Abstract
Differentiation of MHC class II–selected thymocytes toward the CD4+ helper lineage depends on function of the transcription factor ThPOK, whose expression is repressed in CD8+ cytotoxic lineage cells by a transcriptional silencer activity within the distal regulatory element (DRE) in the Thpok gene. Interestingly, the DRE also functions as a transcriptional enhancer. However, how the DRE exerts such dual functionality remains obscure. In this study, we dissected the DRE and identified DNA sequences specifically responsible for enhancer activity, and designated this as the thymic enhancer. Removal of the thymic enhancer from the murine Thpok locus resulted in inefficient ThPOK induction, thereby inducing a redirection toward alternative CD8+ cytotoxic lineage in a proportion of MHC class II–selected cells, even when they express monoclonal MHC class II–restricted transgenic TCR. Thus, regulation of contiguous but separable sequences with opposite function in the DRE plays an important role in precise coupling of TCR signaling with the selection process of two opposite lineages.
Introduction
Two distinct T lymphocyte subsets, CD4+ helper and CD8+ cytotoxic cells, are differentiated from common precursors, the CD4+CD8+ double-positive (DP) thymocytes. MHC class II–selected thymocytes differentiate into CD4+CD8– single-positive (SP) thymocytes committed to the helper lineage, whereas thymocytes expressing MHC class I–restricted TCRs differentiate into CD4−CD8+ SP thymocytes committed to the cytotoxic lineage (1). Thus, TCR specificity for MHC molecules is matched with helper versus cytotoxic lineage choice. Transcription factor ThPOK encoded by the Zbtb7b gene (hereafter referred to as the Thpok gene) is essential for development of CD4+ Th cells. Previous genetic studies have shown that the presence or absence of ThPOK in postselection thymocytes is a crucial factor that discriminates CD4/CD8 lineages (2, 3). Therefore, elucidation of mechanisms that control Thpok expression has been a major subject to understand how cell fate determination is regulated at the transcriptional level.
Kappes and colleagues (4) had identified two relevant cis-regulatory elements in the murine Thpok gene, the distal regulatory element (DRE) and the proximal regulatory element (PRE), which are located ∼3.0 kb upstream and ∼3.6 kb downstream of exon Ia, respectively (Fig. 1A). An enhancer activity within the PRE, referred to as the proximal enhancer (PE), was shown to drive a reporter transgene in the later stage of helper lineage cell development (4, 5). Alternatively, enhancer activity within the DRE was shown to drive reporter transgene expression mainly in CD4+CD8lo thymocytes (4). Thus, enhancer activity in the DRE has been supposed to be responsible for early Thpok induction. Interestingly, however, previous studies demonstrated that the DRE also possesses a transcriptional silencer activity that represses expression of reporter transgene as well as the Thpok gene in cytotoxic lineage cells (4, 5). Thus, the silencer activity in the DRE (referred to as the Thpok silencer in this study) is essential to limit Thpok expression to helper lineage T cells. These observations suggest that a mechanism that controls two opposite functions (silencer and enhancer) in the DRE is important to regulate Thpok expression. However, the molecular basis for such dual functionality of the DRE remains uncharacterized. One could speculate that functional conversion from silencer to enhancer could occur (4), as was reported for the fruit fly dorsal morphogen (6). However, it is also possible that each function is embedded in different DNA sequences. In this study, we identified core sequences responsible specifically for enhancer activity from the DRE and designated them as the “thymic enhancer” (TE). We provide concrete genetic evidence that the TE is important for efficient induction of ThPOK, and consequently to help certain MHC class II–selected thymocytes to appropriately choose the helper lineage fate.
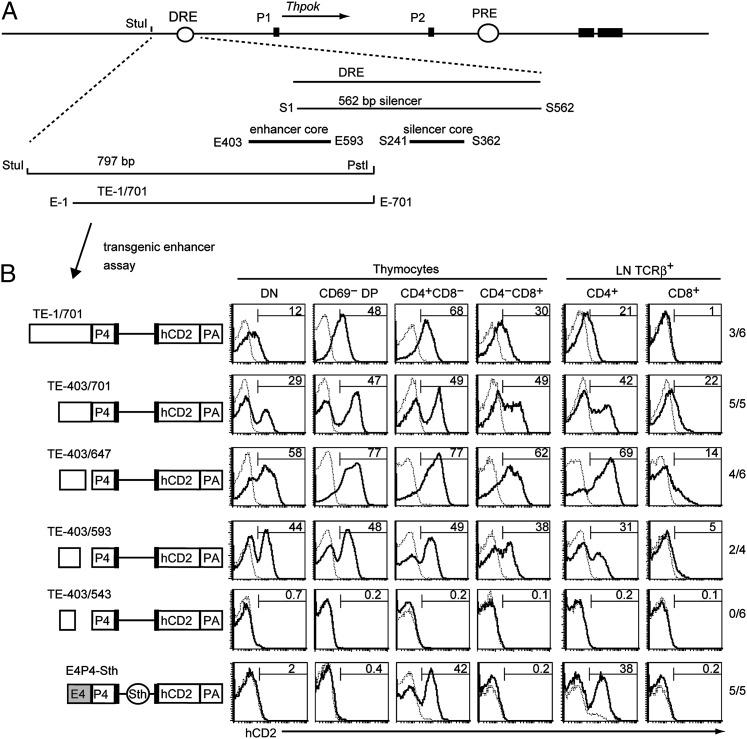
FIGURE 1.
Mapping of core thymic enhancer sequences in the DRE by reporter transgene expression assay. (A) Schematic structure of the murine Thpok locus is shown at the top. Black boxes represent exons. Circle represents the DRE and the PRE. Enlarged schematic structure of the DRE is shown at the bottom. Positions of core sequences for the thymic enhancer and silencer are shown as bold lines labeled as E403–E593 and S241–S362, respectively. (B) Schematic structures of reporter transgenes are shown at the left. E4, P4, Sth, and PA represent the Cd4 proximal enhancer, the Cd4 promoter, the 562-bp Thpok silencer, and SV40 polyA signal, respectively. Open boxes, black boxes, and black line represent the TE fragments, exons, and a part of the intron sequence from the murine Cd4 gene, respectively. Histograms show the expression of the reporter hCD2 gene in the indicated T cell subsets from representative transgenic founders for each construct. The dashed line indicates nontransgenic littermate control. Numbers in the histogram indicate the percentage of cells expressing the reporter transgene. The numbers of transgenic founders expressing reporter gene among the total transgenic founders are indicated at the right.
Materials and Methods
Mice
Thpokgfp (7), Thpokgfp:ΔPE (7), β2m−/− (8), and OT-II TCR transgenic mice (9) have been described. To generate transgenic reporter mice, each of the TE fragments of different lengths obtained by PCR amplification was replaced with the Cd4 proximal enhancer in the pE4P4-hCD2 transgene vector (10) to conjugate with the Cd4 promoter. To generate the E4P4-Sth construct, the 562-bp Thpok silencer (5) was inserted into the HindIII site in the pE4P4-hCD2 vector (10). To generate mice harboring the ΔTE mutation, a targeting vector that deleted the StuI-PstI sequence was transfected into embryonic stem cells harboring the Thpok+/gfp genotype as previously described (7) (Supplemental Fig. 2). All mice were maintained in the animal facility at RIKEN Research Center for Allergy and Immunology and all experiments were performed in accordance with institutional guidelines for animal care.
T cell isolation and flow cytometric analyses
Thymus and lymph nodes were removed from mice at 4–8 wk of age. Cells were stained with the FITC-, PE-, PerCP-, or allophycocyanin-conjugated Abs purchased from BD Biosciences and eBioscience. Multicolor flow cytometry data were collected with FACSCalibur and were analyzed with BD CellQuest (BD Biosciences).
Transfection of luciferase reporter into RM11-1 cells
Several genomic fragments, which were prepared from a 6.4-kb upstream region of exon Ia in the Thpok locus by digestion with appropriate restriction enzymes, were inserted into the pGL4.10 vector (Promega). Ten micrograms of each luciferase vector and an internal control pRL-CMV vector (Promega) were transfected into 5 million RM11-1 cells by electroporation. Luciferase activity was measured 48 h after transfection using the Dual-Luciferase reporter assay system (Promega).
Chromatin immunoprecipitation assay
Preparation of chromatin DNA and primers for the PE were previously described (5). Abs used are control IgG (SC-2025) and anti-Gata3 (SC-268) from Santa Cruz Biotechnology. Primers for TE amplification were 5′-ACCTAGTGGCAGGTAGGAAGCAG-3′ and 5′-GGGTAGCACTATTTATAACTGCGCG-3′.
Results and Discussion
During characterization of Runx-binding sequences in the Thpok gene (5), we noticed that expression of a reporter transgene (Tg) driven by the 6.5-kb region upstream of exon Ia depended on a 1.5-kb StuI-Eco47III fragment encompassing the DRE (Supplemental Fig. 1). Consistent with a previous report (4), enhancer activity in this fragment drove Tg expression mainly in CD4 lineage thymocytes (Supplemental Fig. 1), prompting us to designate the enhancer activity of the DRE as the TE of the Thpok gene. To narrow down sequences responsible for TE activity, we next performed reporter transfection assays with CD4 SP RM11-1 thymoma cells and found that a 797-bp StuI-PstI sequence in close 5′ proximity to the silencer is necessary to enhance reporter gene expression (Fig. 1A, Supplemental Fig. 1).
To examine in vivo function as well as to identify core sequences of the TE, we generated another reporter Tg mice. Several DNA fragments derived from the StuI-PstI sequences were conjugated with the Cd4 promoter (P4), which alone cannot drive Tg expression in the reporter construct (10). A 701-bp and 3′ half of a 298-bp fragment (constructs TE-1/701 and TE-403/701, respectively) could drive hCD2 reporter expression in all thymocyte subsets (Fig. 1B). Further sequential deletion from the 3′ side revealed that the 191-bp TE-403/593 sequences were minimal for enhancer activity in our reporter Tg assay (Fig. 1B). Of note, the levels of hCD2 expression were comparable between CD4SP thymocytes and CD4+ T cells, and hCD2 expression was detected in double-negative and DP thymocytes. Thus, consistent with previous results using the hCD2 promoter (4), the TE lost stage-specificity in conjunction with the heterologous Cd4 promoter. Additionally, in the peripheral T cell pool, the level of hCD2 expression was significantly lower in CD8+ cells than in CD4+ cells with all Tg reporter constructs tested. There are two possible mechanisms that explain this helper lineage-dominant expression: the enhancer activity itself is dominant in CD4+ cells, or silencer activity in the tested TE fragment represses Tg expression in CD8+ cells. However, because helper lineage–dominant expression was not apparent between CD4SP and CD8SP thymocytes, silencer activity, if present, should be active at later stage of CD8 lineage cell differentiation. In contrast, when an entire 562-bp Thpok silencer (Sth) was inserted into the Tg driven by the Cd4 enhancer (E4) and P4 (construct E4P4-Sth), Tg expression was repressed not only in CD8 thymocytes but also in DP thymocytes (Fig. 1B). Thus, full silencer activity in the DRE can repress Tg expression by heterologous enhancer in immature thymocytes. Furthermore, S241–S362 sequences, which do not overlap with the TE-403/593 sequences (Fig. 1A), were sufficient to repress Tg expression in DP and CD8+ SP thymocytes (Supplemental Fig. 1). Even though these results do not formally exclude a possibility of residual silencer activity in the TE-403/593 sequence for repression of Tg expression in CD8+ cells, they fit more with the former possibility. We therefore propose that core sequences necessary for enhancer and silencer activity are likely to be embedded in different DNA sequences within the DRE.
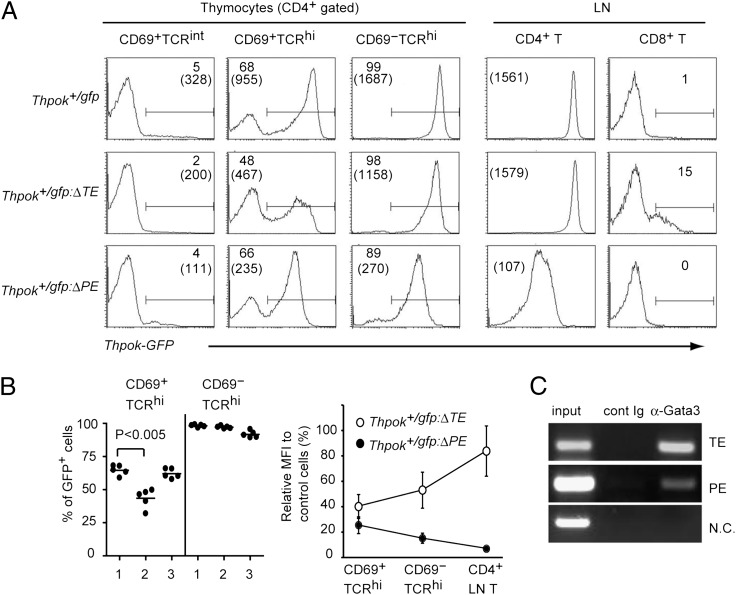
To address the physiological function of the TE in regulating Thpok gene expression and T cell development, we have deleted a 797-bp StuI-PstI region from the Thpok or Thpokgfp reporter allele (7), generating a ThpokΔTE or Thpokgfp:ΔTE allele, respectively (Supplemental Fig. 2). In Thpok+/gfp:ΔTE mice, in which T cell development was supported normally by half dosage of ThPOK protein, GFP expression in the CD4+CD69+TCRhi population decreased in terms of both frequency of GFP+ cells and the amount of GFP per cell compared with those in control cells of Thpok+/gfp mice (Fig. 2). However, the amount of GFP expressed from the Thpokgfp:ΔTE allele increased during maturation to the helper lineage thymocytes and reached a level comparable to that from the control Thpokgfp allele in peripheral CD4+ T cells, whereas deletion of the PE led to a decreased amount of GFP in those cells (Fig. 2A). This result indicates not only an essential role for the TE in efficient induction of the Thpok gene but also distinct stage-specific functions for the TE and PE. The Gata3 transcription factor was shown to be essential for activation of the Thpok gene via binding to a region upstream of exon II and the PE (11). We therefore tested whether Gata3 also binds to the TE by chromatin immunoprecipitation (ChIP) assay. Consistent with recent ChIP-Seq results (12), Gata3 also bound to the TE (Fig. 2B), suggesting that Gata3 may activate the Thpok gene through regulating both the TE and PE activity. Of note, there was low, but significant, GFP expression in some CD8+ T cells in the Thpok+/gfp:ΔTE mice (Fig. 2A), suggesting that sequences additionally deleted with the TE core by the ΔTE mutation are necessary for full silencer activity.
FIGURE 2.
Thymic enhancer is essential for efficient Thpok induction. (A) Histograms showing GFP expression from the Thpokgfp, Thpokgfp:ΔTE, and Thpokgfp:ΔPE alleles in the indicated cell subsets from thymus and lymph node (LN). Numbers in the histogram indicate the percentage of GFP+ cells, and numbers in parentheses indicate mean fluorescence intensity (MFI) of GFP in GFP+ cells. Data are representative of five experiments. (B) Graphs showing statistical analyses of percentage of GFP+ cells (left) and relative MFI to that from the Thpokgfp allele (right) in the indicated cell subsets. Lanes 1, 2, and 3 in the left graph are Thpok+/gfp, Thpok+/gfp:ΔTE, and Thpok+/gfp:ΔPE mice, respectively. (C) Analytical ChIP assay for Gata3 bindings to the TE and PE in the Thpok locus in CD4+ SP thymocytes. The E8I enhancer in the Cd8 gene was used as a negative control (N.C.). One representative result from three independent experiments is shown.
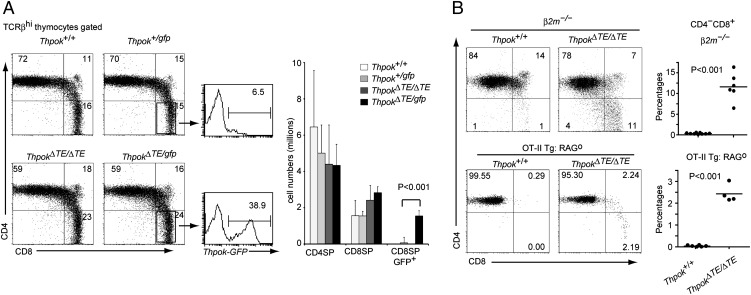
We next examined whether inefficient ThPOK induction due to lack of TE affects the development of MHC class II–selected cells. In ThpokΔTE/ΔTE mice, there was a slight decrease and increase of CD4+CD8− and CD4−CD8+ subsets, respectively, among the TCRβhi thymocyte population (Fig. 3A). Furthermore, in ThpokΔTE/gfp mice in which MHC class II–selected T cells are marked by GFP expression from the Thpokgfp allele that harbors normal regulatory regions (7), the frequency of GFP+ cells was increased in CD8+ SP thymocytes (Fig. 3A). This result suggests a redirected differentiation of some MHC class II–selected cells into the CD8 lineage. Indeed, an emergence of mature CD4−CD8+ T cells was observed in the mature thymocyte population of ThpokΔTE/ΔTE:β2m−/− mice, whereas those cells are absent in control mice (Fig. 3B). These results demonstrated that a small, but significant, proportion of MHC class II–selected cells undergo redirection to the CD8+ cytotoxic lineage by loss of the TE from the Thpok locus.
FIGURE 3.
Thymic enhancer is essential for appropriate helper lineage choice by MHC class II–selected cells. (A) Expression of CD4 and CD8 in TCRβhi mature thymocytes from mice of the indicated genotypes. Numbers in quadrants indicate the percentage of cells in each quadrant. Histograms show GFP expression in the CD4−CD8+ SP thymocytes of Thpok+/gfp and ThpokΔTE/gfp mice. Numbers indicate percentage of GFP+ cells. Data are representative of at least three individual mice of each genotype. Graph shows cell numbers of indicated cell subsets of indicated genotype as mean ± SD. (B) Expression of CD4 and CD8 in mature thymocytes from Thpok+/+:β2m−/− and ThpokΔTE/ΔTE:β2m−/− mice (upper plots) and Thpok+/+ and ThpokΔTE/ΔTE mice harboring OT-II transgene on a Rag1-deficient background (lower plots). Numbers in quadrants indicate the percentage of cells in each quadrant. Percentages of CD4–CD8+ cells in mature thymocyte subsets from each mouse analyzed are shown as a dot in the right panel. Black bar indicates the mean.
Because strong TCR signals have been thought to be involved in helper lineage choice (13) and ThPOK expression (14), differences in TCR affinity to self-peptide would affect the initial amount of ThPOK protein and thereby be involved in cell fate discrimination in ThpokΔTE/ΔTE mice. It was therefore important to test whether redirected differentiation occurs when all thymocytes expressed the identical TCR. To this end, we crossed transgenic mouse strain expressing MHC class II–restricted OT-II TCR (9) with ThpokΔTE/ΔTE mice. To inhibit VDJ recombination of the endogenous Tcr genes, a null mutation of the Rag1 gene was further introduced. Whereas no CD8+ cells expressing OT-II TCR were detected in mature thymocytes from control Thpok+/+ mice, a few CD8+ mature thymocytes emerged in ThpokΔTE/ΔTE mice (Fig. 3B). Thus, partial redirection to CD8 lineage still occurs even when all thymocytes express a monogenic TCR. These results indicate that TCR specificity to self-Ags is not the sole determinant to discriminate CD4/CD8 lineage choice in the ThpokΔTE/ΔTE setting. Supposing that the number of thymic epithelial cells presenting natural selective ligands to transgenic TCR is limited, it is possible that intraclonal competition within the postselection thymocyte population takes place when they all express identical TCR, as was reported during differentiation of regulatory T cells (15). A small proportion of postselection thymocytes that are losers in this competition might receive shorter TCR signals than do the winners. Although such shortened TCR signals would still be sufficient to induce the minimum amount of ThPOK required for helper lineage development in the presence of the TE, they would fail to induce a sufficient amount of ThPOK in the absence of the TE. Our results thus revealed an important requirement for the TE in guiding a small, but significant, proportion of MHC class II–selected thymocytes to appropriately differentiate into the helper lineage via fine-tuning of Thpok gene expression.
In this study, we characterized core sequences responsible for enhancer activity within the DRE and showed that two individual sequences, rather than functional conversion of one element, endow the DRE with dual functionality. Whereas the silencer is dominant and assures helper lineage specificity by inactivation of the Thpok gene, the TE located very near to the silencer is necessary for efficient activation of the Thpok gene, presumably with cooperative help of the PE. Inactivation of the silencer is also involved in efficient Thpok activation. However, given the helper lineage–specific expression of the E4P4-Sth reporter transgene, inactivation of the silencer could occur independently of the TE or PE function. It is important to further unravel molecular mechanisms regulating not only activity of each regulatory region in the DRE but also how multiple regulatory elements work cooperatively to finely tune Thpok expression under TCR signals.
Supplementary Material
Acknowledgments
We are grateful to Wooseok Seo for critical reading of the manuscript.
This work was supported by grants from the Uehara Foundation and by a Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (S) (to I.T.).
The online version of this article contains supplemental material.
- ChIP
- chromatin immunoprecipitation
- DP
- double-positive
- DRE
- distal regulatory element
- PE
- proximal enhancer
- PRE
- proximal regulatory element
- SP
- single-positive
- TE
- thymic enhancer
- Tg
- transgene.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Singer A., Adoro S., Park J. H. 2008. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat. Rev. Immunol. 8: 788–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He X., He X., Dave V. P., Zhang Y., Hua X., Nicolas E., Xu W., Roe B. A., Kappes D. J. 2005. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature 433: 826–833 [DOI] [PubMed] [Google Scholar]
- 3.Sun G., Liu X., Mercado P., Jenkinson S. R., Kypriotou M., Feigenbaum L., Galéra P., Bosselut R. 2005. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat. Immunol. 6: 373–381 [DOI] [PubMed] [Google Scholar]
- 4.He X., Park K., Wang H., He X., Zhang Y., Hua X., Li Y., Kappes D. J. 2008. CD4-CD8 lineage commitment is regulated by a silencer element at the ThPOK transcription-factor locus. Immunity 28: 346–358 [DOI] [PubMed] [Google Scholar]
- 5.Setoguchi R., Tachibana M., Naoe Y., Muroi S., Akiyama K., Tezuka C., Okuda T., Taniuchi I. 2008. Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science 319: 822–825 [DOI] [PubMed] [Google Scholar]
- 6.Jiang J., Cai H., Zhou Q., Levine M. 1993. Conversion of a dorsal-dependent silencer into an enhancer: evidence for dorsal corepressors. EMBO J. 12: 3201–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muroi S., Naoe Y., Miyamoto C., Akiyama K., Ikawa T., Masuda K., Kawamoto H., Taniuchi I. 2008. Cascading suppression of transcriptional silencers by ThPOK seals helper T cell fate. Nat. Immunol. 9: 1113–1121 [DOI] [PubMed] [Google Scholar]
- 8.Koller B. H., Marrack P., Kappler J. W., Smithies O. 1990. Normal development of mice deficient in β2M, MHC class I proteins, and CD8+ T cells. Science 248: 1227–1230 [DOI] [PubMed] [Google Scholar]
- 9.Barnden M. J., Allison J., Heath W. R., Carbone F. R. 1998. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76: 34–40 [DOI] [PubMed] [Google Scholar]
- 10.Sawada S., Scarborough J. D., Killeen N., Littman D. R. 1994. A lineage-specific transcriptional silencer regulates CD4 gene expression during T lymphocyte development. Cell 77: 917–929 [DOI] [PubMed] [Google Scholar]
- 11.Wang L., Wildt K. F., Zhu J., Zhang X., Feigenbaum L., Tessarollo L., Paul W. E., Fowlkes B. J., Bosselut R. 2008. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4+ T cells. Nat. Immunol. 9: 1122–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei G., Abraham B. J., Yagi R., Jothi R., Cui K., Sharma S., Narlikar L., Northrup D. L., Tang Q., Paul W. E., et al. 2011. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity 35: 299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogquist K. A. 2001. Signal strength in thymic selection and lineage commitment. Curr. Opin. Immunol. 13: 225–231 [DOI] [PubMed] [Google Scholar]
- 14.Park K., He X., Lee H. O., Hua X., Li Y., Wiest D., Kappes D. J. 2010. TCR-mediated ThPOK induction promotes development of mature (CD24−) γδ thymocytes. EMBO J. 29: 2329–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bautista J. L., Lio C. W., Lathrop S. K., Forbush K., Liang Y., Luo J., Rudensky A. Y., Hsieh C. S. 2009. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat. Immunol. 10: 610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.