Abstract
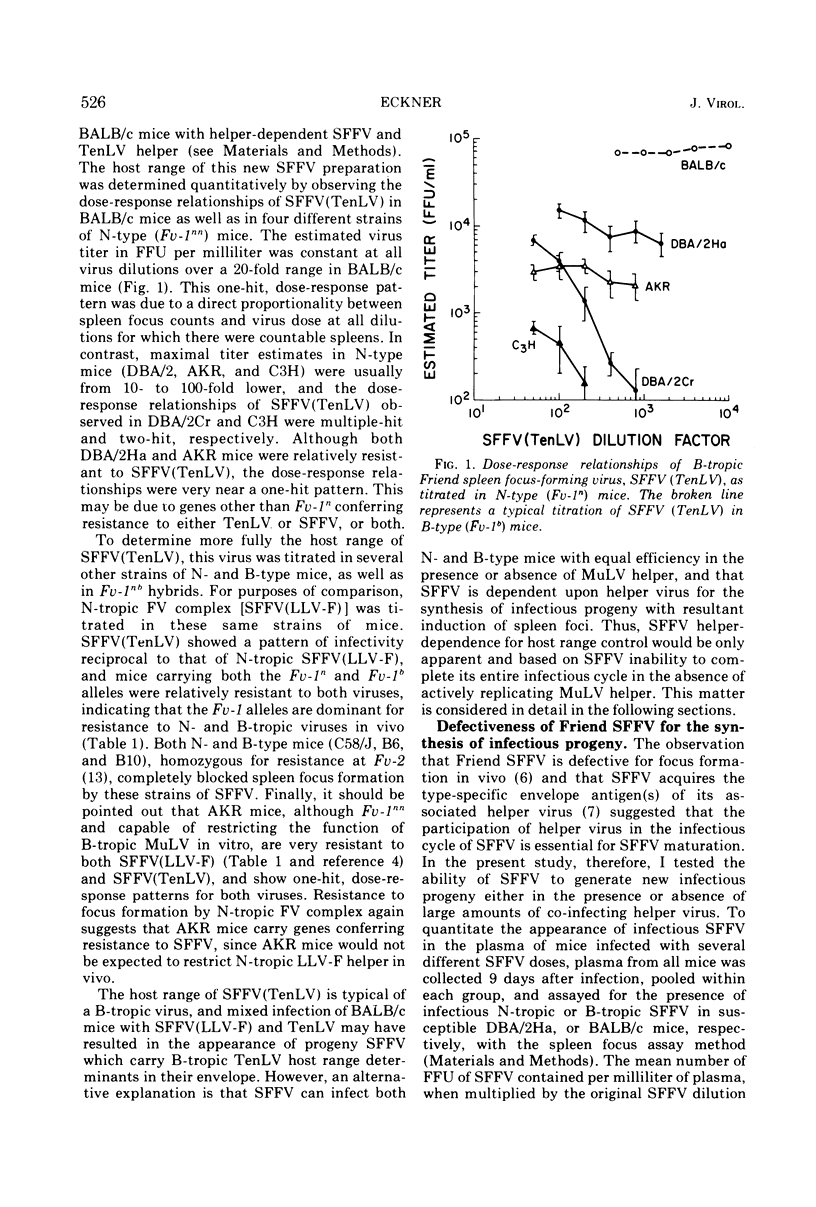
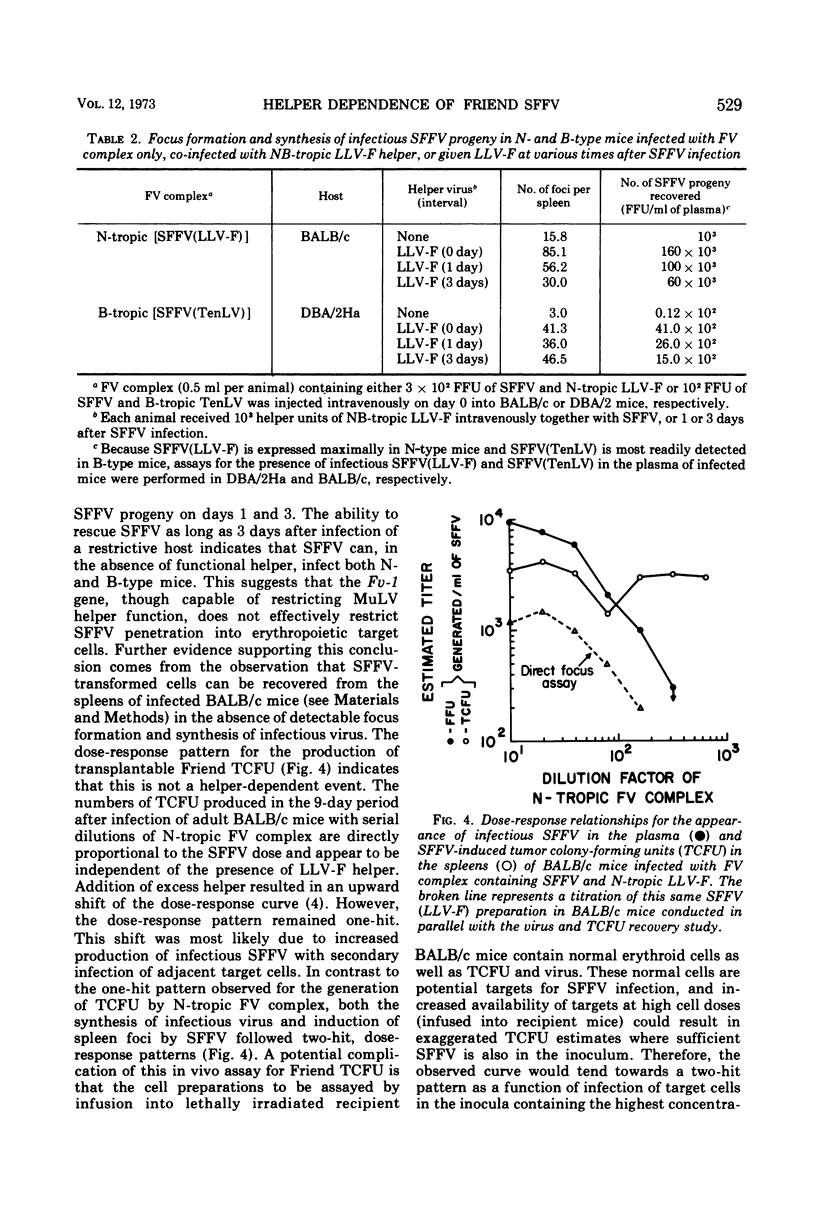
Co-infection of neonatal BALB/c mice with Friend virus (FV) complex (containing defective spleen focus-forming virus [SFFV] and endogenous N-tropic leukemia-inducing helper virus [LLV-F]) and B-tropic Tennant leukemia virus (TenLV) resulted in the inhibition of LLV-F by the Fv-1b gene and recovery of a TenLV pseudotype of SFFV, abbreviated SFFV(TenLV). The host range of this pseudotype was B-tropic, since SFFV(TenLV) was 10 to 100 times more infectious for B-type (Fv-1bb) than for N-type (Fv-1nn) mice. The similar patterns of neutralization of N-tropic and B-tropic SFFV by type-specific murine antisera suggested that the difference in infectivity between these two SFFV preparations did not reside in envelope determinants. Rather, helper control of SFFV's host range was only apparent and dependent upon the ability of associated virus to provide a helper function for late stages in SFFV synthesis. Early stages in SFFV's infectious cycle were shown to be helper independent. The Fv-1 gene did not act at the level of the cell membrane to effectively restrict SFFV infection, since SFFV-induced transformed cells could be detected in the absence of spleen focus formation and SFFV synthesis. Further, the generation of these transformed cells by SFFV followed a one-hit, dose-response pattern, suggesting that SFFV-induced cell transformation is helper independent. Finally, restriction of helper function by Fv-1 may be an intracellular event, because both SFFV and its associated LLV-F helper share common envelope determinants and presumably adsorb onto and penetrate target cells with equal efficiency.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELRAD A. A., STEEVES R. A. ASSAY FOR FRIEND LEUKEMIA VIRUS: RAPID QUANTITATIVE METHOD BASED ON ENUMERATION OF MACROSCOPIC SPLEEN FOCI IN MICE. Virology. 1964 Nov;24:513–518. doi: 10.1016/0042-6822(64)90199-0. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Graf T. Size differences among the high molecular weight RNA's of avian tumor viruses. Virology. 1971 Jan;43(1):214–222. doi: 10.1016/0042-6822(71)90239-x. [DOI] [PubMed] [Google Scholar]
- Eckner B. J., Steeves R. A. Defective Friend spleen focus-forming virus: pseudotype neutralization by helper-specific antisera. Nat New Biol. 1971 Feb 24;229(8):241–243. doi: 10.1038/newbio229241a0. [DOI] [PubMed] [Google Scholar]
- Eckner R. J., Steeves R. A. A classification of the murine leukemia viruses. Neutralization of pseudotypes of Friend spleen focus-forming virus by type-specific murine antisera. J Exp Med. 1972 Oct 1;136(4):832–850. doi: 10.1084/jem.136.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldsteel A. H., Dawson P. J., Kurahara C. In vivo and in vitro recovery of defective Friend virus by various leukemia viruses. Int J Cancer. 1971 Sep 15;8(2):304–309. doi: 10.1002/ijc.2910080216. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Noninfectious RSV deficient in DNA polymerase. Virology. 1971 Jan;43(1):313–316. doi: 10.1016/0042-6822(71)90251-0. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Rowe W. P. Lack of requirement of murine leukemia virus for early steps in infection of mouse embryo cells by murine sarcoma virus. Virology. 1971 Sep;45(3):844–847. doi: 10.1016/0042-6822(71)90211-x. [DOI] [PubMed] [Google Scholar]
- Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970 Jul;45(1):163–169. [PubMed] [Google Scholar]
- Martin G. S., Duesberg P. H. The a subunit in the RNA of transforming avian tumor viruses. I. Occurrence in different virus strains. II. Spontaneous loss resulting in nontransforming variants. Virology. 1972 Feb;47(2):494–497. doi: 10.1016/0042-6822(72)90287-5. [DOI] [PubMed] [Google Scholar]
- May J. T., Somers K. D., Kit S. Defective mouse sarcoma virus deficient in DNA polymerase activity. J Gen Virol. 1972 Aug;16(2):223–226. doi: 10.1099/0022-1317-16-2-223. [DOI] [PubMed] [Google Scholar]
- Mirand E. A., Steeves R. A., Avila L., Grace J. T., Jr Spleen focus formation by polycythemic strains of Friend leukemia virus. Proc Soc Exp Biol Med. 1968 Mar;127(3):900–904. doi: 10.3181/00379727-127-32831. [DOI] [PubMed] [Google Scholar]
- Peebles P. T., Haapala D. K., Gazdar A. F. Deficiency of viral ribonucleic acid-dependent deoxyribonucleic acid polymerase in noninfectious virus-like particles released from murine sarcoma virus-transformed hamster cells. J Virol. 1972 Mar;9(3):488–493. doi: 10.1128/jvi.9.3.488-493.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Rowe W. P., Lilly F. A major genetic locus affecting resistance to infection with murine leukemia viruses. II. Apparent identity to a major locus described for resistance to friend murine leukemia virus. J Exp Med. 1971 Jun 1;133(6):1234–1241. doi: 10.1084/jem.133.6.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIN H. GENETIC CONTROL OF CELLULAR SUSCEPTIBILITY TO PSEUDOTYPES OF ROUS SARCOMA VIRUS. Virology. 1965 Jun;26:270–276. doi: 10.1016/0042-6822(65)90274-6. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W. Studies of genetic transmission of murine leukemia virus by AKR mice. II. Crosses with Fv-1 b strains of mice. J Exp Med. 1972 Nov 1;136(5):1286–1301. doi: 10.1084/jem.136.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Humphrey J. B., Lilly F. A major genetic locus affecting resistance to infection with murine leukemia viruses. 3. Assignment of the Fv-1 locus to linkage group 8 of the mouse. J Exp Med. 1973 Mar 1;137(3):850–853. doi: 10.1084/jem.137.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers K., Kit S. Clonal isolation of murine sarcoma virus (MSV): characterization of virus produced from transformed cells. Virology. 1971 Dec;46(3):774–785. doi: 10.1016/0042-6822(71)90079-1. [DOI] [PubMed] [Google Scholar]
- Steeves R. A., Axelrad A. A. Neutralization kinetics of friend and Rauscher leukemia viruses studied with the spleen focus assay method. Int J Cancer. 1967 May 15;2(3):235–244. doi: 10.1002/ijc.2910020306. [DOI] [PubMed] [Google Scholar]
- Steeves R. A., Eckner R. J., Bennett M., Mirand E. A., Trudel P. J. Isolation and characterization of a lymphatic leukemia virus in the Friend virus complex. J Natl Cancer Inst. 1971 Jun;46(6):1209–1217. [PubMed] [Google Scholar]
- Steeves R. A., Eckner R. J. Host-induced changes in infectivity of Friend spleen focus-forming virus. J Natl Cancer Inst. 1970 Mar;44(3):587–594. [PubMed] [Google Scholar]
- Steeves R. A., Eckner R. J., Mirand E. A., Priore R. L. Rapid assay of murine leukemia virus helper activity for Friend spleen focus-forming virus. J Natl Cancer Inst. 1971 Jun;46(6):1219–1228. [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. A genetic locus for inducibility of C-type in BALB-c cells: the effect of a nonlinked regulatory gene on detection of virus after chemical activation. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2798–2801. doi: 10.1073/pnas.69.10.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouk V., Grundner G., Fenyö E. M., Lamon E., Skurzak H., Klein G. Lack of distinctive surface antigen on cells transformed by murine sarcoma virus. J Exp Med. 1972 Aug 1;136(2):344–352. doi: 10.1084/jem.136.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TENNANT J. R. Derivation of a murine lymphoid leukemia virus. J Natl Cancer Inst. 1962 Jun;28:1291–1303. [PubMed] [Google Scholar]
- Thomson S., Axelrad A. A. A quantitative spleen colony assay method for tumor cells induced by Friend leukemia virus infection in mice. Cancer Res. 1968 Oct;28(10):2104–2114. [PubMed] [Google Scholar]
- Ware L. M., Axelrad A. A. Inherited resistance to N- and B-tropic murine leukemia viruses in vitro: evidence that congenic mouse strains SIM and SIM.R differ at the Fv-1 locus. Virology. 1972 Nov;50(2):339–348. doi: 10.1016/0042-6822(72)90385-6. [DOI] [PubMed] [Google Scholar]



