Abstract
Background
Systemic alterations in arginine bioavailability occur in heart failure (HF) patients with more advanced myocardial dysfunction and poorer clinical outcomes, and improve with beta-blocker therapy.
Methods and Results
We measured fasting plasma levels of L-arginine and related biogenic amine metabolites in 138 stable symptomatic HF patients with left ventricular ejection fraction (LVEF) ≤35% and comprehensive echocardiographic evaluation. Long-term adverse clinical outcomes (death and cardiac transplantation) were followed for 5 years. Lower global arginine bioavailability ratio (GABR, ratio of L-arginine to L-ornithine + L-citrulline) was associated with higher plasma natriuretic peptide levels, more advanced left ventricular diastolic dysfunction, and more severe right ventricular systolic dysfunction (all p<0.001). Patients taking beta-blockers had significantly higher GABR than those off beta-blockers (0.86 [IQR 0.68–1.17] versus 0.61 [0.44–0.89], p<0.001). Subjects with higher GABR experienced fewer long-term adverse clinical events (Hazard Ratio: 0.61 [95%CI 0.43–0.84], p=0.002). In an independent beta-blocker naïve patient cohort, GABR increased following long-term (6 month) beta-blocker therapy (0.89[0.52–1.07] to 0.97 [0.81–1.20], p=0.019).
Conclusions
In patients with chronic systolic heart failure, diminished global L-arginine bioavailability is associated with more advanced myocardial dysfunction and poorer long-term adverse clinical outcomes. GABR levels improved with beta-blocker therapy.
Keywords: heart failure, arginine bioavailability, nitric oxide, natriuretic peptide, prognosis
INTRODUCTION
Endogenous nitric oxide (NO) production plays an important role in the pathophysiology of heart failure1. Despite the many links between endogenous NO and myocardial performance in both normal and failing hearts, precise measurements of NO have not been performed as part of clinical care. This is in part because like many endocrine systems, absolute levels of individual metabolites may not adequately capture the overall metabolic state of these pathways involved. L-arginine is the key substrate of nitric oxide production, and is predominately synthesized in the kidney and liver. The relationships between L-arginine and its products, L-ornithine, and L-citrulline, are complex (Figure 1). Arginases convert L-arginine into urea and L-ornithine, and nitric oxide synthases convert L-arginine into L-citrulline during production of NO. As enzymatic products generated from L-arginine, both L-ornithine and L-citrulline in turn serve as precursors in the synthesis of L-arginine. Hence, diminished availability of L-arginine can be due to either reduced L-arginine transport into the myocardium2 or augmented catabolic pathways, leading to relative L-arginine depletion and concomitant elevated levels of its catalytic products including L-ornithine and L-citrulline. Therefore, the better approximation of the overall balance of functional L-arginine levels can be reflected by steady-state systemic L-arginine bioavailability relative to the degree of L-arginine consumption via the catabolic enzymes arginases and NO synthases. Recognizing this concept, an inverse relationship between measured plasma and red blood cell arginase activity and L-arginine to L-ornithine ratio has been demonstrated in patients with sickle-cell anemia3. In the same study, the L-arginine to [L-ornithine + L-citrulline] ratio (which represents the overall balance between substrate availability of L-arginine and its catabolism by arginase and nitric oxide synthases) have shown to provide important prognostic information3. Therefore, mechanistic insights into the dysregulation of arginine metabolism can be identified and quantified by these steady-state ratios.
Figure 1. Pathway of L-arginine metabolism by nitric oxide synthase and arginase.
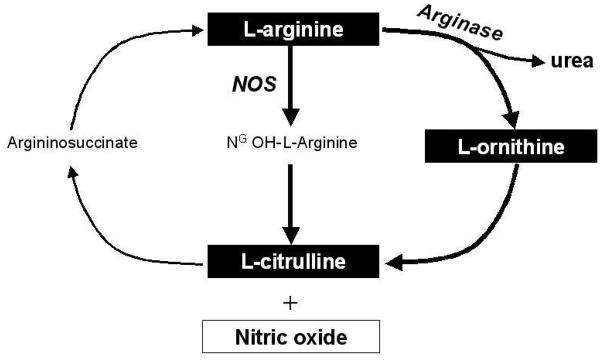
Abbreviations: NOS = nitric oxide synthase
Whether dysregulation of NO and arginine metabolism are intrinsic to heart failure and provide clinically relevant and prognostically valuable information relative to myocardial function and cardiovascular risk is unknown. We therefore sought to examine whether diminished systemic global L-arginine bioavailability ratio (GABR) can reflect a metabolic defect in chronic heart failure, and if so, whether there is prognostic significance in assessing L-arginine bioavailability in this population.
METHODS
Study Design and Subject Population
We analyzed plasma samples from 138 ambulatory patients with stable (>3 months duration) but symptomatic (New York Heart Association [NYHA] functional class II to III) chronic systolic heart failure with available plasma samples between May 1, 2001, and June 30, 2003 as part of the single-center, prospective Assessment of Doppler Echocardiography on Prognosis and Therapy (ADEPT) study. Eligible subjects were 18 to 75 years of age, with left ventricular ejection fraction (LVEF) ≤35%. Subjects were excluded if they had significant primary valvular diseases, or significant hepatic (liver enzymes >5x upper limit of normal) or renal dysfunction (serum creatinine >3.0 mg/dL). Clinical events (all-cause death or cardiac transplantation) were followed for 5 years by telephone follow-up and chart review, with no patients lost to follow-up in this cohort. To compare with non-HF GABR values, blood samples from 73 apparently healthy, age- and gender-matched controls (age of control vs HF cohort: 56±13 vs 56±13, p=0.97; male gender prevalence in control vs HF cohort: 44 [60%] vs 44 [60%], p=1.00) were collected. To test the hypothesis that GABR can be modulated by beta-blocker therapy, we performed serial blood draw at baseline and 6-month follow-up in patients with chronic systolic heart failure (LVEF ≤40%) who were initiating beta-blocker. The Cleveland Clinic Institutional Review Board approved both studies and all subjects gave informed consent.
Sample preparation and analysis of L-arginine metabolites
Plasma samples were obtained after informed consent and were stored at −80°C until analysis. A total of 100 μL of EDTA plasma was combined with 100 μL of 10μM [13C6] arginine in water (internal standard) and mixed by vortexing. The solution was immediately diluted with 550 μL of acetonitrile to precipitate protein. The resulting suspension was centrifuged at 3000 rpm for 15 minutes at 4°C. The supernatant was then transferred via pipette to a labeled 13×100 mm glass test tube and concentrated to dryness using a vortex evaporator. The residue was dissolved in 200 μL of 50% methanol water. 10 μL of the sample solution was injected onto a HPLC column and the levels of arginine and related biogenic amine metabolites quantified by LC/ESI/MS/MS analysis using an API 365 triple quadrupole mass spectrometer (Applied Biosystems, Foster, CA) with Ionics EP 10+ upgrade (Concord, Ontario, CA) interfaced to a Cohesive Technologies Aria LX Series HPLC multiplexing system (Franklin, MA). The amino acids were separated with a 250 × 4.6 mm Rexchrom S5–100-P phenyl column (Product number 728207 Regis Chemical, Morton Grove, IL, USA) equipped with a 1 mm × 15 mm Optimize technologies Opti-Guard Phenyl guard column (part number 10-02-00018 Optimize Technologies, Oregon City, OR, USA). The solvents used were 0.1% formic acid and 10 mM ammonium formate in water (solvent A) and 0.1% formic acid and 10 mM ammonium formate in methanol (solvent B). The gradient used was as follows: the column was first equilibrated with 100% A at 800 μL/min and held at this composition for 0.5 minutes after the injection; a linear gradient was then run to 50% B (50% A) over the next three minutes and held at 50% B for 3.5 minutes at a flow rate of 800 μL/min. At 6.5 minutes the flow rate was increased to 1000 μL/min and the solvent composition was changed to 100% B in a linear fashion over 1 minute. A linear gradient was then run to 100% A at 1000 μL/min over 0.5 minutes and held at this composition and flow rate for 6 minutes. The 8.5-minute duration data window was started at 3.5 minutes after the injection. Mass spectrometric analyses were performed online using electrospray ionization tandem mass spectrometry in the positive ion mode with multiple reaction monitoring. Cone potentials and collision energy were optimized for each analyte. Each analyte monitored demonstrated nearly quantitative recovery, good linearity over multiple orders of magnitude in the concentration range, and intra-assay and inter-assay coefficients of variance <10%. GABR is the ratio of L-arginine to the sum of L-ornithine plus L-citrulline. Estimated glomerular filtration rate (eGFR) was determined by the Modification of Diet in Renal Disease equation. Plasma A-type and B-type natriuretic peptides were analyzed using validated research-based radioimmuoassays performed at the Christchurch Cardioendocrine Research Group as previously reported4, with values slightly lower than that of commercially available assays).5, 6 Plasma myeloperoxidase (MPO) was measured by a sandwich enzyme-linked immunosorbent assay (PrognostiX Inc, Cleveland OH), and high-sensitivity C-reactive protein (hsCRP) was measured by the particle-enhanced immunonephelometry assay (Dade Behring Inc, Deerfield IL), both previously reported in this study cohort7, 8
Transthoracic Echocardiography
Details of transthoracic echocardiography protocol in the ADEPT study have been previously described4. Comprehensive transthoracic echocardiography was performed using commercially available HDI 5000 (Phillips Medical Systems, N.A., Bothell, Washington) and Acuson Sequoia (Siemens Medical Solutions USA Inc., Malvern, Pennsylvania) machines. Two-dimensional and color Doppler imaging was performed in standard parasternal and apical views. Diastolic indices (including pulse-wave Doppler, color M-mode and tissue Doppler imaging) were acquired over ten consecutive beats using sweep speeds of 50 and 100 cm/s using previously described techniques4, 9. Measurements were averaged over three cycles (five cycles for atrial fibrillation), and two experienced individuals who were blinded from the plasma metabolite data made all measurements. The left ventricular ejection fraction (LVEF) and cardiac volumes were measured using Simpson’s biplane method. Assessment of diastolic performance was primarily presented as the ratio of mitral E wave to tissue Doppler mitral septal E′ (E/E′), which had been demonstrated to provide consistent prognostic information9, 10. The degrees of severity of right ventricular systolic dysfunction (RVSD) were determined semi-quantitatively from 0 to 4+, with 3–4 denoted as moderate to severe. Estimated right atrial pressure (RAP) and right ventricular systolic pressure (RVSP) were estimated according to the 2010 American Society of Echocardiography Guidelines for the Echocardiographic Assessment of the Right Heart in Adults11.
Statistical Analysis
Continuous, normally distributed variables are summarized as mean ± standard deviation, while non-normally distributed variables are summarized as median and inter-quartile range (IQR). Clinical variables were compared between groups by Student’s t-test and chi-square test, and Wilcoxon test for variables not normally distributed. Associations of GABR with echocardiographic and clinical indexes were assessed by Spearman’s rank correlation method. The Kruskal-Wallis test (rank sums) was used for comparing ANP and BNP levels across GABR quartiles. The Cox proportional hazards model was used to assess the clinical risks of increasing continuous, standardized increments of GABR using death or cardiac transplantation as predicted outcome. Adjustments for known clinical risk factors of adverse events were added pairwise into the Cox model with GABR and with standard deviation increments. Kaplan-Meier survival plots were calculated from baseline to time of the first adverse clinical event (death or cardiac transplantation) with a follow-up period of 5 years. All statistical analyses were performed using SAS 9.1 and JMP 9.0.0 (SAS Institute, Cary, NC). The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Table 1 shows the baseline characteristics of the study population. In the overall cohort, the median plasma levels of L-arginine, L-ornithine, and L-citrulline were 41.6 μM [IQR: 36.2, 52.8], 20.2 μM [IQR: 15.4, 29.1], and 33.2 μM [IQR: 26.7, 46.3], respectively. The median GABR was 0.77 [IQR: 0.56, 1.06]. In comparison with healthy controls, GABR levels were lower in patients with chronic systolic HF (0.71 [95% CI: 0.50, 0.90] versus 0.92 [0.72, 1.09], p<0.001).
Table 1.
Baseline characteristics of study population (n=138)
| Variable | Value |
|---|---|
| Demographics: | |
| Mean age (years) | 58 ± 14 |
| Male gender, n (%) | 106 (77) |
| African American, n (%) | 22 (16) |
| NYHA class ≥III, n (%) | 44 (32) |
| Ischemic etiology, n (%) | 57 (42) |
| Echocardiographic indices: | |
| Mean LV ejection fraction (%) | 26 ± 6 |
| Mitral E/TDI septal E′ | 19 ± 12 |
| LV end-diastolic volume index (mL/m2) | 112 ± 35 |
| RV systolic dysfunction class ≥ 3+, n (%) | 36 (26) |
| Co-morbidities: | |
| Hypertension, n (%) | 76 (57) |
| Diabetes mellitus, n (%) | 41 (30) |
| Medications: | |
| ACE inhibitors and/or angiotensin receptor blockers, n (%) | 126 (94) |
| Beta-blockers, n (%) | 81 (60) |
| Spironolactone, n (%) | 36 (28) |
| Loop diuretics, n (%) | 105 (78) |
| Christchurch BNP (pM, median [IQR]): | 66 [27, 146] |
| eGFR (mL/min/1.73m2) | 71 ± 26 |
Abbreviations: NYHA = New York Heart Association functional class; LV = left ventricular; TDI = tissue Doppler imaging; RV = right ventricular; ACE = angiotensin converting enzyme; ANP = A-type natriuretic peptide; BNP = B-type natriuretic peptide; eGFR = estimated glomerular filtration rate; IQR = inter-quartile range
In our study cohort of 138 chronic systolic HF patients, patients with GABR above median levels were more likely to be male (93% versus 61%, p <0.01) and were more likely to have underlying ischemic etiology of heart failure (51% versus 33%, p =0.04). Compared to patients with preserved renal function, patients with moderate renal insufficiency (eGFR <60 mL/min/1.73m2) demonstrated lower GABR (0.64 [IQR: 0.43–0.87] versus 0.83 [IQR: 0.59, 1.17], p =0.001). GABR levels did not differ according to presence of diabetes mellitus (p=0.55) or between cachectic and non-cachectic patients (p=0.97).
Patients taking beta-blockers have significantly higher GABR than those not taking beta-blockers at the time of blood draw (GABR: 0.86 [IQR: 0.68, 1.17] versus 0.61 [IQR: 0.44, 0.89], respectively; both p <0.001). It is noteworthy that median L-arginine levels were similar between those taking beta-blockers versus those not on beta-blockers (41.8 μM [IQR: 34.9, 53.6] versus 41.3 μM [IQR: 36.6, 49.4], p =0.665). Furthermore, in the independent cohort of 17 subjects with chronic systolic HF who were beta-blocker naïve, there was a statistically-significant increase in GABR following long-term (6-month) beta-blocker therapy (from 0.89 [0.52, 1.07] to 0.97 [95% CI: 0.81, 1.20], p=0.019).
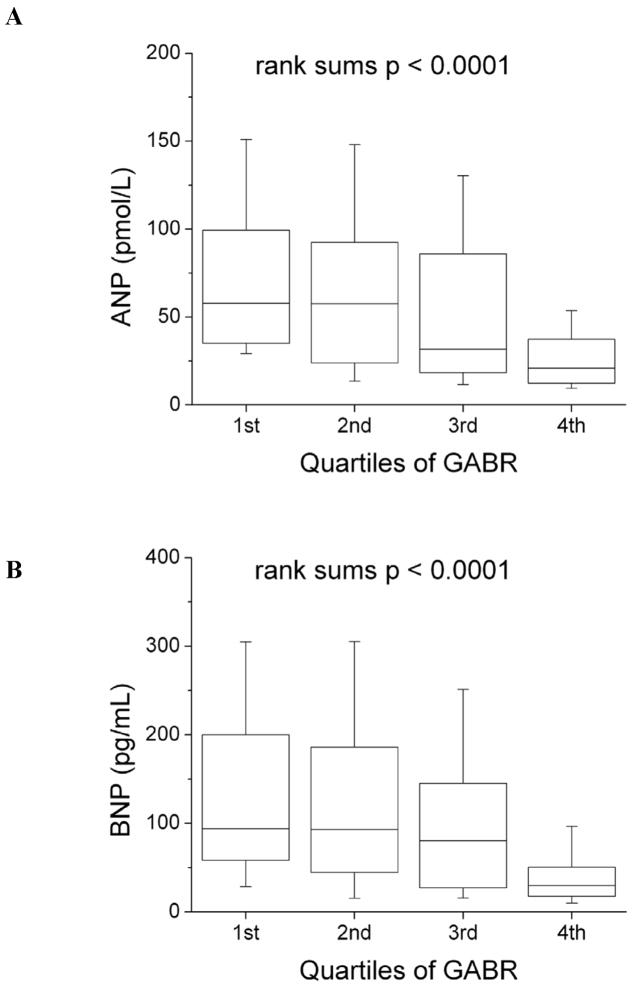
Table 2 illustrates the relationship between L-arginine bioavailability to biochemical and echocardiographic indices of cardiac dysfunction. Plasma natriuretic peptide levels increased with reducing quartiles of GABR (Figure 2). Lower GABR levels were also associated with more advanced diastolic dysfunction, right ventricular systolic dysfunction, but not LV systolic dysfunction or LV dimensions (Table 2). GABR levels were lower in patients with elevated estimated RAP (15 mm Hg) (0.70 [0.47, 0.79] versus 0.87 [0.60, 1.13], p=0.001), and elevated estimated RVSP (≥40 mmHg) (0.59 [0.41, 0.79] versus 0.87 [0.60, 1.20], p<0.001). However, there were no significant correlations between GABR and inflammatory biomarkers such as MPO or hsCRP (Table 2). There were no correlations between L-arginine and any biochemical or echocardiographic indices.
Table 2.
Univariate relationships of biochemical and echocardiographic indexes to global arginine bioavailability ratio (GABR).
| Variable | Spearman’s correlation coefficient | p-value |
|---|---|---|
| Clinical Variables | ||
| ANP | − 0.46 | <0.001 |
| BNP | − 0.40 | <0.001 |
| MPO | − 0.06 | 0.522 |
| hsCRP | − 0.03 | 0.765 |
| eGFR | 0.18 | 0.059 |
| Echocardiographic Variables | ||
| LV ejection fraction | 0.12 | 0.178 |
| LV end-diastolic volume index | − 0.04 | 0.681 |
| LV end-systolic volume index | − 0.06 | 0.520 |
| RV systolic pressure | − 0.32 | <0.001 |
| Mitral E/TDI septal E′ | − 0.31 | <0.001 |
Abbreviations: ANP = atrial type natriuretic peptide; BNP = B-type natriuretic peptide; MPO = myeloperoxidase; hsCRP = high-sensitivity C-reactive protein; eGFR = estimated glomerular filtration rate; LV = left ventricular; RV = right ventricular; TDI = tissue Doppler imaging).
Figure 2. Relationship of L-arginine bioavailability and natriuretic peptide levels according to quartiles of global arginine bioavailability ratio (GABR).
Abbreviations: ANP = atrial natriuretic peptide; BNP = B-type natriuretic peptide
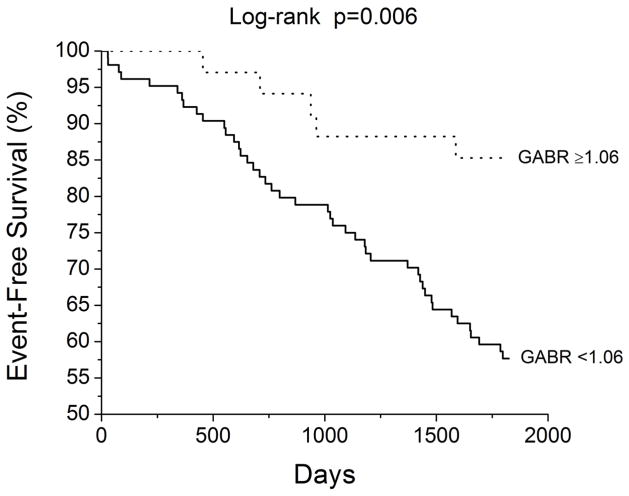
A total of 49 adverse clinical events (death or cardiac transplantation) occurred during a follow-up period of 5 years. According to Cox proportional hazards analysis, higher GABR confers lower unadjusted risk for adverse events, and after adjustment for age, gender, eGFR, beta-blocker use, diabetes mellitus, and echocardiographic measures of cardiac structure and function (Table 3). Figure 3 illustrates Kaplan-Meier survival analysis stratified by high (fourth quartile: GABR≥1.06) and low (first through third quartiles: GABR<1.06) GABR levels similar to previous reports3. In contrast, there was no correlation between absolute plasma L-arginine levels and adverse clinical outcomes.
Table 3.
Multivariate hazard ratios for predicting adverse clinical events for increments in global arginine bioavailability ratio (GABR).
| Variable | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Unadjusted (49 events)* | 0.61 (0.43 – 0.84) | 0.002 |
| Adjusted for Age (years) | 0.60 (0.42 – 0.84) | 0.002 |
| Adjusted for LV ejection fraction (%) | 0.62 (0.43 – 0.86) | 0.004 |
| Adjusted for eGFR (mL/min) | 0.64 (0.44 – 0.90) | 0.009 |
| Adjusted for Diabetes mellitus | 0.62 (0.44 – 0.84) | 0.002 |
| Adjusted for Beta-blocker use | 0.64 (0.44 – 0.89) | 0.007 |
| Adjusted for Mitral E/TDI septal E′ ratio | 0.60 (0.41 – 0.84) | 0.002 |
| Adjusted for elevated RA pressure (15 mmHg) | 0.61 (0.40 – 0.88) | 0.008 |
| Adjusted for RV systolic pressure (mmHg) | 0.57 (0.36 – 0.85) | 0.005 |
| Adjusted for LVEDVi (mL/m2) | 0.60 (0.41 – 0.84) | 0.002 |
| Multivariable model (49 events): | ||
| GABR* | 0.64 (0.43 – 0.93) | 0.019 |
| Age (years)* | 0.91 (0.66 – 1.26) | 0.564 |
| Male gender | 0.80 (0.38 – 1.83) | 0.578 |
| Beta-blocker use | 0.73 (0.39 – 1.35) | 0.309 |
| eGFR (mL/min/1.73m2)* | 0.90 (0.66 – 1.24) | 0.508 |
| Multivariable model (49 events): | ||
| GABR* | 0.52 (0.32 – 0.83) | 0.005 |
| Age (years)* | 1.12 (0.78 – 1.61) | 0.548 |
| LV ejection fraction (%)* | 0.81 (0.56 – 1.18) | 0.269 |
| Mitral E/TDI septal E′* | 0.68 (0.37 – 1.13) | 0.152 |
| RV systolic pressure (mmHg)* | 1.61 (1.12 – 2.27) | 0.010 |
Hazard ratios per 1 standard deviation (SD) increments (1 SD for GABR = 0.38; 1 SD for Age = 13.5 years; 1 SD for eGFR = 25.8 mL/min/1.73m2; 1 SD for LVEF = 5.97%; 1 SD for E/septal E′ = 12.0; 1 SD for RVSP = 13.3 mmHg; 1 SD for LVEDVi = 35.0 mL/m2);
Abbreviation: LV = left ventricular; eGFR = estimated glomerular filtration rate; TDI = tissue Doppler imaging; RA, right atrial; RV = right ventricular; LVEDVi, left ventricular end-diastolic volume index.
Figure 3.
Kaplan-Meier analysis of long-term adverse clinical events stratified by fourth quartile (≥1.06) versus first through third quartile (<1.06) global arginine bioavailability ratio (GABR) levels.
DISCUSSION
We found that estimated L-arginine bioavailability was diminished in patients with increasing severity of chronic heart failure and directly associated with poorer long-term prognosis. Furthermore, our data suggest that the use of beta-blockers was associated with improvement in L-arginine bioavailability. In the aggregate, these findings provide mechanistic understanding of the relationship between L-arginine bioavailability and chronic heart failure, potentially as a mediator of underlying metabolic derangements leading to disease progression or as a product of adaptive and maladaptive responses to cardiac dysfunction. These new data supported the hypothesis that beta-blockade may favorably impact arginine and NO metabolic pathways as a potential mechanism for conferring their clinical benefits in the failing heart.
Our findings parallel results from our group regarding the prognostic value in GABR in patients undergoing elective coronary angiography12, 13, as well as more recently in patients admitted for acute decompensated heart failure14. These data also parallel observations in the sickle cell population, where lower L-arginine bioavailability is associated with greater disease severity3. Diminished plasma L-arginine bioavailability may be associated with underlying vascular dysfunction due to augmented “catabolic pathways” of L-arginine, leading to consumption of L-arginine as a substrate for NO production. Many of the variables associated with lower GABR appear to associate with worsening right-sided congestion leading to passive hepatic and renal congestion (potentially due to right ventricular systolic dysfunction). Since the kidney and liver are major sources of L-arginine, lower GABR may also be explained in part by sub-clinical reductions in L-arginine production during hepatic and renal congestion. Interestingly, direct assessment of plasma levels of L-arginine did not reveal any significant associations with hepatic or renal dysfunction. However, it is also important to emphasize that identifying an association between reduced L-arginine bioavailability and myocardial dysfunction does not establish a causal relationship between the two processes – it is equally likely that disease progression of heart failure may set in train a series of adaptive and maladaptive changes that can result in a decrease in the ratio of arginine to its metabolites.
Previous studies have demonstrated that myocardial expression of inducible nitric oxide synthase is heightened in the failing heart15, and plasma level of stable end-products of NO (nitrite and nitrate, NOx) can be elevated in patients with chronic heart failure16. Furthermore, elevated NOx has been associated with diastolic heart failure as well as restrictive filling patterns in patients with chronic systolic heart failure17, 18. Therefore, it has been assumed that excess NO can be detrimental to cardiac performance. However, the relationship between NOx production and nitric oxide (or L-arginine) availability is complex, and elevated plasma NOx has also been attributed to decreased renal clearance16. Previous studies also have demonstrated that in patients with chronic heart failure, there was a 30% reduction in the trans-cardiac extraction of exogenous [3H] L-arginine compared with healthy controls, suggesting that lower availability of intracellular L-arginine for myocardial NO production may be due to diminished L-arginine transport. Recent data have further suggested a role for arginase-II in modulating cardiac contractility in the failing myocardium19. However, we did not find any significant relationships between biomarkers of systemic inflammation and L-arginine bioavailability.
Incremental to prior reports, our data suggest that a global reduction of arginine bioavailability may be associated with diastolic dysfunction in patients with underlying systolic dysfunction. The exact mechanism is not clear, but it has long been recognized that patients with non-ischemic heart failure may still demonstrate myocardial metabolism-perfusion mismatch by positron emission tomography assessment20. Therefore, microvascular or regional ischemia from diminished substrate availability leading to NO deficiency may be present, leading to the presence of worsening diastolic dysfunction. GABR also was significantly associated with right-sided systolic dysfunction. This is consistent with recent observations that right-sided heart failure was associated with a metabolic defect21, 22, and the propensity for improvement with beta-adrenergic blockade following metabolic restoration23, 24.
Beta-blocker therapy may reduce sympathetic tone in heart failure, thereby relieving the heightened demand for L-arginine consumption in NO-dependent vasodilation25. We observed that at baseline beta-blocker use is associated with higher baseline GABR, and the notable improvement in GABR levels following initiation of beta-blockers is also consistent with this hypothesis. These data are intriguing and hypothesis-generating, but should be interpreted with caution as beta-blocker therapy was not randomly assigned in this descriptive association. Nevertheless, improvement of GABR associated with beta-blocker therapy in the independent cohort is consistent with previous findings in the setting of diabetes mellitus whereby multifactorial risk factor modification can be associated with improvement in GABR in those with less advanced disease states26. Hence the ability to monitor and detect such metabolic improvement as surrogate for therapeutic response warrants further investigations.
Study Limitations
There were no direct invasive or non-invasive vascular measures to demonstrate the functional consequences of diminished GABR. It is also likely that there are unknown confounding metabolic pathways that may affect the metabolite levels analyzed in our study, which can only be confirmed by stable isotope or radioisotope studies to account for competing arginine biosynthesis and degradation pathways. It is conceivable that underlying hepatic dysfunction may also alter the plasma sample measurements. Furthermore, our cohort was underpowered to definitively determine prognostic value of our measurements, and the plasma metabolite levels as well as outcomes could be altered by subsequent medical therapies despite a relatively uniform treatment approach in a single-center setting. Nevertheless, understanding the mechanistic underpinnings of metabolic and vascular abnormalities associated with heart failure is an important endeavor, since patients presenting with vascular dysfunction have significant disease burden and poor prognosis. Our observations highlight the feasibility of gaining insight into metabolic phenotypes of heart failure by measuring systemic levels of metabolites in the arginine-NO pathway, as well as the promise of targeting altered metabolic pathways of arginine metabolism in improving clinical outcomes of this devastating and costly disease. Further studies are needed to confirm the prognostic role of L-arginine bioavailability in predicting long-term clinical outcomes as observed in our chronic heart failure cohort.
CONCLUSION
In patients with chronic systolic heart failure, diminished global L-arginine bioavailability can be associated with more advanced left ventricular diastolic dysfunction, worse right ventricular systolic dysfunction, and poorer long-term adverse clinical outcomes.
Acknowledgments
FUNDING SOURCES
The original ADEPT study was funded by the American Society of Echocardiography, and received partial funding from GlaxoSmithKline Pharmaceuticals, and Roche Diagnostics Inc. This work was supported by National Institutes of Health grants P01HL076491, P01HL77107, P01HL098055, R01HL103866, R01HL70621, R01HL103931, and P20HL113452, Foundaton LeDucq, the American Heart Association Ohio Valley Affiliates (0465266B), and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439-06).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loyer X, Heymes C, Samuel JL. Constitutive nitric oxide synthases in the heart from hypertrophy to failure. Clin Exp Pharmacol Physiol. 2008;35:483–8. doi: 10.1111/j.1440-1681.2008.04901.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaye DM, Parnell MM, Ahlers BA. Reduced myocardial and systemic L-arginine uptake in heart failure. Circ Res. 2002;91:1198–203. doi: 10.1161/01.res.0000047506.52381.90. [DOI] [PubMed] [Google Scholar]
- 3.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Troughton RW, Prior DL, Pereira JJ, Martin M, Fogarty A, Morehead A, et al. Plasma B-type natriuretic peptide levels in systolic heart failure: importance of left ventricular diastolic function and right ventricular systolic function. J Am Coll Cardiol. 2004;43:416–22. doi: 10.1016/j.jacc.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 5.Yandle TG, Espiner EA, Nicholls MG, Duff H. Radioimmunoassay and characterization of atrial natriuretic peptide in human plasma. J Clin Endocrinol Metab. 1986;63:72–9. doi: 10.1210/jcem-63-1-72. [DOI] [PubMed] [Google Scholar]
- 6.Yandle TG, Richards AM, Gilbert A, Fisher S, Holmes S, Espiner EA. Assay of brain natriuretic peptide (BNP) in human plasma: evidence for high molecular weight BNP as a major plasma component in heart failure. J Clin Endocrinol Metab. 1993;76:832–8. doi: 10.1210/jcem.76.4.8473392. [DOI] [PubMed] [Google Scholar]
- 7.Tang WH, Shrestha K, Van Lente F, Troughton RW, Martin MG, Borowski A, et al. Usefulness of C-Reactive Protein and Left Ventricular Diastolic Performance for Prognosis in Patients With Left Ventricular Systolic Heart Failure. Am J Cardiol. 2007 doi: 10.1016/j.amjcard.2007.08.038. in press. [DOI] [PubMed] [Google Scholar]
- 8.Tang WH, Tong W, Troughton RW, Martin MG, Shrestha K, Borowski A, et al. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007;49:2364–70. doi: 10.1016/j.jacc.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 9.Troughton RW, Prior DL, Frampton CM, Nash PJ, Pereira JJ, Martin M, et al. Usefulness of tissue doppler and color M-mode indexes of left ventricular diastolic function in predicting outcomes in systolic left ventricular heart failure (from the ADEPT study) Am J Cardiol. 2005;96:257–62. doi: 10.1016/j.amjcard.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 10.Yu CM, Sanderson JE, Marwick TH, Oh JK. Tissue Doppler imaging a new prognosticator for cardiovascular diseases. J Am Coll Cardiol. 2007;49:1903–14. doi: 10.1016/j.jacc.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 11.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 86–8. [DOI] [PubMed] [Google Scholar]
- 12.Tang WH, Wang Z, Cho L, Brennan DM, Hazen SL. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol. 2009;53:2061–7. doi: 10.1016/j.jacc.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sourij H, Meinitzer A, Pilz S, Grammer TB, Winkelmann BR, Boehm BO, et al. Arginine bioavailability ratios are associated with cardiovascular mortality in patients referred to coronary angiography. Atherosclerosis. 2011;218:220–5. doi: 10.1016/j.atherosclerosis.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 14.Shao Z, Wang Z, Shrestha K, Thakur A, Borowski AG, Sweet W, et al. Pulmonary hypertension associated with advanced systolic heart failure: dysregulated arginine metabolism and importance of compensatory dimethylarginine dimethylaminohydrolase-1. J Am Coll Cardiol. 2012;59:1150–8. doi: 10.1016/j.jacc.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haywood GA, Tsao PS, von der Leyen HE, Mann MJ, Keeling PJ, Trindade PT, et al. Expression of inducible nitric oxide synthase in human heart failure. Circulation. 1996;93:1087–94. doi: 10.1161/01.cir.93.6.1087. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein RD, Zhang X, Zhao G, Forfia P, Tuzman J, Ochoa F, et al. Mechanisms of nitrate accumulation in plasma during pacing-induced heart failure in conscious dogs. Nitric Oxide. 1997;1:386–96. doi: 10.1006/niox.1997.0150. [DOI] [PubMed] [Google Scholar]
- 17.Yu CM, Fung PC, Chan G, Lai KW, Wang Q, Lau CP. Plasma nitric oxide level in heart failure secondary to left ventricular diastolic dysfunction. Am J Cardiol. 2001;88:867–70. doi: 10.1016/s0002-9149(01)01894-x. [DOI] [PubMed] [Google Scholar]
- 18.Saitoh M, Osanai T, Kamada T, Matsunaga T, Ishizaka H, Hanada H, et al. High plasma level of asymmetric dimethylarginine in patients with acutely exacerbated congestive heart failure: role in reduction of plasma nitric oxide level. Heart Vessels. 2003;18:177–82. doi: 10.1007/s00380-003-0715-y. [DOI] [PubMed] [Google Scholar]
- 19.Steppan J, Ryoo S, Schuleri KH, Gregg C, Hasan RK, White AR, et al. Arginase modulates myocardial contractility by a nitric oxide synthase 1-dependent mechanism. Proc Natl Acad Sci U S A. 2006;103:4759–64. doi: 10.1073/pnas.0506589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Neill JO, McCarthy PM, Brunken RC, Buda T, Hoercher K, Young JB, et al. PET abnormalities in patients with nonischemic cardiomyopathy. J Card Fail. 2004;10:244–9. doi: 10.1016/j.cardfail.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Piao L, Marsboom G, Archer SL. Mitochondrial metabolic adaptation in right ventricular hypertrophy and failure. J Mol Med (Berl) 2010;88:1011–20. doi: 10.1007/s00109-010-0679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redout EM, Wagner MJ, Zuidwijk MJ, Boer C, Musters RJ, van Hardeveld C, et al. Right-ventricular failure is associated with increased mitochondrial complex II activity and production of reactive oxygen species. Cardiovasc Res. 2007;75:770–81. doi: 10.1016/j.cardiores.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Bogaard HJ, Natarajan R, Mizuno S, Abbate A, Chang PJ, Chau VQ, et al. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med. 2010;182:652–60. doi: 10.1164/rccm.201003-0335OC. [DOI] [PubMed] [Google Scholar]
- 24.Tatli E, Kurum T, Aktoz M, Buyuklu M. Effects of carvedilol on right ventricular ejection fraction and cytokines levels in patients with systolic heart failure. Int J Cardiol. 2008;125:273–6. doi: 10.1016/j.ijcard.2007.07.166. [DOI] [PubMed] [Google Scholar]
- 25.Fukuchi M, Hussain SN, Giaid A. Heterogeneous expression and activity of endothelial and inducible nitric oxide synthases in end-stage human heart failure: their relation to lesion site and beta-adrenergic receptor therapy. Circulation. 1998;98:132–9. doi: 10.1161/01.cir.98.2.132. [DOI] [PubMed] [Google Scholar]
- 26.Tripolt NJ, Meinitzer A, Eder M, Wascher TC, Pieber TR, Sourij H. Multifactorial risk factor intervention in patients with Type 2 diabetes improves arginine bioavailability ratios. Diabet Med. 2012;29:e365–8. doi: 10.1111/j.1464-5491.2012.03743.x. [DOI] [PubMed] [Google Scholar]