Dear Sirs,
Ataxia telangiectasia (A-T) is a rare, autosomal recessive disorder of childhood characterized by progressive cerebellar ataxia, telangiectasia, and immune defects, and is caused by mutations in the ataxia-telangiectasia mutated (ATM) gene [1]. The incidence of the disease is about 1 in 40,000 to 100,000 births [2]. A-T has been reported worldwide [3], but reports of this disease in Africa are rare and generally limited to clinical description.
We describe a Malian family with parental consanguinity (Fig. 1a) and three of ten children presenting with cerebellar symptoms in early childhood. Two patients, 14- and 10-year-old boys, had normal births and development until age 2, when they presented with progressive gait difficulty, including difficulty stopping when running and falls. They both later developed slurred speech, weakness, and decreased coordination of the upper extremities. No sensory or bladder difficulty was noted. Family history was remarkable for a grandfather who died at age 75 and had balance problems since he was a teenager.
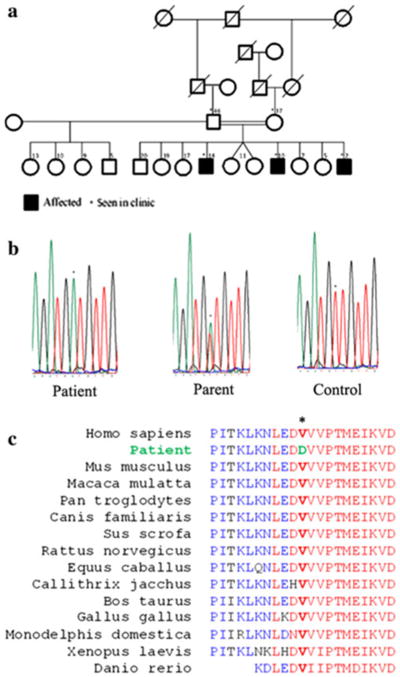
Fig. 1.
Pedigree and genetic features of the family. a Pedigree of the family showing consanguinity, and unaffected (white) and affected (black) individuals. b Sequencing shows a homozygous T7985A mutation (asterisk) in ATM. c ATM protein alignment in various species shows high conservation of the substituted Val2662 (asterisk)
Neurological examination of both patients showed an ataxic gait, markedly reduced hand coordination, and nystagmus on fixation and lateral gaze. They had slight distal weakness and atrophy in the lower legs. Reflexes were normal to reduced, and the Babinski sign was absent. The older brother had scoliosis. Cardiologic examination and testing were normal. Brain CT scan showed cerebellar atrophy with prominent cisterna magna. Vitamin E and beta and gamma tocopherol serum levels were normal. Genetic testing for Friedreich's ataxia was negative.
A follow-up clinical assessment showed oculomotor apraxia, ocular telangiectasia, and square wave jerks. The parents noted that the patients had recurrent diarrhea and upper respiratory infections.
These new findings were in favor of ataxia telangiectasia (Table 1). Additional blood testing showed high alphafetoprotein (AFP) levels and low IgA, IgE, and IgG2 levels. Also, aspartate and alanine aminotransferases (AST, ALT), and C-reactive protein (CRP) were elevated in the two patients, suggesting liver dysfunction. Genetic analysis of the ATM gene identified a novel homozygous single-nucleotide substitution at position c.7985T > A (Fig. 1b), predicting the amino acid substitution V2662D. Five available unaffected siblings did not have this sequence variant. The V2662 residue lies in a predicted ATP binding domain [3] and is conserved across a broad range of vertebrate species (Fig. 1c). In addition, this non-conservative amino acid change yields a score of –3 according to the BLOSUM 62 substitution matrix [4], and was not found in 100 ethnically matched controls, suggesting that the mutation found here is likely deleterious.
Table 1. Summary of clinical and laboratory findings in patients.
| Patient | Age (years) | Age at onset (years) | Sex | Clinical findings | Laboratory findings | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Cerebellar ataxia | Ocular apraxia | Telangiectasia | Recurrent infections | Choreoathetosis | Alpha-fetoprotein (n < 8.5 ng/ml) | Immunodeficiency | Cerebellar atrophy | ||||
| IV.8 | 14 | 2 | M | Yes | Yes | Yes | Yes | Yes | 733.2 | Yes | Yes |
| IV.11 | 10 | 2 | M | Yes | Yes | Yes | Yes | Yes | 752.4 | Yes | Yes |
| IV.14 | 2 | 2 | M | Yes | No | No | No | No | 135.9 | NA | NA |
N normal, NA not available
More recently, the parents noticed that their 2-year-old son also had an ataxic gait. His AFP levels were elevated, but he had no telangiectasias. This highlights the usefulness of AFP testing in the diagnosis of A-T, as previously discussed [5].
Recurrent upper respiratory infections due to immune deficiency [6] occur in ataxia telangiectasia, but the two older patients also presented with frequent diarrhea, which may represent an associated infectious disease specific to the region in Mali where the patients live. Increased cancer susceptibility has been associated with A-T [7], however, hemato-oncological examination showed no signs of malignancy in our patients. In addition, abdominal and inguinal echography showed no tumors.
Although cases of A-T have been reported in populations with African ancestry [3, 8] and in North Africa [9–11], reports of this disease in sub-Saharan Africa have been limited to clinical characterization [12, 13]. We report here genetically confirmed A-T with a novel mutation in this region, and add to the global spectrum of this disease.
Our study shows that hereditary neurological diseases may not be uncommon in this region of Africa, although limited expertise and lack of diagnostic tools might lead to their underestimation and neglect.
Acknowledgments
We are grateful to the subjects and their families for participating in this study. We thank Alison La Pean for help with patient characterization, Dr. Aldiouma Guindo at the University of Bamako, Mali for the use of his laboratory, Dr. Barrington Burnett at the Neurogenetics Branch, NINDS for help with editing, and James Nagle and Deborah Kauffmann at the NINDS DNA sequencing facility for help with sequencing. This work was supported by intramural funds from the NINDS at NIH.
Footnotes
Conflicts of interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard: This study has been approved by the appropriate ethics committee and has therefore been performed in accordance with the ethical standards laid down in 1964 Declaration of Helsinki.
Contributor Information
Guida Landouré, Email: glandoure@gmail.com, Service de Neurologie, Centre Hospitalier Universitaire du Point “G”, B.P: 333, Bamako, Mali; Neurogenetics Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA.
Fanny Mochel, INSERM UMR S975, Brain and Spine Institute, Hôpital La Salpêetriére, Paris, France; AP-HP, Department of Genetics, Hôpital La Salpêtriére, Paris, France; University Pierre and Marie Curie, Paris, France.
Katherine Meilleur, Neurogenetics Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA.
Madani Ly, Service d'Hémato-oncologie, Centre Hospitalier Universitaire du Point “G”, Bamako, Mali.
Modibo Sangaré, Neurogenetics Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA.
Nouhoum Bocoum, Faculté de Médecine, de Pharmacie et d'Odonto-stomatologie, Universite de Bamako, Bamako, Mali.
Koumba Bagayoko, Faculté de Médecine, de Pharmacie et d'Odonto-stomatologie, Universite de Bamako, Bamako, Mali.
Thomas Coulibaly, Service de Neurologie, Centre Hospitalier Universitaire du Point “G”, B.P: 333, Bamako, Mali.
Amadou M. Sarr, Laboratoire d'analyses médicales BIOTECH, Torokorobougou, Bamako, Mali
Hamidou O. Bâ, Service de Cardiologie, Centre Hospitalier Universitaire de Gabriel Touré, Bamako, Mali
Souleymane Coulibaly, Service de Psychiatrie, Centre Hospitalier Universitaire du Point “G”, Bamako, Mali.
Cheick O. Guinto, Service de Neurologie, Centre Hospitalier Universitaire du Point “G”, B.P: 333, Bamako, Mali
Mahamadou Touré, Service de Radiologie, Centre Hospitalier “Mere-Enfant” le Luxembourg, Bamako, Mali.
Moussa Traoré, Service de Neurologie, Centre Hospitalier Universitaire du Point “G”, B.P: 333, Bamako, Mali.
Kenneth H. Fischbeck, Neurogenetics Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA
References
- 1.Savitsky K, Bar-Shira A, Gilad S, et al. A single ataxia telangiectasia gene with a product similar to P1-3 kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 2.Sedgwick RP, Boder E. Ataxia telangiectasia. In: Vinken PJ, Bruyn GW, Klawans HL, editors. Handbook of clinical neurology. Vol. 16. Elsevier; Amsterdam: 1991. pp. 347–423. [Google Scholar]
- 3.Demuth I, Dutrannoy V, Marques W, Jr, et al. New mutations in the ATM gene and clinical data of 25 AT patients. Neurogenetics. 2011;12:273–282. doi: 10.1007/s10048-011-0299-0. [DOI] [PubMed] [Google Scholar]
- 4.Henikoff S, Henikoff JG. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci USA. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhagen MM, Abdo WF, Willemsen MA, et al. Clinical spectrum of ataxia-telangiectasia in adulthood. Neurology. 2009;73:430–437. doi: 10.1212/WNL.0b013e3181af33bd. [DOI] [PubMed] [Google Scholar]
- 6.Waldmann TA, Broder S, Goldman CK, Frost K, Korsmeyer SJ, Medici MA. Disorders of B cells and helper T cells in the pathogenesis of the immunoglobulin deficiency of patients with ataxia telangiectasia. J Clin Invest. 1983;71:282–295. doi: 10.1172/JCI110768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swift M, Morrell D, Massey RB, Chase CL. Incidence of cancer in 161 families affected by ataxia-telangiectasia. N Eng J Med. 1991;325:1831–1836. doi: 10.1056/NEJM199112263252602. [DOI] [PubMed] [Google Scholar]
- 8.Swift M, Morrell D, Cromartie E, et al. The incidence and gene frequency of ataxia-telangiectasia in the United States. Am J Hum Genet. 1986;39:573–583. [PMC free article] [PubMed] [Google Scholar]
- 9.Shridharan R, Radhakrishnan K, Ashok PP, Mousa ME. Prevalence and pattern of spinocerebellar degenerations in northeastern Libya. Brain. 1985;108:831–843. doi: 10.1093/brain/108.4.831. [DOI] [PubMed] [Google Scholar]
- 10.Belaoui M, Barbouche MR, Sassi A, et al. Primary immunodeficiency in Tunisia: study of 152 cases. Arch Pediatr. 1997;4:827–831. doi: 10.1016/s0929-693x(97)88145-6. [DOI] [PubMed] [Google Scholar]
- 11.Triki C, Feki I, Meziou M, Turki H, Zahaf A, Mhiri C. Clinical, biological and genetic study of 24 patients with ataxia-telangiectasia from southern Tunisia. Rev Neurol (Paris) 2000;156:634–637. [PubMed] [Google Scholar]
- 12.Wagstaff LA, Joffe R. Ataxia-telangiectasia in a South African Bantu child. S Afr Med J. 1969;43:662–664. [PubMed] [Google Scholar]
- 13.Aiyesimoju AB, Osuntokun BO, Bademosi O, Adeuja AO. Hereditary neurodegenerative disorders in Nigerian Africans. Neurology. 1984;34:361–362. doi: 10.1212/wnl.34.3.361. [DOI] [PubMed] [Google Scholar]