Abstract
Background
A chronic wound is defined as an area where the skin is not intact that fails to heal within eight weeks. Such wounds usually develop on the lower limbs as a complication of diabetes, venous insufficiency, or inadequate arterial perfusion. Most of the roughly 45 000 limb amputations performed in Germany each year are necessitated by non-healing chronic wounds.
Methods
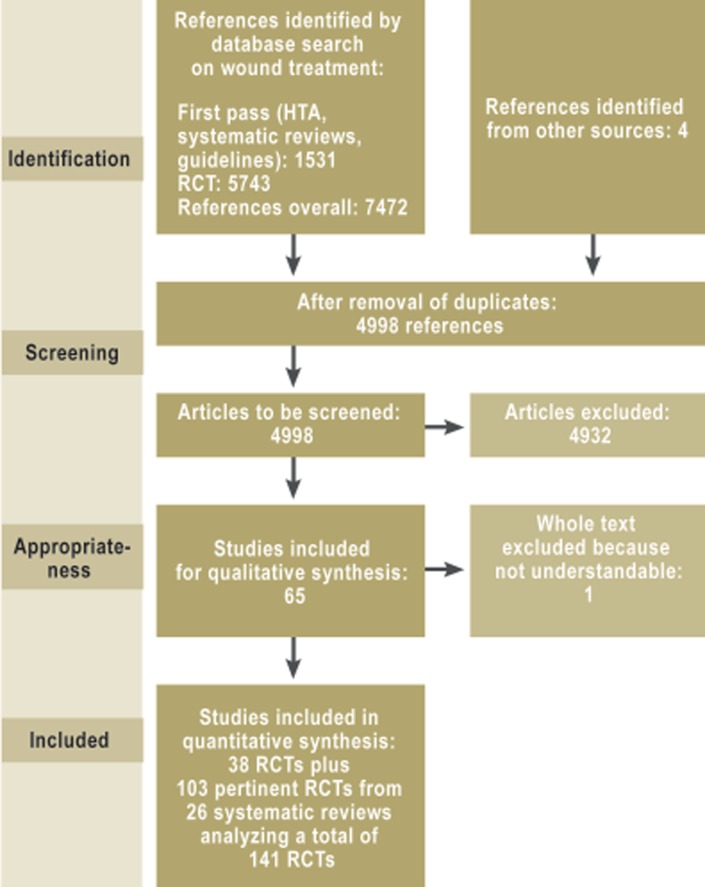
In the development of this S3 guideline, a systematic search was performed that yielded 4998 references including 38 randomized, controlled trials and 26 systematic reviews, which were used as the basis for the recommendations and statements made in the guideline. Twelve member societies of the umbrella Association of Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, AWMF), as well as the German Association of Nursing Science (Deutsche Gesellschaft für Pflegewissenschaft, and patient representatives participated in the consensus rounds in which the guideline’s recommendations and statements were agreed upon.
Results
This guideline contains seven evidence-based recommendations and 30 good clinical practice (GCP) recommendations. Evidence-based recommendations are given in favor of hydrogel, hyperbaric oxygenation, and integrated care, and against the use of medicinal honey and growth factors. Terms are defined precisely in order to ease communication and to specify what is meant by “wound debridement” (a procedure performed by a physician) as opposed to cleansing a wound. Under the premise of preventing pain, exudation, and maceration, local therapeutic agents can be chosen on the basis of the scientific evidence, the patient’s preference, the physician’s experience, and the wound situation. Costs should also be considered.
Conclusion
Scant evidence is available to answer many of the relevant questions about chronic wounds. There are valid data in support of hyperbaric oxygen and integrated care. More research is needed.
Chronic wounds are often treated unsystematically in routine practice, even though successful wound healing depends to a large extent on continuity in the re-evaluation of the state of the wound and reassessment of the treatment strategy.
Chronic wounds are associated with
In addition, they restrict patients’ everyday activities and mobility and cause emotional distress. As far as quality of life is concerned, systematic reviews have shown that pain is the most serious physical impairment of all (1, 3– 6).
Inadequate venous return is the cause of about 1.2% of all days lost from work in Germany. About 1% of the total cost of inpatient medical care in Germany is spent on the treatment of venous leg ulcers (7). On average, one patient in three has a recurrence (8). It does seem, however, that the incidence and prevalence of venous leg ulcers are both lower than they were reported to be in the 1970s. Large-scale studies in the Rhineland region of Germany have revealed a current prevalence of about 0.08%, which would imply that about 50 000 to 80 000 persons in the country suffer from this condition.
Chronic wounds on the lower limbs can arise because of arterial hypoperfusion (arterial leg ulcers), often in combination with diabetes (diabetic foot ulcers). The prevalence of peripheral arterial hypoperfusion in the overall population is 3% to 10%, depending on the definition. 15% to 20% of persons over age 70 have peripheral arterial occlusive disease (PAOD) (9). No reliable figures are available for the prevalence or incidence of stage IV PAOD or of leg ulcers of mixed arterial and venous pathogenesis.
Studies of diabetic foot ulcers in various countries have yielded prevalence figures ranging from 2% to 10% of the diabetic population, with an annual incidence of 2% to 6% (10).
Foot ulcers can lead, in the worst case, to amputations of toes, the foot, or the entire lower limb. According to data from the German AOK health insurance company, amputations are carried out in about 29 000 diabetic patients in Germany every year (11).
Although no precise epidemiological data are available on the frequency of recurrence of chronic wounds, individual studies have shown that both diabetic foot ulcers and venous insufficiency ulcers tend to recur, particularly when peripheral arterial hypoperfusion is also present (12). Recurrent diabetic foot ulcers are the most likely of all to necessitate amputation (in up to 60% of cases) (13).
Methods
This S3 guideline was created in accordance with generally recognized quality criteria (14) under the aegis of the German Association for Wound Healing and Wound Treatment (Deutsche Gesellschaft für Wundheilung und Wundbehandlung e.V.) in collaboration with 11 societies belonging to the Association of Scientific Medical Societies in Germany (AWMF, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften), the German Association of Nursing Science, and patient representatives. The persons who developed the guideline thus came from multiple medical disciplines and other health occupations involved in the treatment of wounds; the participants were selected because of their expertise and because they had minimal personal conflicts of interest.
A comprehensive set of key questions about the current methods of cleaning and dressing wounds and about various physical treatments served as the basis for a literature search and assessment performed externally by Kleijnen Systematic Reviews Ltd. (UK).
Individual guideline authors carried out systematic literature searches on further subjects, such as patient preferences or organization. In total, 4998 publications on wound-treatment interventions were retrieved, including the reports of 38 randomized controlled trials that were used to develop the guideline. In addition, 103 randomized controlled trials from 26 systematic reviews were used. The effects on clinical endpoints such as wound healing, reduction of wound size, pain, and complications were systematically assessed according to the GRADE scheme (15), which also provides an overview of the quality of the evidence. On the basis of this evidence, the participants issued graded recommendations that were developed in multiple consensus-building sessions (Table).
Table. Evidence levels and recommendation grades in the S3 guideline on the local treatment of chronic wounds.
| Type of study | Quality levelaccording to GRADE (34) | Recommendation gradeand illustrative example |
| Systematic reviews (meta-analysis) and randomized controlled therapeutic trials | High (It is highly unlikely that further research will alter confidence in the observed treatment effect.) | Recommendation grade A Example: “Treatment X clearly should / should not be used” |
| Randomized controlled trials with an intermediate risk of systematic error | Moderate (Further research is likely to have a major impact on our confidence in the observed treatment effect. The treatment effect may turn out to be different than observed in this trial.) | Recommendation grade B Example: “Treatment X should / should not be used” |
| Randomized, controlled trials with a high risk of systematic error | Low (Further research will very likely have a major impact on our confidence in the observed treatment effect. The treatment effect will probably be different than observed in this trial.) | Recommendation grade O Example: “Treatment X can be used” |
In areas where scientific evidence could not be obtained or was not considered essential, good clinical practice (GCP) recommendations were issued by consensus.
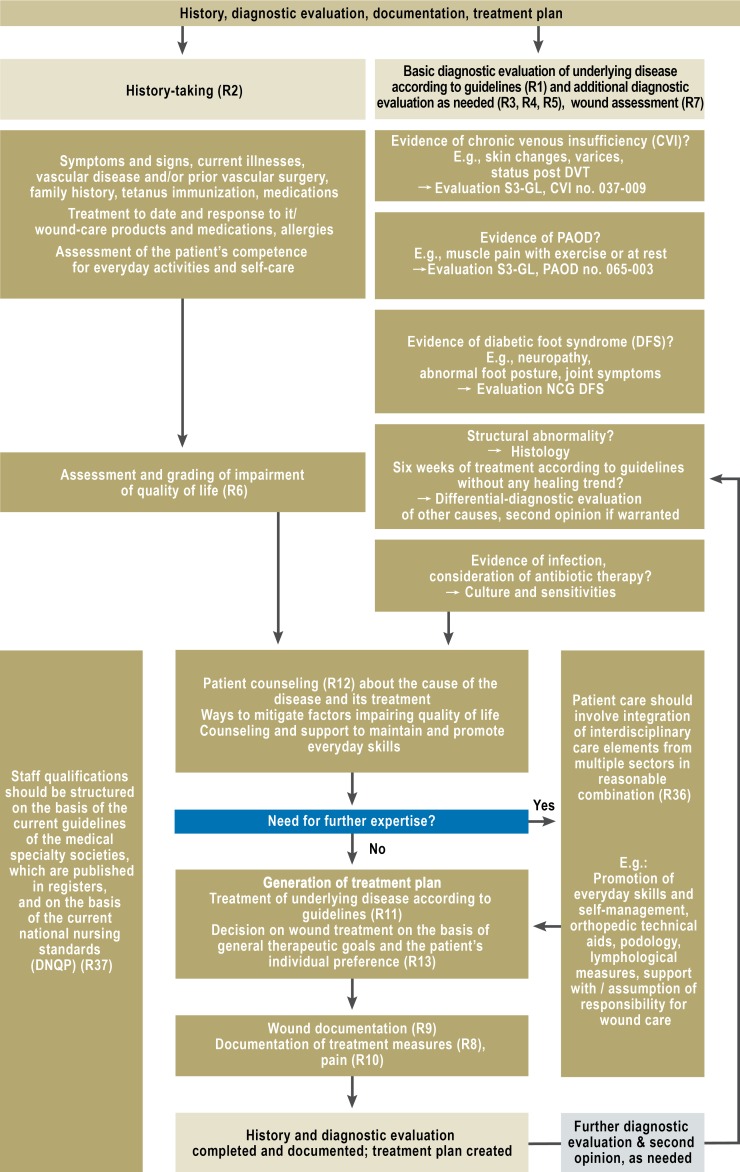
On the basis of the recommendations and statements for which a consensus could be obtained, an algorithm was created that encapsulates the basic principles of diagnostic assessment and wound treatment (Figures 1 and 2 and eFigure 1). The development of this guideline is described in a comprehensive guideline report (16).
Figure 1.
Algorithm for wound-specific diagnostic
evaluation and history-taking
R, recommendation;
DVT, deep vein thrombosis;
DNQP, German Network for Quality Development in Nursing (Deutsches Netzwerk Qualitätsentwicklung in der Pflege);
PAOD, peripheral arterial occlusive disease;
NCG, national care guideline
S3-GL, S3 guideline
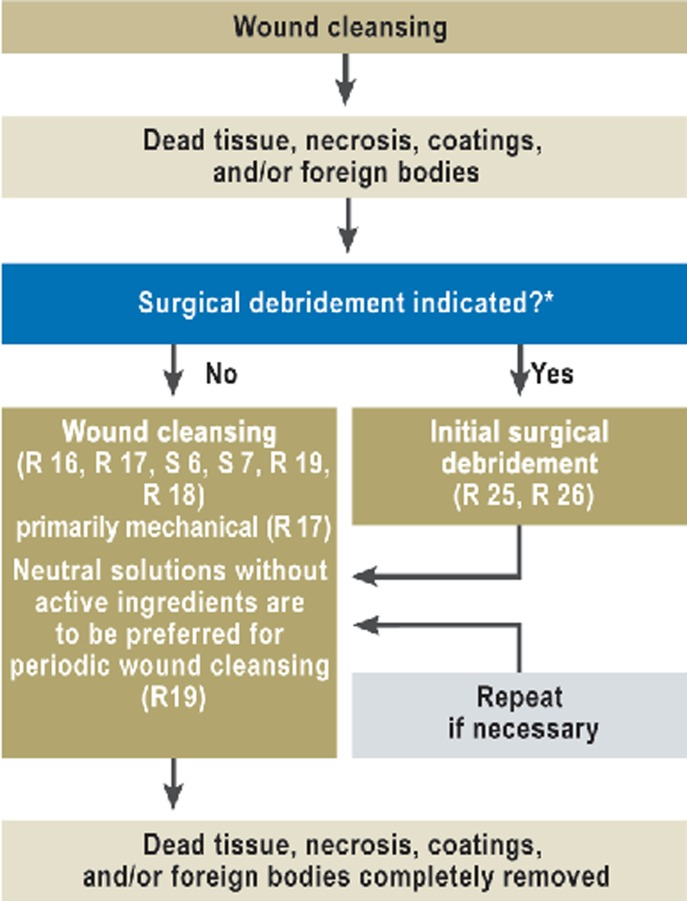
Figure 2.
Algorithm for wound cleansing
R, recommendation;
S, statement;
*for more detailed information, cf. eFigure 1
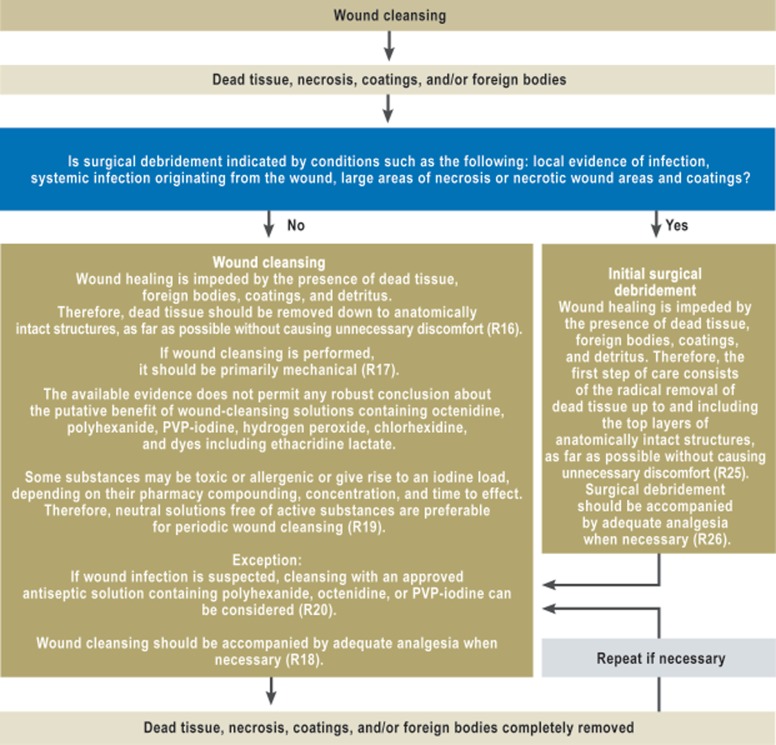
eFigure 1.
Algorithm for wound cleansing
R, recommendation
Diagnostic assessment and documentation
The clinical approach to chronic wounds begins with history-taking and the diagnostic assessment of the underlying disease, which should be performed as recommended in the guidelines of the relevant medical specialty society. The general procedure is shown in the algorithm for history-taking and diagnostic assessment (Figure 1).
A recommendation of major importance is that, if no trend toward wound healing is evident after six weeks of treatment in conformity with the guidelines, there should be further differential-diagnostic assessment for other potential causes of impaired wound healing. In case of doubt, a second opinion should be obtained (GCP).
Moreover, micriobiological testing is recommended only when antibiotic treatment of a bacterial infection emanating from the wound is being considered.
Adequate documentation is very important and serves as the basis of communication among treating personnel, as well as of quality management and billing. At a minimum, documentation must include the (established or suspected) cause of impaired wound healing, the size of the wound, the visual appearance of the wound surface and its edge and surroundings, the treatment(s) ordered and provided, and any changes made in the treatment.
The guideline also contains definitions and explanations of terms. For example, the proper use of the term “debridement” is explained. This word is often used inappropriately for lesser procedures; a clear distinction is drawn between debridement and simple wound cleansing.
Wound cleansing and surgical debridement
The literature search did not reveal any high-grade evidence (see Table) for either wound cleansing or wound debridement, yet there was a strong consensus among the experts that wound healing is impaired by the presence of dead tissue, foreign bodies, coatings, and detritus. The recommendation for initial treatment is, therefore, that dead tissue should be radically removed up to and including the top layers of intact anatomical structures (as far as possible without causing unnecessary discomfort). This is called debridement. The indications for surgical debridement include signs of local infection, systemic spread of infection from the wound, and large areas of necrosis or coating of the wound. Pain should be treated appropriately when necessary.
In distinction to surgical debridement, wound cleansing is defined as the removal of dead tissue, necrosis, wound coatings, and/or foreign bodies down to the anatomically intact structures, with preservation of granulation tissue. Like debridement, wound cleansing serves the purpose of complete removal of all elements impeding wound healing. It should be performed primarily mechanically and with accompanying analgesic medication when needed.
The literature does not provide evidence of any advantage for one kind of rinsing solution over another. A consensus statement was issued that rinsing with unsterile solutions or with non-sterilely filtered tap water carries the risk of introducing microbial pathogens. There is no evidence that Ringer’s lactate is any more efficacious as a rinsing solution than normal saline.
Wound-rinsing solutions with chemical additives (polyhexanide, octenidine, hypochlorite, H2O2, ethacridine lactate, and dye solutions) have not been shown to promote wound healing any better than normal saline. For non-infected wounds, the use of antiseptic substances (some of which, e.g., iodine gauze, have aggressive effects) is not recommended, because there is no evidence that these substances promote wound healing (relative risk [RR] 1.06; 95% confidence interval [CI] 0.819–1.375) or prevent infection (RR 1.06; 95% CI 0.87–1.3). Neutral solutions without any added active chemicals are recommended for active periodic wound cleansing. The use of approved antiseptic agents can be considered if infection is suspected.
“Passive periodic wound cleansing”—a term newly coined in the context of the current S3 guideline—refers to supplementary methods, performed as part of the wound cleansing process, that take place underneath a wound covering (secondary dressing). These include, for example, enzymatic or osmotic methods or wet cleansing compresses. In general, there is insufficient evidence to support the use of such methods.
A beneficial effect on the healing of diabetic foot ulcers has been demonstrated only for hydrogel; the evidence for this is of moderate quality (see Table for grading of evidence levels) and is derived from two clinical trials (RR 1.83, 95% CI 1.19-2.82) (17, 18). The authors hypothesize that the observed benefit may be due to promotion of the body’s intrinsic autolysis process by rehydration. Therefore, the guideline contains the recommendation that hydrogel can be used to treat diabetic foot syndrome (DFS) when rehydration is required, e.g., when there is dryness causing pain, when deep structures such as tendons or bone are exposed, or when dry residual wound coating is present. A consensus among the experts supports the extension of this recommendation to venous and arterial leg ulcers as well.
Dry areas of necrosis should not be rehydrated because of the risk of wet gangrene (GCP).
The guideline contains an explicit recommendation against the use of honey, because there is good evidence (19, 20) that honey does not accelerate wound healing (RR 1.15; 95% CI 0.96-1.38), while significantly more patients being treated with it complain of pain (RR 2.53; 95% CI 1.53-4.18) (19).
Wound cleansing with fly larvae (maggots) is faster than with hydrogel, but also significantly more painful. There is no significant difference between these two techniques with respect to the percentage of healed wounds at six to twelve months (RR 1.14; 95% CI 0.86–1.53) (21) (Figure 2; see eFigure 1 for long version).
Wound care products and topical application
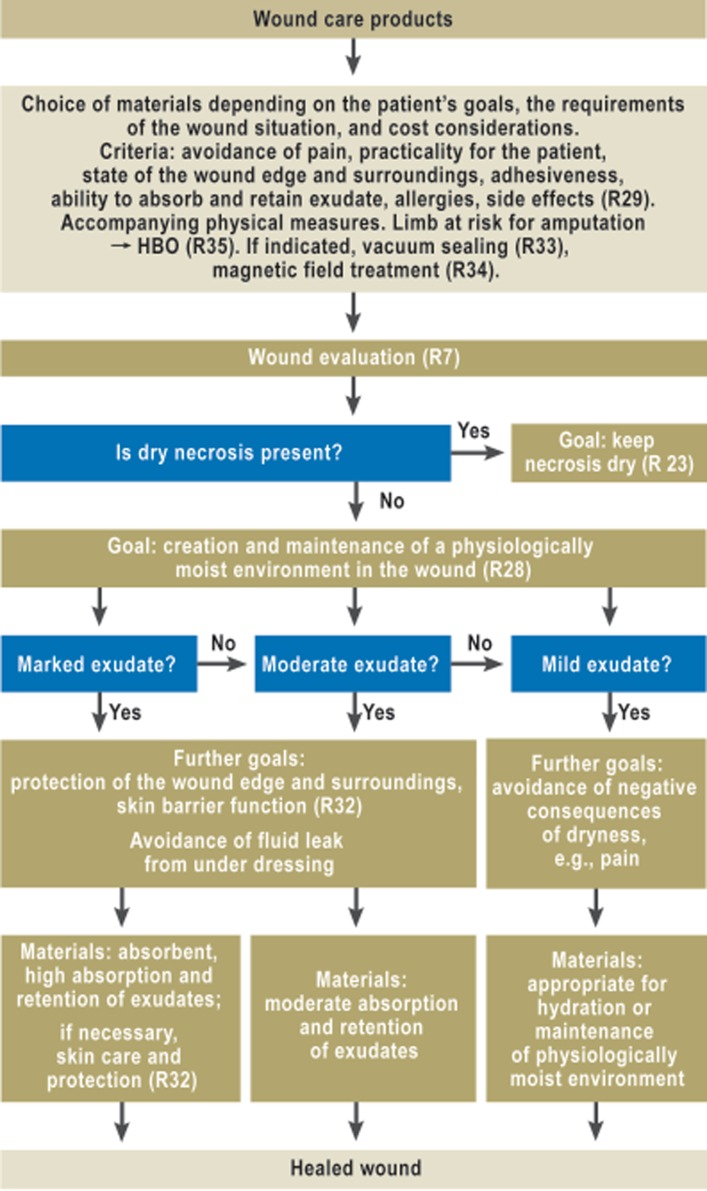
The spectrum of available wound care products includes, for example, hydrocolloids, film overlays, foams, microfiber dressings, alginates, and polyacrylates, which are sold in various combinations of materials.
Wound fillers (e.g., alginates) are substances with which deep wounds can be filled; in contrast, hydrocolloids and films are laid over wounds. Some materials, such as foams, can be used in either manner, while others are offered in combination form, together with an active agent.
There was inadequate evidence to support any recommendation for or against most of the product groups used as wound care products, whether or not they contain an active ingredient. The experts, therefore, agreed to seek consensus on certain criteria for the selection of wound care products, to be issued as good clinical practice recommendations. These criteria include, for example,
avoidance of pain,
practicality for the patient,
strength of adhesion,
absorption and retention of exudate,
avoidance of maceration.
The choice of wound care products depends, among other things, on the requirements of the wound situation, the patient’s goals, and cost considerations.
A physiologically moist environment in the wound should be created or maintained.
Wound care products containing silver do not promote wound healing to any significantly better extent than those that do not contain silver. This was the conclusion of four systematic reviews and nine randomized, controlled trials that fulfilled the criteria of the systematic literature search.
In vitro studies have shown that silver is both bactericidal and cytotoxic. There has not yet been any detailed comparative test of the various forms of silver-containing products that are currently sold (elementary-metallic, silver salts, ion exchangers).
Nor does the available evidence, some of which is of high quality (Table) (22), support any benefit on wound healing, or the prevention of infection, from cadexomer-iodine, PVP-iodine ointment, PVP-iodine gel, or PVP-iodine gauze. Less scientifically robust studies do, however, provide evidence of these substances’ toxicity, allergenicity, and iodine loading. Thus, in view of their questionable benefits and possible complications, these products are not recommended for use in the treatment of non-infected wounds.
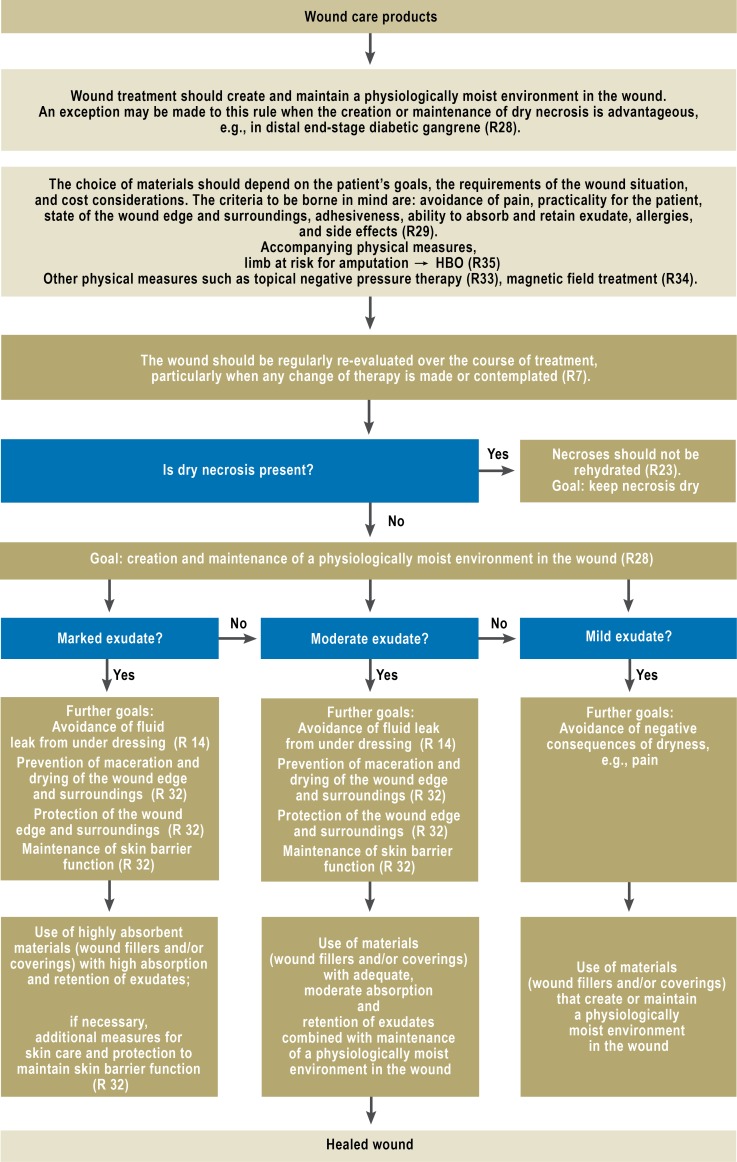
In general, the local treatment of wounds must be based on knowledge of the materials used and their proper application, including their indications and contraindications and their allergenic and toxic potential (Figure 3; eFigure 2).
Figure 3.
Algorithm for wound care products
R, recommendation; HBO, hyperbaric O2
eFigure 2.
Algorithm for wound coverings and ointments
R, recommendation
HBO, hyperbaric O2
Accompanying physical measures
Various physical techniques, including vacuum therapy, stimulating current, shock-wave therapy, and magnetic-field therapy, are said to accelerate wound healing.
In recent years, vacuum sealing has come into very widespread use. The evaluation of the evidence for this technique is based on several systematic reviews (23– 26).
The available data are insufficient to provide a basis for any clear recommendation in favor of vacuum sealing. The benefit of the technique has been demonstrated to date only with respect to surrogate variables such as reduction of wound size (standardized mean difference [SMD] 0.45; 95% CI 0.87–0.04) (26). Vacuum sealing can therefore be considered as an aid to wound dressing with the goal of a reduction of its depth or volume (grade 0 recommendation).
Magnetic-field therapy can be considered for the accompanying treatment of venous ulcers; here, however, the recommendation (grade 0) is based only on low-grade evidence (cf. Table) (RR 2.025; 95% CI 1.055 to 2.768) (27). The optimal duration and frequency of application and the optimal field intensity have yet to be determined.
There is good evidence for the efficacy of whole-body pressure-chamber therapy (hyperbaric oxygen therapy, HBO) in the treatment of diabetic foot ulcers for which other forms of treatment have not brought about complete healing (RR 2.14; 95% CI 1.18-3.88) (28). Moderately good evidence (Table) supports therapeutic benefit with respect to the endpoint that is perhaps most important to patients, i.e., reduction of the rate of major amputations (RR 0.31; 95% CI I 0.13-0.71) (29). On the basis of these data, the guideline contains a grade B recommendation that hyperbaric oxygen therapy should be used as an additional treatment option for patients with diabetic foot syndrome in whom all possible revascularization measures have been tried without success and the danger remains that the limb may have to be amputated.
No RCTs of adequate quality are available to support any evidence-based judgment of the putative clinical utility of ultrasound therapy, water-filtered infrared A light, low-energy lasers, or shock-wave therapy.
Care of patients with chronic wounds by caregivers from multiple sectors and professions
The literature review that was performed for the guideline revealed moderately good evidence (30) that patients with chronic ulcers who receive combined and integrated treatment by caregivers from multiple sectors and professions experience a therapeutic benefit from such treatment, with improvement in endpoints that patients consider important, including quality of life, everyday activities, and pain. Although the results of randomized and controlled trials cannot necessarily be extrapolated to the German health-care system as a whole, there is similar evidence within the German health care system that structured care has, indeed, improved outcomes for diabetic patients (31, 32).
There was, therefore, a strong consensus among the experts for the following recommendation: “Patients should receive integrated treatment from caregivers from multiple sectors and professions, with the individual elements combined in a reasonable manner (grade B recommendation).” Examples of such elements include coordination by a single center, quality assurance, and cooperation across sectors and professions.
Strengths and limitations of this guideline
In the preparation of this guideline, the various methods of local treatment of chronic wounds were assessed for the first time ever with strict methods of evaluation and with the participation of all professions involved in wound care. The analysis of the evidence showed that it is entirely possible to carry out high-quality RCTs in this field (e.g., [21, 22, 28]; Figure 4).
Figure 4.
Evaluation of scientific evidence
R, recommendation; HTA, health technology assessment; RCT, randomized controlled trial
Nonetheless, the guideline group found only very few RCTs whose methods were sound enough to make their conclusions robust, perhaps because the certification procedures for medicinal products in this field do not yet require any proof of therapeutic benefit (33). Graded recommendations (A, B, or O) are given in this guideline only where supported by adequate evidence from randomized, controlled studies or meta-analyses. Wherever this is not the case, the guideline makes statements and good clinical practice recommendations rather than graded recommendations.
High-quality evidence allowing a clear, positive recommendation (i.e., at least grade B) was found to exist only for HBO therapy and for integrated care strategies. In all cases where insufficient evidence was available from randomized controlled trials, the guideline group took care to formulate its recommendations cautiously and responsibly. Whenever an expert consensus could be obtained, criteria are given in the form of good clinical practice recommendations and statements, which are intended to serve as a practical aid to clinicians in combination with the highly detailed discussion of the evidence found in the guideline. The guideline should help make the treatment of wounds an area of well-founded medical expertise, in which clinical practice is based on scientific evidence and interdisciplinary collaboration rather than on supposed knowledge derived principally from advertising.
The guideline itself has been published on the website of the AWMF (in German). A short version is now in preparation.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Dr. Rüttermann and Dr. Maier-Hasselmann state that they have no conflict of interest.
M. Burckhardt has received honoraria for lecturing at continuing education presentations of the Transfernetzwerk Bildung and the PFAD-Akademie (two private associations providing continuing education for health professionals).
B. Nink-Grebe has received funding from the Medi company for performing sponsored clinical trials on its behalf and for a study that she initiated concerning compression therapy.
References
- 1.Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients’ quality of life. Health QualLife Outcomes. 2007;5 doi: 10.1186/1477-7525-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller-Buhl U, Leutgeb R, Bungartz J, Szecsenyi J, Laux G. Expenditure of chronic venous leg ulcer management in German primary care: results from a population-based study. Int Wound J. 2012 Feb 28; doi: 10.1111/j.1742-481X.2012.00942.x. doi: 10.1111/j.1742-481X.2012.00942.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Persoon A, Heinen MM, van der Vleuten CJ, de Rooij MJ, van de Kerkhof PC, van AT Leg ulcers: a review of their impact on daily life. JClinNurs. 2004;13:341–354. doi: 10.1046/j.1365-2702.2003.00859.x. [DOI] [PubMed] [Google Scholar]
- 4.Deutsches Netzwerk für Qualitätsentwicklung in der Pflege (DNQP) Osnabrück. 2008. Expertenstandard zur Pflege von Menschen mit chronischen Wunden. [Google Scholar]
- 5.Gonzalez-Consuegra RV, Verdu J. Quality of life in people with venous leg ulcers: an integrative review. J Adv Nurs. 2011;67:926–944. doi: 10.1111/j.1365-2648.2010.05568.x. [DOI] [PubMed] [Google Scholar]
- 6.Briggs M, Flemming K. Living with leg ulceration: a synthesis of qualitative research. JAdvNurs. 2007;59:319–328. doi: 10.1111/j.1365-2648.2007.04348.x. [DOI] [PubMed] [Google Scholar]
- 7.Bosquanet N FP. The new international challenge. 1996. Venous Disease; pp. 6–9. [Google Scholar]
- 8.Leitlinien der Deutschen Gesellschaft für Phlebologie. AWMF online. 2010. Diagnostik und Therapie des Ulcus cruris venosum. [Google Scholar]
- 9.Lawall H, Diehm C. Diagnostik und Therapie der peripheren arteriellen Verschlusskrankheit (PAVK) S3-LL der Deutschen Gesellschaft für Angiologie. Bearbeitungsstand. www.leitlinien.de. 2009 Apr 27; [Google Scholar]
- 10.German Agency for Quality in Medicine (ÄZQ) Edited by Gin. Diabetic foot - prevention and therapy 60. 2008. Type-2-diabetes 2006 - National disease management guideline. [Google Scholar]
- 11.Maier-Hasselmann A, Wilm S. Epidemiologie. In: S3-Leitlinie: Lokaltherapie chronischer Wunden bei Patienten mit den Risiken periphere arterielle Verschlusskrankheit, Diabetes mellitus, chronisch venöse Insuffizienz. Edited by Deutsche Gesellschaft für Wundheilung und Wundbehandlung e.V. 2012: 1-287 [Google Scholar]
- 12.Laible J, Mayer H, Evers GC. Prevalence of ulcus cruris in home care nursing. An epidemiological study in North Rhine-Westphalia. Pflege. 2002;15:16–23. doi: 10.1024/1012-5302.15.1.16. [DOI] [PubMed] [Google Scholar]
- 13.Morbach S, Furchert H, Groblinghoff U, et al. Long-Term prognosis of diabetic foot patients and their limbs: Amputation and death over the course of a decade. Diabetes Care. 2012;35:2021–2027. doi: 10.2337/dc12-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deutsches Instrument zur methodischen Leitlinien-Bewertung (DELBI) Fassung 2005/2006 + Domäne 8 2008. www.leitlinien.de/leitlinienmethodik/leitlinienbewertung/delbi.
- 15.Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an american college of chest physicians task force. Chest. 2006;129:174–181. doi: 10.1378/chest.129.1.174. [DOI] [PubMed] [Google Scholar]
- 16.Burckhardt M, Gregor S, Kleijnen J, et al. Leitlinienreport S3-Leitlinie Lokaltherapie chronischer Wunden bei Patienten mit den Risiken periphere arterielle Verschlusskrankheit, Diabetes mellitus, chronische venöse Insuffizienz. AWMF. 2012 [Google Scholar]
- 17.d’Hemecourt PA, Smiell JM, Karim MR. Sodium carboxymethylcellulose aqueous-based gel vs becaplermin gel in patients with nonhealing lower extremity diabetic ulcers. Wounds: A compendium of clinical research & practice. 1998;10:69–75. [Google Scholar]
- 18.Jensen JL, Seeley J, Gillin B. Diabetic foot ulcerations. A controlled, randomized comparison of two moist wound healing protocols: Carrasyn Hydrogel Wound dressing and wet-to-moist saline gauze. AdvWound Care. 1998;11:1–4. [PubMed] [Google Scholar]
- 19.Jull A, Walker N, Parag V, Molan P, Rodgers A. Randomized clinical trial of honey-impregnated dressings for venous leg ulcers. Br J Surg. 2008;95:175–182. doi: 10.1002/bjs.6059. [DOI] [PubMed] [Google Scholar]
- 20.Gethin G, Cowman S. Bacteriological changes in sloughy venous leg ulcers treated with manuka honey or hydrogel: an RCT. J Wound Care. 2008;17:241–247. doi: 10.12968/jowc.2008.17.6.29583. [DOI] [PubMed] [Google Scholar]
- 21.Dumville JC, Worthy G, Soares MO, et al. VenUS II: a randomised controlled trial of larval therapy in the management of leg ulcers. Health Technology Assessment. 2009;13:1–206. doi: 10.3310/hta13550. [DOI] [PubMed] [Google Scholar]
- 22.Jeffcoate WJ, Price PE, Phillips CJ, et al. Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes. Health Technology Assessment. 2009;13 doi: 10.3310/hta13540. [DOI] [PubMed] [Google Scholar]
- 23.Peinemann F, Sauerland S. Negative-pressure wound therapy: systematic review of randomized controlled trials. Dtsch Arztebl Int. 2011;108(22):381–389. doi: 10.3238/arztebl.2011.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medical Advisory Secretariat Ontario Health Technology Advisory Committee. Vacuum assisted closure therapy for wound closure. Toronto, Ontario: Ontario Ministry of Health Long-term Care; 2006. [Google Scholar]
- 25.Ubbink DT, Westerbos SJ, Nelson EA, Vermeulen H. A systematic review of topical negative pressure therapy for acute and chronic wounds. BrJ Surg. 2008;95:685–692. doi: 10.1002/bjs.6238. [DOI] [PubMed] [Google Scholar]
- 26.Institute for Quality Efficiency in Health Care. Final report NO4-03. 4/2006. Vakuumversiegelung von Wunden. [Google Scholar]
- 27.Jeran M, Zaffuto S, Moratti A, Bagnacani M, Cadossi R. Pemf stimulation of skin ulcers of venous origin in humans preliminary report of a double blind study. Electromagnetic Biology and Medicine. 1987;6:181–188. [Google Scholar]
- 28.Londahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care. 2010;33:998–1003. doi: 10.2337/dc09-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kranke P, Bennett M. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database of Systematic Reviews: Reviews. 2004;(1) doi: 10.1002/14651858.CD004123.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Edwards H, Courtney M, Finlayson K, Shuter P, Lindsay E. A randomised controlled trial of a community nursing intervention: improved quality of life and healing for clients with chronic leg ulcers. Journal of Clinical Nursing. 2009;18:1541–1549. doi: 10.1111/j.1365-2702.2008.02648.x. [DOI] [PubMed] [Google Scholar]
- 31.Stock S, Drabik A, Buscher G, et al. German diabetes management programs improve quality of care and curb costs. Health Aff (Millwood) 2010;29:2197–2205. doi: 10.1377/hlthaff.2009.0799. [DOI] [PubMed] [Google Scholar]
- 32.Dorresteijn JA, Kriegsman DM, Valk GD. Complex interventions for preventing diabetic foot ulceration. CochraneDatabaseSystRev. 2010;1 doi: 10.1002/14651858.CD007610.pub2. CD007610. [DOI] [PubMed] [Google Scholar]
- 33.Burckhardt M, Meyer G. Die Leitlinie zur Lokaltherapie chronischer Wunden. Vertrauenswürdige Praxishilfe. Pflegezeitschrift. 2012;65:326–328. [PubMed] [Google Scholar]
- 34.Kunz R, Burnand B, Schünemann HJ. The GRADE System. An international approach to standardize the graduation of evidence and recommendations in guidelines. Der Internist. 2008;49:673–680. doi: 10.1007/s00108-008-2141-9. [DOI] [PubMed] [Google Scholar]