal study and the children in each of the 4 groups were unique.
Each participant underwent neurodevelopmental evaluation. All children were tested during the day by graduate students or the senior developmental psychologist trained and certified to administer the testing measures. Parents remained with their children during the testing session, which routinely took one to two hours to complete. For the 18-22 month children, the Bayley Scales of Infant Development-III (4) Cognitive and Language Scales, which combines the scores from the Receptive and Expressive Language subscales, was administered. For the 36-47 month old children, the Wechsler Preschool and Primary Scale of Intelligence-III (5), which provided a measure of verbal ability and performance-based cognitive ability, was administered.
MRI scanning was performed at night during natural sleep (all children born term) or with chloral hydrate sedation (50 mg/kg orally), which was used only for children born preterm who did not fall asleep naturally. Parents remained with the children during the scanning. Once children were asleep, scanning took 45 to 60 minutes to complete. Head phones were placed on children’s ears for noise protection.
All MRI scans were performed on a Siemens 3 T Trio Tim scanner using the standard 12-channel phased array head coils provided with the system. Sagital T1-weighted anatomical images were obtained with a multi-echo three dimensional Magnetization Prepared Rapid Acquisition Gradient Echo sequence (MPRAGE). The technical details of procedures used for segmenting the brain into different tissue structures has been described previously (3). The public domain algorithm ‘FreeSurfer’ (http://surfer.nmr.mgh.harvard.edu) was used for brain segmentation (6,7). The method consisted of the removal of imaged non-brain tissue using a hybrid watershed/ surface deformation procedure, automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures, tessellation of the gray matter white matter boundary, automated topology correction, and surface deformation following intensity gradients. This optimally placed the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class. An essential analysis step required for children of this age was that every scan required visual inspection for accuracy of regional segmentation. We found that automatic segmentation of images of pediatric brains often missed areas of the anterior temporal and orbital frontal lobes. Thus all scans were evaluated for accuracy and manually corrected when necessary. Brains were separated into thirteen anatomic regions (third ventricle, lateral ventricle, anterior-cingulate, cingulate amygdala, caudate, thalamus, hippocampus, cerebellum white matter, frontal, cerebral white matter, corpus callosum, temporal, and parietal) and analysis was performed on normalized total brain volume. These major areas were selected as they are most associated with injury in preterm children and also associated with language and cognitive development (8).
Statistical Analyses
Data were analyzed using statistical analysis software Version 9 (SAS 9.2 Institute Inc, Cary, North Carolina). Differences in developmental testing for the 18-22 and 36-47 month preterm and full term children were examined using t-tests. Repeated measures analysis of variance was performed with the 13 regions as a repeated factor between children born preterm and term as well as between the term and preterm groups at 18-22 months and 36-47 months. Post-hoc analysis was performed to assess age by group interactions on volumes. Analysis of covariance was completed to assess the impact of medical and psychosocial variables on regional brain volumes.
RESULTS
As expected, birth weight and gestational age differed significantly between the term and preterm groups. A difference was also found in family income between the preterm 18-22 month olds and the other three groups. At 3 years the term children weighed significantly more than the preterm children however no differences were found between the groups for head size at either age. Ethnicities included Hispanic, Black, Native American and White children as presented in Table 1 with other demographic characteristics. The 18-22 month old children born preterm (age adjusted for gestation) scored significantly lower than the term children on the Bayley Scales of Infant Development-III language scales though there were no significant differences on the cognitive scale. The 36-47 month old children born preterm scored significantly lower on the Wechsler Preschool and Primary Scale of Intelligence-III Verbal and Performance scales than the children born term (see Table 2).
Table 1.
Demographic Information for the 18-22 month and 36-47 month preterm and term children
| 18-22 month Preterm n=16 |
18-22 month Term n=10 |
36-47 month Preterm n=12 |
36-47 month Term n=10 |
|
|---|---|---|---|---|
| Birth Weight (grams) | 1082.1 (194.9)** | 3115.6 (359.9) | 1152.2 (245.6)** | 3345.2 (641.5) |
| Gestation Age | 28.1 (1.5)** | 39.4 (1.1) | 29.7 (1.3)** | 38.1 (2.6) |
| Test Age (months) | 20.2 (1.5) | 21.4 (1.5) | 43.1 (3.4) | 41.1 (3.6) |
| Mother Age (years) | 29.6 (5.5) | 32.4 (4.2) | 31.2 (6.9) | 35.7 (2.5) |
| Gender (% female) | 50% | 40% | 8% | 10% |
| Family Income | 1.1 (1.2)* | 4.9 (3.0) | 3.3 (2.3) | 3.0 (3.6) |
| Ethnicity: White | 3 (18.75%) | 4 (40%) | 3 (25%) | 5 (50%) |
| Black | 1 (6.25%) | 0 | 0 | 0 |
| Hispanic | 9 (56.25%) | 5 (50%) | 8 (67%) | 5 (50%) |
| Native American | 3(18.75%) | 1 (10%) | 1 (8%) | 0 |
p<0.001 or
p<0.05 for preterm less than term groups
Note: Family Income: 1-<$10,000, 2-$10,000-$20,000, 3- $20,000-$30,000, 4-$30,000-$40,000, 5-$40,000-$50,000, 6- $50,000-$60,000, 7->$60,000
Table 2.
Developmental Test scores for the 18-22 month and 36-47 month groups
| 18-22 month Preterm |
18-22 month Term |
36-47 month Preterm |
36-47 month Term |
|
|---|---|---|---|---|
| BSID-III Cognitive | 93.4 (12.1) | 101.5 (8.8) | ||
| BSID-III Language | 85.9 (11.5)* | 96.5 (8.4) | ||
| WIPPSI – Verbal IQ | 91.3 (12.3)* | 105.7 (14.6) | ||
| WPPSI – Perform IQ | 91.3 (12.2)* | 100.2 (10.6) | ||
| Weight (pounds) | 24.0 (3.0) | 26.1 (2.8) | 31.2 (5.1)* | 35.4 (2.5) |
| Head Circumference cm | 47.7 (2.2) | 47.1 (2.5) | 50.2 (1.6) | 50.7 (1.7) |
p<.05 for preterm less than term group
Note. BSID-III is the Bayley Scales of Infant Development, 3rd Edition and WPPSI is Wechsler Preschool and Primary Scale of Intelligence- 3rd Edition. Scores are means and standard deviations.
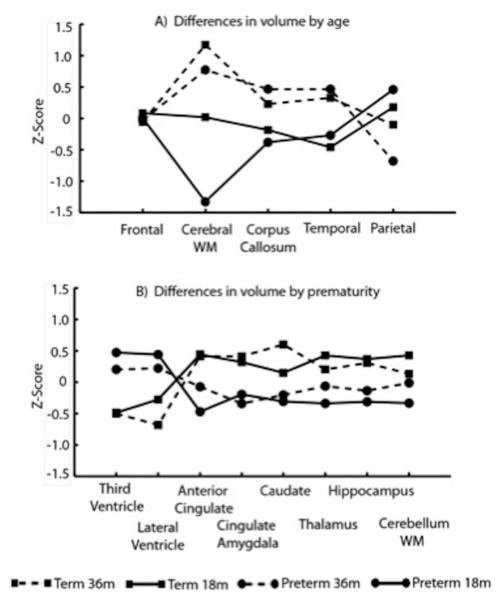
Repeated measures analysis of variance was significant by region by subject group (prematurity compared to term born) (p<.0001). Post hoc analysis of variance indicated that the parietal region (p=.02), cerebral white matter (p<.0001), third ventricle (p=.05) and lateral ventricle volumes (p=.03) were significantly different among the four groups (Table 3). The 18-22 month old children born preterm had significantly smaller cerebral white matter volume compared to other groups (18-22 month old children born term, 36-47 month old children born term, and 36-47 month old children born preterm). The 18-22 month term group also had smaller cerebral white matter volume compared to the 36-47 month term group. The 18-22 month old children born preterm had significantly larger parietal volume than the 36-47 month old children born preterm and term. The 18-22 month old children born preterm had significantly larger third ventricle volumes compared to the children born term at both 18-22 months and 36-47 months. Both the 18-22 month old and 36-47 month old children born preterm had significantly larger lateral ventricle volumes compared to the 36-47 month old children born term. A trend toward significance for age was found in mean differences for the corpus callosum and temporal region volumes (Figure 1A) in contrast to the other region volumes which differed due to prematurity (Figure 1B).
Table 3.
Normalized regional cerebral volume means and standard deviations
| Region | 18-22 month Preterm |
18-22 month Term |
36-47 month Preterm |
36-47 month Term |
|---|---|---|---|---|
| Frontal | 10.25 (0.84) | 10.33 (1.30) | 10.20 (0.80) | 10.18 (0.76) |
| Cerebral WMp<0.0001 | 20.65 (1.82)C | 23.10 (2.21)B | 24.48 (2.05)AB | 25.21 (0.82)A |
| Corpus Callosum | 0.17 (0.34) | 0.18 (0.03) | 0.23 (0.12) | 0.21 (0.44) |
| Temporal | 6.20 (0.66) | 6.10 (0.61) | 6.60 (0.43) | 6.52 (0.32) |
| Parietal p=0.02 | 5.75 (0.50)A | 5.59 (0.87)AB | 5.10 (0.54)B | 5.42 (0.24)B |
| 3rd Ventricle p=0.05 | 0.08 (0.04) A | 0.05 (0.01)B | 0.08 (0.04)A | 0.05 (0.01)B |
| Lateral Ventricle p=0.03 | 1.06 (0.70)A | 0.67 (0.29)AB | 0.94 (0.64)A | 0.45 (0.23)B |
| Anterior Cingulate | 0.57 (0.05) | 0.66 (0.12) | 0.61 (0.10) | 0.65 (0.10) |
| Cingulate Amygdala | 1.81 (0.14) | 1.90 (0.25) | 1.78 (0.13) | 1.91 (0.17) |
| Caudate | 1.28 (0.13) | 1.34 (0.13) | 1.29 (0.10) | 1.39 (0.12) |
| Thalamus | 0.88 (0.10) | 0.94 (0.06) | 0.90 (0.07) | 0.92 (0.04) |
| Hippocampus | 0.52 (0.08) | 0.57 (0.04) | 0.54 (0.07) | 0.56 (0.04) |
| Cerebellum WM | 1.86 (0.23) | 2.07 (.30) | 1.95 (0.37) | 1.99 (0.23) |
Note: ABC – superscripts group means with same letter were not significantly different in post hoc pair wise comparisons
Figure 1.
Cerebral volumes (z-scores) for regions by 4 groups (18-22 and 36-47 month preterm and term children) showing differences due to age (A) and due to prematurity (B). Lines are connected for visual clarity.
Analysis of covariance was completed to better understand if medical and psychosocial factors were related to the four regions found to be significant in the repeated measures analysis of variance. Gender, gestational age, days on ventilation, small for gestational age, test age and income were all not significant when put in the model for measures of cerebral, parietal, lateral ventricle and third ventricle volumes.
Correlations were used to better understand the relationship between the lateral ventricle and third ventricle to other brain regions. A significant negative correlation was found between the lateral ventricle and frontal volume for the 18-22 month (p=0.04, r= −0.52) and the 36-47 month (p=0.0006, r= −0.84) preterm groups. Significant negative correlations were also found for the lateral ventricle and hippocampus volumes for both the 18-22 month (p=0.0001, r= −0.81) and 36-47 month (p=0.01, r= −0.68) preterm groups. The lateral ventricle volumes were negatively correlated with the parietal region volumes (p=0.025, r= −0.56) for the 18-22 month preterm group and for caudate volume for the 36-47 month preterm group (p=0.015, r= −0.68).
A significant negative correlation was found between third ventricle volume and hippocampus volume for the 18-22 month (p=0.01, r= −0.62) and 36-47 month (p=0.01, r= −0.70) preterm groups. The third ventricle also had significant negative associations with temporal volume for both 18-22 month (p=0.04, r= −0.52) and 36-47 month (p=0.005, r= −0.75) preterm groups. The 36-47 month preterm group had significant negative correlations with third ventricle volume for the frontal (p=0.0003, r= −0.87) and caudate (p=0.047, r= −0.58) volumes. In addition the 18-22 month preterm group had significant negative correlations with the third ventricle volume and the corpus callosum (p=0.047, r= −0.50), and thalamus (p=0.035, r= −0.53).
DISCUSSION
The purpose of the current study was to identify regional structural differences associated with both prematurity and age. Children born preterm who were 18-22 and 36-47 months of age were hypothesized to have smaller regional brain volumes and larger ventricles compared to children born term of similar ages. When comparing regional brain volumes, group-related differences in overall cerebral white matter volumes were found. The 18-22 month old preterm group had significantly smaller cerebral white matter volume compared to all three other groups, consistent with findings of previous studies showing reduced global white matter volume in children born preterm compared to children born term (9-11).The finding that 18-22 month old children born preterm also had smaller cerebral white matter volume compared to 36-47 month old children born preterm was also expected given that white matter volume has been found to increase in childhood in both children born preterm and full-term (12). Interestingly, the fact that cerebral white matter volume between the 36-47 month old preterm and term groups were not significantly different suggest that that cerebral white matter of children born preterm may have normalized with time and reached the same volume as their term counterparts.
Our study found that 18-22 month old children born preterm had significantly larger parietal volume than the 36-47 month old children born preterm. Although the current study is cross-sectional in nature, this finding suggests possible age-related changes. Previous studies examining age-related changes in parietal volume in older children and adolescents report a similar reduction in parietal gray matter volume in children born preterm and term, resulting from the process of dendritic ‘pruning’ and synapse elimination. Similar to our findings, previous studies have also found a reduction in parietal volume across childhood in children born preterm and term (12-13). Giedd and colleagues (14) performed a large-scale longitudinal neuroimaging study, following children born term from 4 to 21 years of age, and found that parietal grey matter volume increased in pre-adolescence and was followed by a reduction in volume, starting at 12 years of age. Although our study is cross-sectional in nature, the fact that we found that 36-47 month old children born preterm had smaller parietal volumes than their 18-22 month old counterparts suggests that the process of dendrite pruning and synapse elimination could be occurring. Previous studies which found a reduction in parietal volume in childhood, such as Giedd et al.’s study (14), found that this reduction occurred around pre-adolescence in children born term. Our study found a volume difference between the 18-22 months and 36-47 months, but only in those children born preterm. It is possible that the process of dendritic pruning and synapse elimination may be disrupted in children born preterm and may possibly occur at differing time points than children born term. Future longitudinal studies examining age-related changes in cerebral volume among young children born preterm may help clarify our findings.
Findings also indicated that children born preterm had significantly larger third ventricle volumes compared to the children born term at both 18-22 months and 36-47 months, and that both the 18-22 month old and 36-47 month old children born preterm had significantly larger lateral ventricle volumes compared to the 36-47 month old children born term. Enlarged ventricles in children born preterm compared to children born term have been well documented (15). Larger lateral ventricles, for instance, have been found in children born preterm (15-16), at 8 years of age (17), and at 15 years of age (15). The enlarged lateral and third ventricles are thought to be due to the subcortical tissue loss associated with prematurity (15)
In order to better understand the possible effects of enlarged ventricles on other brain regions, we explored the relationship between ventricular and regional brain volumes in both the 18-22 month and 36-47 month old preterm groups. Significant negative correlations were found between the lateral ventricle and the frontal lobe, hippocampus, and cerebellum cortex volumes for both the 18-22 month and 36-47 month preterm groups. In addition, the corpus callosum and parietal lobe volumes were negatively correlated with the lateral ventricle volumes for the 18-22 month preterm group. When examining correlations with the third ventricles, third ventricle volumes were significantly and negatively correlated with frontal lobe and hippocampus volumes for the 18-22 month and 36-47 month preterm groups. In the 36-47 month preterm group, significant negative correlations between the third ventricle and the cerebellum cortex, temporal, and parietal volumes were also found. It is possible that some of the significant correlations found between ventricles and regional brain volumes were the result of early white matter damage that is associated with prematurity (18). For example, as the lateral ventricle increases in size, neighboring structures, such as the frontal and parietal lobes and the hippocampus, are likely to be impacted. The correlations that were found between the lateral ventricle volume and hippocampus, frontal lobe, parietal lobe, and corpus callosum volumes might be due to the proximity of these areas to the lateral ventricles. The correlations found between the lateral ventricle size and the cerebellum volume and the correlations between the third ventricle size and regional brain volumes may, on the other hand, may be a marker of more general brain injury. Since children born preterm are often born during the third trimester of pregnancy, a period during which the germinal matrix is in the process of developing, it is possible that, along with damage found in the ventricular system, other areas of the developing brain are impacted (19).
There were several limitations to this study. A longitudinal design would be ideal. The number of children in the four groups was small, and though there were a disproportionate number of males in the 36-47 month groups it did not appear to have impacted the results according to the analysis of covariance completed. In addition we were unable to obtain MRI sequences such as diffusion tensor imaging that might more fully characterize the degree of white matter myelination. A further limitation is that our relatively small subject numbers precluded analysis of the relationship between brain volume and developmental assessments. Finally, individual variation is an important issue in any clinical study, however our study population showed the expected range of head circumference and weight, and also as expected our preterm children scored significantly lower on developmental testing compared to term children at both age groups which is consistent with the literature.
In conclusion this study is one of the few studies that used MRI scanning with young children born preterm and term. Our finding of the different growth patterns of the cerebral white matter dependent on prematurity and age underscores the need to use imaging earlier in the life of a child at risk for developmental delays such as children born preterm. Often a study will do an early scan in the Newborn Intensive Care nursery with limited follow-up. Most studies have used imaging in early school age or adolescent. Imaging in combination with developmental testing may help us more adequately diagnose learning disorders in this at-risk group. Further investigation of the development of brain regions in young preterm children using a longitudinal study design with the same child evaluated at specific time periods would be important. This could help us better understand when the earliest point in development is to detect early learning deficits. Associations between regional brain volumes and developmental skills require further exploration, in order to enhance early diagnosis of developmental problems and refine intervention techniques that are used with children born preterm.
Acknowledgment
We would like to acknowledge the work of our doctoral students and MIND Research Network staff Susanne Duvall, Erica Montague, Lynette Silva, Judith Segall, Diana South and Cathy Smith who worked many nights scanning the children and completing developmental testing. We would also like to thank the CTSC nurses assisting with the recruitment and study coordination including Becky Montman, Carol Hartenberger, Mashid Rhoohi and Conra Backstrom Lacy. We also would like to thank the many families who traveled long distances and persevered late into the night to get their children to sleep through the scans used to complete this study.
This research was supported by a grant from the Department of Energy # DE-FG02-08ER64581, and the University of New Mexico Clinical Translational Science Center (1UL1RR031977-01).
Footnotes
Author Contributions
Jean Lowe is the first author, and Peggy MacLean and John Phillips are also primary authors contributing to the conception and design of the study, the analysis and interpretation of the data and writing and revising of the article. Robin Ohls assisted with the writing and editing of the article. Joy VanMeter helped test the children, and draft and revise the article. Clifford Qualls provided assistance with statistical analysis and editing the results section. Arvind Caprihan was responsible for the technical support for the imaging and accuracy of the data from the scans.
Declaration of Conflicting Interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Ethical Approval
The following study was approved by the Institutional Review Board of the University of New Mexico.
Contributor Information
Jean R. Lowe, Department of Pediatric, Division of Neonatology, University of New Mexico, MSC03 2220, Albuquerque, NM 87131-5313.
Peggy C. MacLean, Center for Development and Disability, University of New Mexico, Albuquerque, N.M. 87106 Phone: 505-331-3010; pmaclean@salud.unm.edu.
Arvind Caprihan, The Mind Research Network, Albuquerque, New Mexico 87106 Phone: 505-363-0845; acaprihan@mrn.org.
Robin K Ohls, Department of Pediatric, Division of Neonatology, University of New Mexico Albuquerque, New Mexico, USA, 87131-5313 Phone: 505-272-6410; rohls@salud.unm.edu.
Clifford Qualls, Clinical and Translational Science Center, University of New Mexico, Albuquerque, New Mexico 87131-5313 Phone: 505-272-5551; cqualls@salud.unm.edu.
Joy VanMeter, The Mind Research Network, Albuquerque, New Mexico 87106 Phone: 505-515-4141; jvanmeter@mrn.org
John Phillips, Department of Neurology, University of New Mexico, Albuquerque, New Mexico 87131-5313 Phone: 505-272-8247; jjphillips@salud.unm.edu.
REFERENCES
- 1.Soria-Pastor S, Padilla N, Zubiaurre-Elorza L, et al. Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics. 2009;124:e1161–e1170. doi: 10.1542/peds.2009-0244. [DOI] [PubMed] [Google Scholar]
- 2.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 3.Lowe J, Duvall SW, MacLean PC, et al. Comparison of structural magnetic resonance imaging and development in toddlers born very low birth weight and full-term. J Child Neurology. 2011;26(5):593–598. doi: 10.1177/0883073810388418. [DOI] [PubMed] [Google Scholar]
- 4.Bayley N. Psychological Corporation. 3rd Edition Harcourt Assessment, Inc; San Antonio, TX: 2006. Bayley Scales of Infant Development. [Google Scholar]
- 5.Wechsler D. Psychological Corporation. 3rd Edition San Antonio, TX: 2002. Manual for Wechsler Preschool and Primary Scale of Intelligence. [Google Scholar]
- 6.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 7.Fischl B, Salat D, Busa E, et al. Whole brain segmentation. Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 8.Tzarouchi LC, Astrakas LG, Xydis V, et al. Age-related grey matter changes in preterm infants: An MRI study. NeuroImage. 2009;47:1148–1153. doi: 10.1016/j.neuroimage.2009.03.072. [DOI] [PubMed] [Google Scholar]
- 9.Counsell SJ, Edwards AD, Chew ATM, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- 10.Kesler SR, Reiss AL, Vohr B, et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008;152:513–520. doi: 10.1016/j.jpeds.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson BS, Anderson AW, Ehrenkranz R, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 111:939–948. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- 12.Ment LR, Kesler SR, Vohr B, et al. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics. 2009;123:503–511. doi: 10.1542/peds.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- 14.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 15.Nosarti C, Al-Asady MHS, Frangou S, et al. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125:1616–23. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 16.Maalouf EF, Duggan PJ, Rutherford MA, et al. Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. J Pediatr. 1999;135:351–357. doi: 10.1016/s0022-3476(99)70133-2. [DOI] [PubMed] [Google Scholar]
- 17.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 18.Brunssen SH, Harry GJ. Diffuse white matter injury and neurologic outcomes of infants born very preterm in the 1990’s. J Obstet Gynecol Neonat Nur. 2007;36:386–395. doi: 10.1111/j.1552-6909.2007.00156.x. [DOI] [PubMed] [Google Scholar]
- 19.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]