Abstract
The present study was undertaken to test the efficacy of immunization with the native major outer membrane protein (nMOMP) of C. trachomatis mouse pneumonitis (MoPn) serovar in combination with a novel immunostimulatory adjuvant consisting of CpG oligodeoxynucleotide (ODN) linked to the nontoxic B subunit of cholera toxin (CTB-CpG) to elicit a protective immune response to C. trachomatis. High levels of Chlamydia specific IgG antibodies were detected in the sera from BALB/c mice immunized intramuscularly and subcutaneously (i.m.+s.c.) with the nMOMP/CTB-CpG vaccine or with nMOMP adjuvanted with a mixture of CT and CpG ODN (CT + CpG). Further, these immunization schemes gave rise to significant T-cell mediated Chlamydia-specific immune responses. No Chlamydia-specific humoral or cell-mediated immune responses were detected in the control mice vaccinated with ovalbumin together with either CTB-CpG or CT + CpG. Following an intranasal challenge with C. trachomatis the groups of mice immunized with nMOMP plus CTB-CpG, CT + CpG or live C. trachomatis were found to be protected based on their change in body weight and lung weight as well as number of inclusion forming unit recovered from the lungs, as compared with control groups immunized with ovalbumin plus either adjuvants. Interestingly, IFN-γ-producing CD4+, but not CD8+, T-cells showed a significant correlation with the outcomes of the challenge. In conclusion, nMOMP in combination with the novel adjuvant CTB-CpG elicited a significant antigen specific antibody and cell-mediated immune responses as well as protection against a pulmonary challenge with C. trachomatis.
Keywords: Chlamydia trachomatis, vaccine, CTB-CpG adjuvant
INTRODUCTION
Chlamydia trachomatis is worldwide the leading cause of bacterial sexually transmitted diseases [1]. Newborns at the time of delivery can acquire ocular and respiratory infections from an infected mother. In addition, in countries with poor hygienic conditions, it causes trachoma resulting from scarring of the conjunctiva. Annually in the USA approximately 4-5 million new genital infections are reported [2]. The majority of these occur in teenagers and individuals under 30 years of age. Although effective antimicrobial therapy is available, the treatment has been largely unsuccessful in halting the spread of infection. This is most likely, at least in part, due to the high rate of asymptomatic infections that may persist for months to years [3]. Thus, the development of an effective vaccine to prevent C. trachomatis infections is urgently needed.
The most utilized model to test vaccine protocols is the C. trachomatis mouse pneumonitis (MoPn) serovar (also called Chlamydia muridarum). This serovar was originally isolated from the lungs of mice inoculated with throat washings from humans with respiratory infections [4]. Respiratory or genital inoculation of mice with this organism has been found to induce infections that closely parallel those observed in humans. Using this murine model a vaccine formulated with the native major outer membrane protein (nMOMP) has been found to induce significant protection against an intranasal and a genital challenge [5, 6].
A novel immunostimulatory adjuvant based on the nontoxic B subunit of cholera toxin (CT) linked to CpG oligodexynucleotide (CTB-CpG) was recently developed by Adamsson et al. [7]. CT is a multi-subunit macromolecule composed structurally and functionally of the A and B subunits [8]. Subunit A has ADP-ribosylating activity and by enhancing intracellular cAMP levels alters ion transport resulting in water and chloride losses from the intestines with subsequent diarrhea [9]. The B subunit binds to the cell’s ganglioside GM1 receptor facilitating the entry of the A subunit into the cytosol where it binds to NAD and catalyzes the ADP-ribosylation of the Gsα protein [10]. CT is a very strong mucosal adjuvant when co-administered with antigens. CT exerts its adjuvant effect by inducing antigen-specific CD4+ Th1- and Th2-type cells that produce high levels of IFN-γ, IL-4, IL-5, IL-6 and IL-10 [11]. In addition to T cell immunity, CT is able to stimulate the development of systemic IgG1 and IgG2a, as well as mucosal secretory IgA antibody responses [12]. The inherent toxicity of CT residing in its A-subunit precludes its use as an adjuvant in humans. However, the non-toxic B subunit pentamer (CTB) can be given safely by different routes to humans but is not by itself a strong mucosal adjuvant.
CpG binds to TLR9, which is located on the endosomal membranes and signals through recruitment of the adaptor molecule myeloid differentiation factor 88 resulting in the induction of a strong Th1 polarized immune response. The adjuvant effect of CpG has been extensively studied and CpG has now reached advanced human trials [13, 14]. It has recently been shown that the immunostimulatory/adjuvant effect of CpG ODN is significantly enhanced when chemically conjugated to CTB [7, 15, 16]. The increased immunostimulatory property of the CTB-CpG conjugate may be due to increased uptake of coupled CpG ODN by target cells and also by CTB/GM1-directed intracellular transport into the endoplasmic reticulum [15].
In this study, we tested a novel vaccine formulation containing the nMOMP and the CTB-CpG adjuvant. BALB/c mice immunized with this vaccine developed strong humoral and cell mediated immune responses. Furthermore, the immunized animals were significantly protected against a respiratory challenge with C. trachomatis. These results warrant further exploration on the potential of nMOMP plus the novel adjuvant CTB-CpG as vaccine candidate to counter Chlamydia infection.
MATERIALS AND METHODS
C. trachomatis stocks
The C. trachomatis MoPn strain Nigg II was obtained from the American Type Culture Collection (ATCC; Manassas, VA) and was grown in HeLa-229 cells using Eagle’s minimal essential medium supplemented with 5% fetal bovine serum (FBS) as described [17]. Purified elementary bodies (EB) were stored at −70°C in 0.2 M sucrose, 20 mM sodium phosphate (pH 7.4), and 5 mM glutamic acid (SPG) [17]. Bacterial stocks were titrated in HeLa-229 cells.
Preparation of the C. trachomatis native MOMP (nMOMP)
Extraction and purification of the native C. trachomatis MoPn MOMP (nMOMP) were performed as previously described [5, 6]. The purified nMOMP was refolded by dialysis in 0.1 M phosphate buffer (pH 7.8) containing 2 mM reduced glutathione, 1 mM oxidized glutathione (Sigma, St. Louis, MO), 1 mM EDTA, and 0.05% Z3-14. The protein was then concentrated and fixed with 2% glutaraldehyde (Sigma, St. Louis, MO) at room temperature for 2 min. Glycine (Bio-Rad Laboratories) was added to stop the reaction. The nMOMP was concentrated with a Centricon-10 filter (Millipore Corp., Bedford, MA) and dialyzed against a solution containing 20 mM phosphate buffer (pH 7.4), 0.15 M NaCl and 0.05% Z3-14 before using it to immunize mice.
Conjugation of CpG ODN to CTB
CpG ODN 1826, a 20-mer containing two copies of an optimal mouse CpG motif (TCCATG ACG TTC CTG ACG TT) with complete PS backbone (Coley Pharmaceutical Group, Ontario, Canada) and recombinant CTB protein (Operon, Ebersberg, Germany) were used for conjugation [7]. The conjugation of CpG ODN to CTB was performed by mixing thiolated ODN with maleimide-activated CTB at room temperature overnight, followed by purification by gel filtration through Superdex 200 (Amersham Biosciences, Uppsala, Sweden). The high molecular peak was collected and protein concentration was determined using a protein assay (micro BCA; Pierce, Rockford, IL), with recombinant CTB as a reference protein. Concentration of nucleic acid was determined by measuring the absorbance at 260 nm. The CTB-CpG conjugate was analyzed by SDS-PAGE and CTB and CpG ODN in the conjugate were visualized with Coomassie Blue (Sigma-Aldrich, St. Louis, MO) and SYBR Green (Molecular Probes, Paisley, UK), respectively. The GM1-binding property of CTB-CpG was confirmed by a solid-phase GM1 ELISA.
Immunization and challenge of mice
Three-week-old female BALB/c (H-2d) mice were purchased from Charles River Laboratories (Wilmington, MA) and were housed at the University of California, Irvine, Vivarium. All animal protocols were approved by the University of California, Irvine, Animal Care and Use Committee.
Animals were immunized by the intramuscular (i.m.) and subcutaneous (s.c.) routes with nMOMP (10 μg/mouse/immunization) in combination with CTB-CpG (20 μg/mouse/immunization) or 1 μg/mouse/immunization of CT (Sigma-Aldrich, St. Louis, MO), a non-toxic dose for mice, plus 10μg CpG ODN 1826 (Coley Pharmaceutical Group, Ontario, Canada). As negative controls, groups of mice were immunized with the same amount of ovalbumin (Sigma, St. Louis, MO) in combination with CTB-CpG or CT + CpG ODN. The mice were boosted twice at 2-week intervals with the same vaccine preparations. Positive control mice were immunized by the intranasal (i.n.) route once with 104 inclusion forming units (IFU) of the C. trachomatis MoPn strain. Mice were challenged i.n. with 104 IFU of the C. trachomatis MoPn at four weeks after the last boost. Fourteen mice were employed for each experimental group.
Characterization of the humoral response by enzyme-linked immunosorbent assay (ELISA), Western blot assay and in vitro neutralization
Blood was collected by periorbital puncture and all the immunoassays were performed with pooled sera from each group. The Chlamydia-specific antibody titers were determined by an ELISA as previously described [17]. In brief, each well of 96-well plates was coated with 100 μl per well of C. trachomatis MoPn EB containing 10 μg/ml of protein in PBS to which serial dilutions of serum were added. Following incubation at 37°C for 1 hr, the plates were washed, and horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (Ig), IgG1, IgG2a (BD Pharmingen, San Diego, CA), IgG2b, IgG3 (Southern Biotechnology Associates, Birmingham, AL), IgG (mixed components of IgG1, IgG2a, IgG2b and IgG), or IgA (ICN Pharmaceuticals, Aurora, Ohio) was applied, and then incubated at 37°C for 1 hr. The plates were washed, and the binding was measured in an ELISA reader (Labsystem Multiscan; Helsinki, Finland) at 405 nm using 2, 2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonate) as the substrate.
Western blot was performed as previously described with C. trachomatis EB as the antigen [5]. Approximately 30 μg of purified EB were loaded on a 7.5-cm-wide slab polyacrylamide gel. Following transfer to nitrocellulose membrane, the nonspecific binding was blocked with BLOTTO (bovine lacto transfer technique optimizer; 5% [wt/vol] nonfat dry milk, 2mM CaCl2, 50 mM Tris-HCl [pH8.0]) for 2 hrs at room temperature, and then serum samples diluted 1:100 were added to the membrane and incubated overnight at 4°C. Then, the membrane was washed and incubated with horseradish peroxidase-conjugated goat anti-mouse antibody for 1 hr, followed by visualization of the bands by developing with 0.01% hydrogen peroxide and the substrate 4-chloro-1-naphthol. A 1:5 dilution of mouse monoclonal antibodies against the C. trachomatis serovar 40-kDa MOMP (mAb MoPn-40; generated in our laboratory) acted as a positive control.
The in vitro neutralization assay was performed according to the protocol by Peterson et al. [18]. Briefly, five-fold serial dilutions of the serum were made in PBS. Duplicate dilutions were incubated for 45 min at 37°C with 104 IFU of the C. trachomatis MoPn. The mixtures were then inoculated by centrifugation onto HeLa-229 cell monolayers that were grown in 15 × 45-mm glass shell vials. The monolayers were incubated for 30 hrs and subsequently fixed with methanol. The inclusions were stained using a cocktail of monoclonal antibodies to Chlamydia prepared in our laboratory [17]. A horseradish peroxidase conjugated goat anti-mouse antibody was added and developed with 0.01% H2O2 and 4-chloro-naphthol (Sigma-Aldrich, St. Louis, MO). The results are expressed as percent inhibition relative to the control sera.
Preparation of splenocytes
Spleens were disrupted with a 1 ml syringe plunger on a 40 μm nylon cell strainer (BD Falcon, BD Biosciences, Bedford, MA) using 5 ml of sterile PBS with 2% FBS. Erythrocytes were lysed with ammonium chloride buffer (BD Pharm Lyse, BD Biosciences). Single-cell suspensions were washed and the cell viability was assessed by trypan blue exclusion [17].
In vitro CD4+ splenic lymphocyte proliferative assay (LPA)
To assess the T-cell memory response after the immunization, in vitro LPA was performed before challenge. In brief, the spleens from three mice from each group were collected and pooled, and CD4+ splenic T-cells were isolated using anti-mouse CD4 particles-DM as described by the manufacturer (BD Biosciences, San Jose, CA). Accessory cells for antigen presentation (APCs) were prepared by irradiating (3,000 rads; 137Cs) syngeneic unseparated splenocytes. APCs (105/well) were incubated with C. trachomatis MoPn EB at a 1:5 ratio, at 37 °C for 2 hrs. Concanavalin A (ConA; Sigma-Aldrich, St. Louis, MO) was used as positive stimulant at a concentration of 5 μg/ml, and tissue culture media served as a negative control. CD4+ cells were added at a 1:1 ratio to APCs. RPMI 1640 supplemented with 5% FBS, 2% L-glutamine, and 0.5% 2-Mercaptoethanol (RPMI 1640-5% FBS) was used as culture media. The total volume per well was 200 μl. At the end of 72 hrs of incubation, 1.0 μCi of [methyl-3H] thymidine (47 Ci/mmol; Amersham, Arlington Heights, IL) in 25 μl of RPMI 1640-5% FBS was added per well, and the incorporation of [3H] was measured using a scintillation counter (Beckman Instruments, Fullerton, CA).
Ex vivo analysis of cytokines production by CD4+- or CD8+-memory T-cells with flow cytometry
Single-cell suspensions were prepared from splenocytes as described above. Approximate 107 of splenocytes were re-suspended in RPMI 1640-5% FBS and incubated with EB at a ratio of 1:1 at 37° in a 5% CO2 incubator for 1 h. Brefeldin A (10 μg/ml; Sigma, St. Louis, MO) was added and the incubation continued for 5 hrs. Cells were harvested, washed and re-suspended in flow cytometry buffer (PBS, 0.2% BSA, 0.05% NaN3) [19]. Non-specific binding of antibodies was inhibited by blocking Fc receptors with anti-CD16 (Fc block; eBioscience) for 10 min on ice. For the 4-color staining protocols, the cells were first stained for cell surface markers after antibody concentrations were optimized using appropriate fluorochrome-labeled isotype-matched control antibodies. Fluorescein isothiocyanate (FITC) anti-CD44 (clone Pgp-1), PerCp anti-CD8a (clone 53-6.7), and Allophycocyanin (APC) anti-CD4 (clone RM4-5) were used for surface staining. Subsequently, the cells were intracellularly stained (ICS) with phycoerythrin (PE) IFN-γ (clone XMG1.2) or IL-4 (clone 11B11) following the manufacturer instruction [20]. All the antibodies were purchased from eBioscience (San Diego, CA). Ten thousand total events were counted using an analytical BD FACSCalibur flow cytometer (Becton-Dickinson). Data analyses were performed using FlowJo software, v8.7.3 (Tree Star, Ashland, OR).
Evaluation of the infection following the i.n. challenge
After the intranasal challenge the mice were weighed daily for 10 days [5]. At day 10 post-challenge the mice were euthanized, their lungs harvested and placed in 5 ml of SPG. The lungs were homogenized, and serial 10-fold dilutions were inoculated onto Hela-229 cells grown in 48-well tissue culture plates. Following centrifugation the plates were incubated for 30 hrs at 37°C in a 5% CO2 incubator. Inclusions were stained with a cocktail of monoclonal antibodies prepared in our laboratory [17].
Statistical analyses
The Mann-Whitney U test was used to compare the numbers of C. trachomatis IFU, and the Student t-test was performed to compare CD4+ T-cell proliferative response (cpm), lung weight and body weight change of mice at day-10 post-challenge. To compare the daily changes in mean body weight between each group of mice during the 10 days following the i.n. challenge, repeated measures ANOVA was conducted. A pairwise correlation analysis was performed to assay whether the protection of the vaccines against the C. trachomatis i.n. challenge based on, the numbers of IFU recovered from lungs, lung weight and body weight change of mice, is related with IFN-γ-producing effector T-cells. Due to only 5 study groups, i.e., 5 data points available for each parameter, Pearson’s correlation analysis is not sensitive enough to detect the potential relatedness between parameters. Therefore, a bootstrap scheme was implemented with R program [21] to calculate the p-values of correlation for any two parameters. Suppose the correlation coefficient of two parameters is ρ. We fixed the first parameter and randomly sample the second parameter with replacement from data. Set the correlation coefficient of the first parameter and the simulated second parameter as . We repeated this process for n times, e.g. 10000 times. The empirical p-value for ρ is then defined as , where I is an indicator variable with
RESULTS
Antibody response following immunization
Here, we investigated the vaccination of mice with nMOMP and CTB-CpG or CT + CpG ODN in comparison with, an i.n. immunization with live C. trachomatis, the gold standard, on induction of protection to a subsequent i.n. challenge with this pathogen. To this end, groups of female Balb/c mice were immunized three times with nMOMP in combination with either CTB-CpG or a mixture of CT + CpG ODN via the i.m. and s.c. routes. Positive control mice were inoculated by the i.n. route once with 104 IFU of the C. trachomatis MoPn strain. The mice were then examined for their humoral and cellular immunity as well as protection four weeks after the final immunization.
Serum samples collected the day before the intranasal challenge were tested by ELISA, Western blot, and for the presence of neutralizing antibodies. As shown in Table 1, high Chlamydia-specific IgG antibody titers were observed in the groups of mice immunized with nMOMP using either CTB-CpG or CT + CpG ODN as adjuvants (25,600 and 51,200, respectively). In the control mice immunized i.n. with live C. trachomatis MoPn, the IgG titer was also high (12,800). In these three groups of mice the Chlamydia-specific IgA antibody titer was approximately 400. The two control groups immunized with ovalbumin and either adjuvants had no detectable Chlamydia-specific IgG or IgA antibodies in their sera.
Table 1. Serum antibody titer the day before the i.n. challenge with C. trachomatis MoPn.
| Vaccine | Anti-C. trachomatis MoPn ELISA titer |
Neutralizing titer |
|||||
|---|---|---|---|---|---|---|---|
| IgG | IgG1 | IgG2a | IgG2b | IgG3 | IgA | ||
| nMOMP + CTB-CpG | 25,600 | 3,200 | 25,600 | 12,800 | 25,600 | 400 | <50 |
| nMOMP + CT + CpG ODN | 51,200 | 6,400 | 25,600 | 25,600 | 25,600 | 400 | 50 |
| Ovalbumin + CTB-CpG | <100 | <100 | <100 | <100 | <100 | <100 | <50 |
| Ovalbumin + CT + CpG ODN | <100 | <100 | <100 | <100 | <100 | <100 | <50 |
| C. trachomatis | 12,800 | 1,600 | 25,600 | 12,800 | 12,800 | 400 | 1,250 |
The ratio of the IgG2a/IgG1 was used to evaluate the immune response predominantly to Th1 or Th2. In the group of mice immunized with nMOMP/CTB-CpG, the ratio was 8 (25,600/3,200), while the group immunized using nMOMP/CT + CpG ODN, had the ratio of 4 (25,600/6,400). The highest ratio was observed in the group of mice immunized i.n. with live Chlamydia (16; 25,600/1,600). As expected, the negative control groups of animals that received ovalbumin had no detectable IgG2a or IgG1 antibodies to Chlamydia in their sera.
The in vitro neutralizing antibody titer in the mice vaccinated with the nMOMP and CT + CpG ODN was 50, while no neutralizing antibodies were detected in the mice vaccinated with the nMOMP and CTB-CpG adjuvant. In the positive control group inoculated i.n. with live Chlamydia the neutralizing titer in serum was 1,250. The serum samples from the mice vaccinated with ovalbumin were used as background controls.
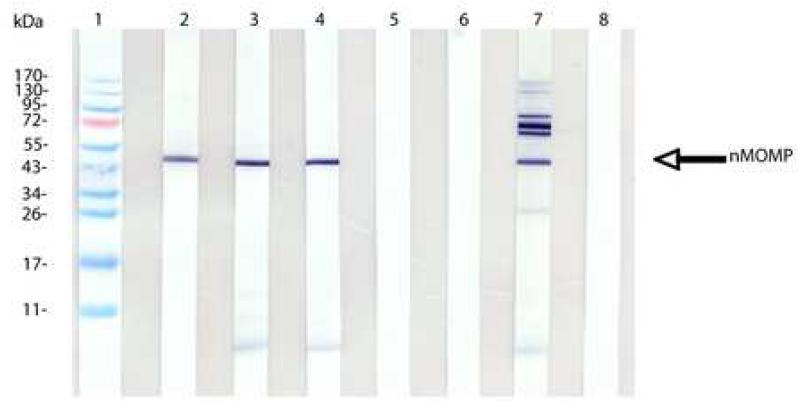
The Western blot in Fig. 1 shows that mice immunized with the nMOMP and CTB-CpG or with CT + CpG ODN developed antibodies against the nMOMP, as shown by a band with a MW of 40 kDa, and LPS. Animals immunized i.n. with live C. trachomatis also produced antibodies to the nMOMP. In addition, antibodies to bands with a molecular mass higher than 100 kDa, the 60-kDa cysteine rich protein (crp), the 28 kDa and LPS were detected. Control mice immunized with ovalbumin instead of the nMOMP did not show any bands in the blot. No protein bands were detected in any of the serum samples collected before immunization.
Figure 1. Western blot analysis of serum samples from the day before i.n. challenge.
C. trachomatis MoPn EB were used as the antigen. Lane 1, molecular weight standards. Lane 2, control mAb MoPn-40. Lanes 3 to 7, sera from mice immunized with MOMP and CTB-CpG-1826 (lane 3); with MOMP and CT + CpG-1826 (lane 4); with ovalbumin and CTB-CpG-1826 (lane 5); with ovalbumin and CT + CpG-1826 (lane 6); with C. trachomatis MoPn EB (lane 7). Lane 8, serum from d0.
In vitro splenic CD4+ T-cells proliferative response following immunization
The splenic CD4+ T-cell immune response for each group of mice was first evaluated by an in vitro LPA the day before the challenge. Results are summarized in Table 2. Splenic CD4+ T-cells from the mice immunized with the nMOMP and CTB-CpG exhibited a significant lymphoproliferative response when stimulated with EB compared to the response of the control mice immunized with ovalbumin and the same adjuvant (5.3 × 103 versus 1.6 × 103 cpm, p < 0.001). The CD4+ T-cells isolated from the mice vaccinated with the nMOMP and CT + CpG ODN also showed a higher lymphoproliferative response to EB stimulation than the control mice immunized with ovalbumin and CT + CpG ODN (3.9 × 103 versus 2.5 × 103 cpm), however, there was no statistical significance between these two groups (p > 0.05). The lymphoproliferative response of the mice vaccinated with the nMOMP and CTB-CpG (5.3 × 103 cpm) was significantly stronger than that of the mice immunized with the nMOMP and CT + CpG ODN (3.9 × 103 cpm; p < 0.05). As expected, the more robust lymphoproliferative response was observed in the mice immunized i.n. with live C. trachomatis (21.4 × 103 cpm).
Table 2. CD4+ T-cell response the day before the i.n. challenge with C. trachomatis MoPna.
| Vaccine | CD4+ T-cell proliferative response (× 103 cpm) to |
|
|---|---|---|
| EB | Medium | |
| nMOMP + CTB-CpG | 5.3 ± 0.01b, c | 0.3 ± 0.05 |
| nMOMP + CT + CpG ODN | 3.9 ± 0.8d | 0.3 ± 0.1 |
| Ovalbumin + CTB-CpG | 1.6 ± 0.5 | 0.5 ± 0.16 |
| Ovalbumin + CT + CpG ODN | 2.5 ± 1.1 | 0.5 ± 0.2 |
| C. trachomatis | 21.4 ± 3.0 | 0.2 ± 0.02 |
The values are means ± SE for triplicate cultures. The data are from one of the experiments representative of duplicate experiments. UV-inactivated C. trachomatis MoPn EB were added at a ratio of EB to T cells of 1:5.
p < 0.05 as determined by the Student t test for the comparison with the group immunized with nMOMP + CT + CpG ODN
p < 0.001 as determined by the Student t test for the comparison with the group immunized with ovalbumin + CTB-CpG
p > 0.05 as determined by the Student t test for the comparison with the group immunized with ovalbumin with adjuvant of CT + CpG ODN
Ex vivo intracellular levels of IFN-γ and IL-4 in CD4+- and CD8+-T cells following the i.n. challenge with C. trachomatis MoPn
To directly detect the source of IFN-γ and IL-4 produced by effector memory T-cells, ex vivo intracellular flow cytometric analyses of IFN-γ and IL-4 were performed on pooled splenocytes from each group collected at 10 days after the intranasal challenge.
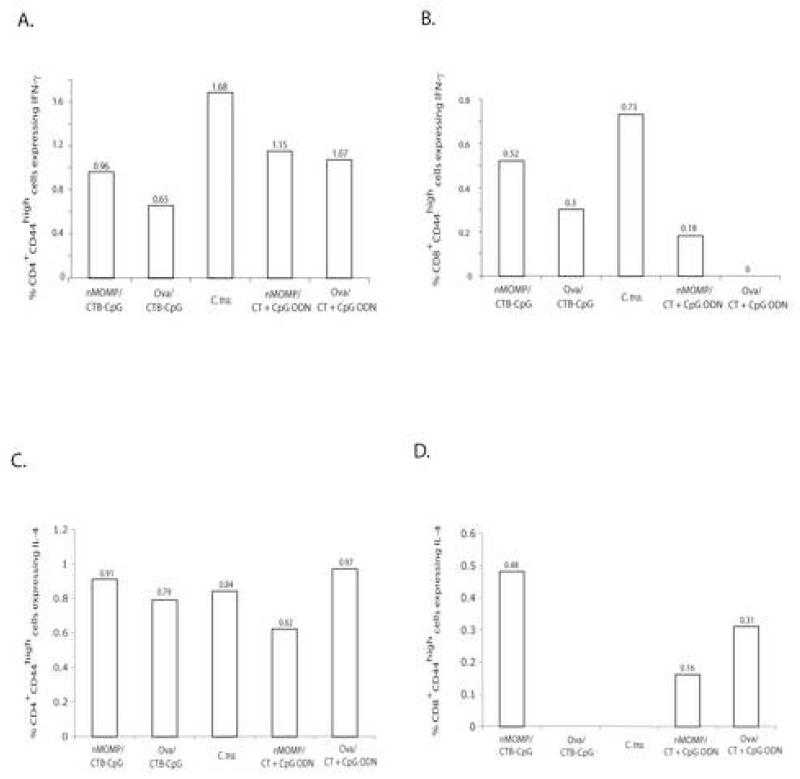
In the mice vaccinated with the nMOMP and CTB-CpG the percentage of IFN-γ producing CD4 effector memory T-cells (IFN-γ+CD4+CD44+: 0.96%) and of IFN-γ producing CD8 effector memory T-cells (IFN-γ+CD8+CD44+: 0.52%) was high when compared with the control mice immunized with ovalbumin and the same adjuvants (IFN-γ+CD4+CD44+: 0.65% and IFN-γ+CD8+CD44+: 0.3%) (Fig. 2A-B). Similar results were obtained when the percentage of IFN-γ producing CD4 and CD8 effector memory T-cells were compared between the mice immunized with the nMOMP and CT + CpG ODN (IFN-γ+CD4+CD44+: 1.15% and IFN-γ+CD8+CD44+: 0.18%) versus the group immunized with ovalbumin and the same adjuvants (IFN-γ+CD4+CD44+: 1.07% and IFN-γ+CD8+CD44+: 0%). The highest populations of IFN-γ+CD4+CD44high and IFN-γ+CD8+CD44high cells were detected in the mice vaccinated with live C. trachomatis MoPn (CD4: 1.68% and CD8: 0.73%)
Figure 2. Flow cytometry assay performed using splenocytes isolated from the mice at day 10 after the i.n. C. trachomatis challenge.
A. CD4+CD44high splenic T-cells were gated, and the frequency of positive intracellular IFN-γ staining in CD4+CD44high splenic T-cells was exposed.
B. CD8+CD44high splenic T-cells were gated, and the frequency of positive intracellular IFN-γ staining in CD8+CD44high splenic T-cells was exposed.
C. CD4+CD44high splenic T-cells were gated, and the frequency of positive intracellular IL-4 staining in CD4+CD44high splenic T-cells was exposed.
D. CD8+CD44high splenic T-cells were gated, and the frequency of positive intracellular IL-4 staining in CD8+CD44high splenic T-cells was exposed.
As shown in Fig. 2C-D, intracellular IL-4 production was found in 0.91% CD4+CD44high and 0.48% CD8+CD44high T-cells of the mice vaccinated with the nMOMP and CTB-CpG, and in 0.79% CD4+CD44high and 0% CD8+CD44high T-cells of the control mice vaccinated with ovalbumin and CTB-CpG. In contrast, 0.62% CD4+CD44high and 0.16% CD8+CD44high T-cells produced intracellular IL-4 in the mice vaccinated by the nMOMP and CT + CpG ODN. However, 0.97% CD4+CD44high and 0.31% CD8+CD44high T-cells produced IL-4 in the control mice immunized with ovalbumin and CT + CpG ODN. There were 0.84% IL4+CD4+CD44high and no detectable IL4+CD8+CD44high T-cells found in mice that were inoculated i.n. with C. trachomatis. These results indicate that IL-4 production was most likely non-specific to C. trachomatis.
Changes in body weight following the i.n. challenge
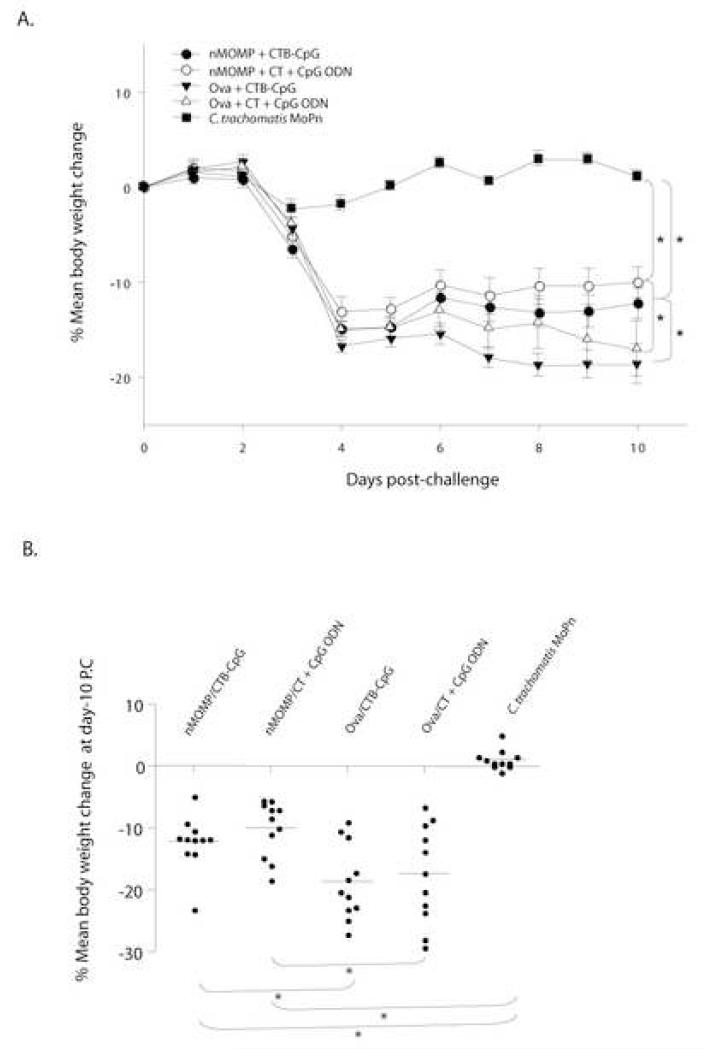
The changes in mean body weight of each group of mice following the i.n. challenge are shown in Fig. 3. Mice immunized with the nMOMP and CTB-CpG or with the nMOMP plus CT + CpG ODN lost weight from the 2nd through the 4th day after the challenge and then slowly regained some of their weight. At day 10 after the i.n. challenge these two groups of mice had lost 12% and 10% of their initial body weights, respectively. The corresponding control animals immunized with ovalbumin instead of the nMOMP had lost significantly more body weight (19% and 17%, respectively; p < 0.05). No significant difference in body weight change was observed between the mice vaccinated with the nMOMP and CTB-CpG and the group immunized with the nMOMP plus CT + CpG ODN (p > 0.05). The mice vaccinated i.n. with live EB showed a transient minimal drop in body weight up to the third day after i.n. challenge and then quickly recovered it.
Figure 3. Changes in body weight following the i.n. challenge.
A. Percentage change of mean body weight following the i.n. C. trachomatis challenge. *, p< 0.05 as determined by the Repeated Measures ANOVA.
B. Percentage change of mean body weight of each group at day 10 after the i.n. C. trachomatis challenge. Mean is shown as a short line and a solid dot represents the weight of each individual mouse. *, p< 0.05 as determined by the Student’s t-test.
Assessment of lung infection following the i.n. challenge
Ten days after the i.n. challenge the mice were euthanized and their lungs were weighed, homogenized and cultured. The weight of the lung was used as a parameter to assess the overall infection and inflammatory reaction. As shown in Table 3, the mean weight of the lungs from the mice immunized with the nMOMP and CTB-CpG or with the nMOMP and CT + CpG ODN was 0.30+0.01 g and 0.27+0.03 g, respectively (p>0.05). In comparison with the corresponding control mice immunized with ovalbumin instead of the nMOMP , the average weight of the lungs was 0.33+0.01 g and 0.35+0.01 g, respectively (p < 0.05). In the positive control group inoculated i.n. with live EB the mean weight of the lungs was 0.22±0.01 g.
Table 3. Lung weight and number ofC. trachomatis MoPn IFU recovered from the lungs following the i.n. challenge.
| Vaccine | Lung weight (gr.; mean ± SE) | No. IFU lung culture Median (range) × (106) |
|---|---|---|
| nMOMP + CTB-CpG | 0.30 ± 0.01 a, b | 1.63 (0.67-21.06)d,e |
| nMOMP + CT + CpG ODN | 0.27 ± 0.03c | 1.32 (0.02-11.77)f |
| Ovalbumin + CTB-CpG | 0.33 ± 0.01 | 55.45 (1.30-181.72) |
| Ovalbumin + CT + CpG ODN | 0.35 ± 0.01 | 55.34 (15.07-192.05) |
| C. trachomatis | 0.22 ± 0.01 | <0.00005 |
p > 0.05 as determined by the Student t test for the comparison with the group immunized with nMOMP + CT + CpG ODN
p < 0.05 as determined by the Student t test for the comparison with the group immunized with ovalbumin + CTB-CpG
p < 0.05 as determined by the Student t test for the comparison with the group immunized with ovalbumin + CT + CpG ODN
p > 0.05 as determined by the Mann Whitney U-test for the comparison with the group immunized with nMOMP + CT + CpG ODN
p < 0.05 as determined by the Mann Whitney U-test for the comparison with the group immunized with ovalbumin + CTB-CpG
p < 0.05 as determined by the Mann Whitney U-test for the comparison with the group immunized with ovalbumin + CT + CpG ODN
The yields of chlamydial IFU recovered from the lungs at 10 days post-challenge are shown in Table 3. From the mice vaccinated with the nMOMP and CTB-CpG a median number of 1.6 × 106 IFU were recovered per pair of animal lungs of this group. This yield of IFU was significantly lower in comparison with the number of IFU from the control group of mice vaccinated with ovalbumin and CTB-CpG (55.4 × 106 IFU; p < 0.05). From the mice immunized with the nMOMP and CT + CpG ODN we recovered 1.3 × 106 IFU, whereas the corresponding control mice immunized with ovalbumin and the same adjuvant 55.3 × 106 IFU were recovered (p < 0.05). The difference in the number of IFU recovered from the mice immunized with the nMOMP/CTB-CpG and nMOMP/CT + CpG ODN is not statistically significant (p > 0.05). No detectable Chlamydia was recovered from the lungs of the mice immunized with live organisms.
Correlation analyses between splenic T-cell proliferation assay before challenge and protection after challenge with C. trachomatis MoPn
A bootstrap scheme was performed to analyze the correlation between the in vitro CD4+ T-cells’ memory response before challenge and the protection readouts conducted 10 days post-challenge with C. trachomatis MoPn. As illustrated in Table 4, the empirical p values of correlation were calculated between any two parameters from the following readouts. The CD4+ T-cells proliferation in vitro, the frequency of IFN-γ-producing CD4+ effector memory (IFN-γ+CD4+CD44high) T-cells and IFN-γ-producing CD8+ effector memory (IFN-γ+CD8+CD44high) T-cells as assessed by ex vivo ICS, C. trachomatis MoPn IFU recovered from lung tissues, and the mean body weight change. The frequency of IFN-γ+CD4+CD44high showed strong significant correlation with the proliferative CD4+ T-cells (p < 0.001), and inverse correlation with the number of C. trachomatis MoPn IFU recovered from the lungs (p < 0.05), and the mean body weight loss (p < 0.001). However, the frequency of IFN-γ+CD8+CD44high T-cells only exhibited a significant inverse correlation with the mean body weight loss (p < 0.001), but not with number of IFU recovered from the lungs (p > 0.05). In addition, a significant correlation between the number of C. trachomatis MoPn IFU recovered from the lungs and the mean body weight change of the mice was observed (p < 0.001).
Table 4. Correlation of immunological parameters and protection.
p value conducted by pairwise correlation analysisa between the proliferative response (cmp) of CD4+ T-cells before challenge, the frequency of IFN-γ-producing memory T-cells (IFN-γ+CD4+CD44high and IFN-γ+CD8+CD44high), the number of C. trachomatis MoPn IFU recovered from the lungs, and percentage in mean body weight change of the mice at day 10 post i.n. challenge
| cmp of CD4+ T- cells |
IFN-γ+CD4+CD44high (%) |
IFN-γ+CD8+CD44high (%) |
IFU in lung | Δ Mean body weight (%) |
|
|---|---|---|---|---|---|
| cmp of CD4+ T-cells | --- | 9×10−13 | 1×10−9 | 0.026 | 3×10−15 |
| IFN-γ+CD4+CD44high (%) |
--- | --- | 3×10−3 | 1×10−6 | 2×10−32 |
| IFN-γ+CD8+CD44high (%) |
--- | --- | --- | 0.193 | 7×10− |
| IFU in lung | --- | --- | --- | --- | 3×10−4 |
| Mean body weight change (%) |
--- | --- | --- | --- | --- |
The empirical p values were derived from a bootstrapped correlation scheme. The statistical significant level is set to 0.05. Except for the number (p value) with underline, all the p values showed the statistical significance in the pairwise correlation analysis.
DISCUSSION
Most traditional vaccines comprising of live or inactivated whole pathogens possess intrinsic adjuvant activity as they contain pathogen associated molecules such as LPS, and microbial nucleic acids with the ability to activate TLR and other pattern recognition receptors in the host cells. This in turn leads to induction of a coordinated set of innate and adaptive immune responses [22]. The current use of highly purified subunit vaccines, such as synthetic peptides and recombinant proteins, that lack inherent adjuvanticity, has resulted in the need to develop new and safe immunomodulatory adjuvants to enhance and direct antigen specific humoral as well as cellular immunity.
Recently, a novel immunomodulatory agent based on CpG ODN linked to CTB (CTB-CpG) was developed [7]. Here, we utilized this non-toxic adjuvant in combination with the C. trachomatis nMOMP to establish its ability to protect mice. In this study we showed that a vaccine consisting of CTB-CpG and the nMOMP partially protected mice against a respiratory challenge with C. trachomatis. The protection afforded with this vaccine was equivalent to that elicited by a vaccine that used whole CT plus CpG ODN as adjuvant. This finding is of importance as the holotoxin CT, although highly potent, is precluded for human use as adjuvant due to its inherent toxicity. The non toxic pentameric CTB, which binds to the ganglioside GM1 receptor, found on the surface of all nucleated cells [10] was shown to be safe for human use via different routes [23-27]. Recently, Nystrom-Asklin et al. [16] have shown that a vaccine adjuvanted with the CTB-CpG was able to induce protective immunity in mice against a mucosal pathogen, namely Helicobacter pylori.
In 2002 Pal et al. [5] tested the same nMOMP preparation using CpG+Alum as adjuvants. In contrast to the strong Th1 response observed here using CTB-CpG or CT+CpG, the use of CpG+Alum elicited a more balanced Th1/Th2 response. Following the i.n. challenge, the use of CpG+Alum resulted in a stronger protection in comparison to the one observed here. For example, the mice immunized using CpG+Alum lost less body weight than the groups immunized using CTB-CpG or CT+CpG. In addition, the animals immunized with CpG+Alum appeared to be better protected based on the lung weight and number of IFU recovered from the lungs. However, it is important to point out that while the lungs have both a systemic and a mucosal component the genital tract is mainly mucosal and therefore, protection using CTB-CpG or CT+CpG may be stronger than utilizing CpG+Alum in that animal model. As expected, in both experiments the protection achieved by immunization with live Chlamydia was more robust than that obtained with the subunits vaccines.
In this study, we found that IFN-γ production by memory CD4 T-cells (CD4+CD44high) from immunized mice, after C. trachomatis MoPn intranasal challenge, showed a strong correlation with CD4 T-cells’ proliferative activity. Furthermore, both proliferation of CD4 T-cells and IFN-γ production from these memory CD4 T-cells showed a strong inverse correlation with C. trachomatis MoPn IFU recovered from the lungs and loss of body weight in mice. In contrast, IFN-γ production from memory CD8 T-cells (CD8+CD44high) after challenge only showed an inverse correlation with body weight loss but not with C. trachomatis MoPn IFU recovered from the lungs. These results support the notion that IFN-γ producing CD4 T-cells, but not CD8 T cells response plays a critical role in protection against a respiratory challenge with C. trachomatis in mice vaccinated with the nMOMP and either CTB-CpG or CT + CpG ODN. Neither CD4 T-cells proliferation nor any other outcome at post-challenge showed statistical correlation with IL-4 production. These findings are in line with the previous reports from experimental models, which indicate that CD4 Th1 cells, B cells and antibodies are mainly responsible for mediating the clearance of a C. trachomatis infection, while CD8 T-cells do not appear to play a significant role. More specifically, protection in the C. trachomatis MoPn infection model is most likely mediated by CD4+ T-cells that produce IFN-γ, as mice deficient in MHC class II molecules [28], CD4 [28, 29], IL-12 [30], IFN-γ [31] or IFN-γ receptor [32] can not control the infection. Similarly, mice depleted of C. trachomatis MoPn-specific CD4+ T-cells [29] could not clear the infection. Adoptive transfer of C. trachomatis MoPn-specific CD4+ Th-1 cell clones, but not Th-2 cell clones, was also shown to protect nude mice against C. trachomatis MoPn infection [33]. It is in general accepted that CD8 T-cells are neither sufficient nor necessary to confer protective immunity in the murine model of chlamydial genital infection [34-36]. Although the mechanisms are not fully elucidated it is assumed that the protection is at least partially mediated by the production of IFN-γ [37-40].
The vaccines comprising of the nMOMP and either CTB-CpG or CT plus CpG ODN elicited a strong humoral response. A high IgG2a to IgG1 ratio and a high titer of IgG3 indicated a predominant Th1 type response [43, 44]. Similarly, a high IgG2a/IgG1 ratio was observed in mice immunized i.n. with live Chlamydia [17, 45]. Interestingly, the antibodies elicited by vaccines based on the nMOMP had limited ability to neutralize EB in vitro, when compared with the antibodies found in the mice inoculated i.n. with live C. trachomatis. A previous study reported that high titers of C. trachomatis-specific antibody do not correlate with the resolution of infection in humans and, in fact, were correlated with increased severity of sequelae of infection, such as tubal infertility [46]. Su et al. [47] reported that antibodies are particularly important in local recall immunity, as mice deficient in antibodies are less resistant to re-infection than mice with local anti-chlamydial antibodies. Similarly, Morrison et al. [48] showed that antibodies contribute significantly to protection against a genital tract re-infection but not against a primary infection. On the other hand, Pal et al. [49, 50] have shown that passive immunization with IgA and IgG monoclonal antibodies can protect mice against genital and respiratory tract infections with Chlamydia. This finding is supported by an earlier epidemiological observation in infected humans that showed an inverse correlation between IgA levels in cervical secretions and the amount of Chlamydia recovered from the cervix [51].
In conclusion, we report here that a vaccine consisting of the chlamydial nMOMP and CTB-CpG, a novel non-toxic immunomodulatory adjuvant, induced protection against a pulmonary challenge with C. trachomatis as effective as that elicited using nMOMP in combination with whole CT + CpG ODN. These results may have implications for the development of a vaccine against Chlamydia infection in humans.
ACKNOWLEDGEMENTS
This study was supported by Public Health Service grant AI-32248 from the National Institute of Allergy and Infectious Diseases, the Swedish Research Council (Vr), the Swedish International Development Cooperation Agency (Sida-SAREC), University of Gothenburg Vaccine Research Institute (GUVAX) and Dr. Z Jia was supported by the UCI SPECS program grant NIH UO1CA114810.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Global prevalence and incidence of selected curable sexually transmitted diseases: overview and estimates. World Health Organization; Geneva: 2001. [Google Scholar]
- [2].National Surveillance Data for Chlamydia, Gonorrhea, and Syphilis. ; 2007. Centers for Disease Control and Prevention; United States: Trends in reportable sexually transmitted diseases in the United States, 2007; pp. 1–7. [Google Scholar]
- [3].Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, et al. Prevalence of chlamydial and gonococcal infections among young adults in the United States. Jama. 2004;291(18):2229–36. doi: 10.1001/jama.291.18.2229. [DOI] [PubMed] [Google Scholar]
- [4].Nigg C. An Unidentified Virus Which Produces Pneumonia and Systemic Infection in Mice. Science. 1942;95(2454):49–50. doi: 10.1126/science.95.2454.49-a. [DOI] [PubMed] [Google Scholar]
- [5].Pal S, Davis HL, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein by use of CpG oligodeoxynucleotides as an adjuvant induces a protective immune response against an intranasal chlamydial challenge. Infection and immunity. 2002;70(9):4812–7. doi: 10.1128/IAI.70.9.4812-4817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infection and immunity. 2005;73(12):8153–60. doi: 10.1128/IAI.73.12.8153-8160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Adamsson J, Lindblad M, Lundqvist A, Kelly D, Holmgren J, Harandi AM. Novel immunostimulatory agent based on CpG oligodeoxynucleotide linked to the nontoxic B subunit of cholera toxin. J Immunol. 2006;176(8):4902–13. doi: 10.4049/jimmunol.176.8.4902. [DOI] [PubMed] [Google Scholar]
- [8].Fujihashi K, Koga T, van Ginkel FW, Hagiwara Y, McGhee JR. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine. 2002;20(19-20):2431–8. doi: 10.1016/s0264-410x(02)00155-x. [DOI] [PubMed] [Google Scholar]
- [9].Spangler BD. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56(4):622–47. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Snider DP. The mucosal adjuvant activities of ADP-ribosylating bacterial enterotoxins. Crit Rev Immunol. 1995;15(3-4):317–48. doi: 10.1615/critrevimmunol.v15.i3-4.70. [DOI] [PubMed] [Google Scholar]
- [11].Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155(10):4621–9. [PubMed] [Google Scholar]
- [12].Marinaro M, Boyaka PN, Finkelman FD, Kiyono H, Jackson RJ, Jirillo E, et al. Oral but not parenteral interleukin (IL)-12 redirects T helper 2 (Th2)-type responses to an oral vaccine without altering mucosal IgA responses. The Journal of experimental medicine. 1997;185(3):415–27. doi: 10.1084/jem.185.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang H, Rayburn E, Zhang R. Synthetic oligodeoxynucleotides containing deoxycytidyl-deoxyguanosine dinucleotides (CpG ODNs) and modified analogs as novel anticancer therapeutics. Current pharmaceutical design. 2005;11(22):2889–907. doi: 10.2174/1381612054546707. [DOI] [PubMed] [Google Scholar]
- [14].Nouri-Aria KT. Recent progress in allergen immunotherapy. Iran J Immunol. 2008;5(1):1–24. doi: 10.22034/iji.2008.17096. [DOI] [PubMed] [Google Scholar]
- [15].Harandi AM, Holmgren J. CpG DNA as a potent inducer of mucosal immunity: implications for immunoprophylaxis and immunotherapy of mucosal infections. Curr Opin Investig Drugs. 2004;5(2):141–5. [PubMed] [Google Scholar]
- [16].Nystrom-Asklin J, Adamsson J, Harandi AM. The adjuvant effect of CpG oligodeoxynucleotide linked to the non-toxic B subunit of cholera toxin for induction of immunity against H. pylori in mice. Scand J Immunol. 2008;67(5):431–40. doi: 10.1111/j.1365-3083.2008.02085.x. [DOI] [PubMed] [Google Scholar]
- [17].Pal S, Fielder TJ, Peterson EM, de la Maza LM. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infection and immunity. 1994;62(8):3354–62. doi: 10.1128/iai.62.8.3354-3362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Peterson EM, Zhong GM, Carlson E, de la Maza LM. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infection and immunity. 1988;56(4):885–91. doi: 10.1128/iai.56.4.885-891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].De Pascalis R, Taylor BC, Elkins KL. Diverse myeloid and lymphoid cell subpopulations produce gamma interferon during early innate immune responses to Francisella tularensis live vaccine strain. Infection and immunity. 2008;76(9):4311–21. doi: 10.1128/IAI.00514-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].eBioscience. Intracellular Cytokine Staining Protocol. 2008 [Google Scholar]
- [21].Department of Statistics and Mathematics of the Wu Wien Available from: http://www.r-project.org/
- [22].Xu D, Liu H, Komai-Koma M. Direct and indirect role of Toll-like receptors in T cell mediated immunity. Cellular & molecular immunology. 2004;1(4):239–46. [PubMed] [Google Scholar]
- [23].Karlsen TH, Sommerfelt H, Klomstad S, Andersen PK, Strand TA, Ulvik RJ, et al. Intestinal and systemic immune responses to an oral cholera toxoid B subunit whole-cell vaccine administered during zinc supplementation. Infection and immunity. 2003;71(7):3909–13. doi: 10.1128/IAI.71.7.3909-3913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stanford M, Whittall T, Bergmeier LA, Lindblad M, Lundin S, Shinnick T, et al. Oral tolerization with peptide 336-351 linked to cholera toxin B subunit in preventing relapses of uveitis in Behcet’s disease. Clinical and experimental immunology. 2004;137(1):201–8. doi: 10.1111/j.1365-2249.2004.02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wassen L, Jertborn M. Kinetics of local and systemic immune responses after vaginal immunization with recombinant cholera toxin B subunit in humans. Clinical and diagnostic laboratory immunology. 2005;12(3):447–52. doi: 10.1128/CDLI.12.3.447-452.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Holmgren J, Adamsson J, Anjuere F, Clemens J, Czerkinsky C, Eriksson K, et al. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunology letters. 2005;97(2):181–8. doi: 10.1016/j.imlet.2004.11.009. [DOI] [PubMed] [Google Scholar]
- [27].Sanchez J, Holmgren J. Cholera toxin structure, gene regulation and pathophysiological and immunological aspects. Cell Mol Life Sci. 2008;65(9):1347–60. doi: 10.1007/s00018-008-7496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infection and immunity. 1995;63(12):4661–8. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infection and immunity. 2000;68(12):6979–87. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Perry LL, Feilzer K, Caldwell HD. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and - independent pathways. J Immunol. 1997;158(7):3344–52. [PubMed] [Google Scholar]
- [31].Wang S, Fan Y, Brunham RC, Yang X. IFN-gamma knockout mice show Th2-associated delayed-type hypersensitivity and the inflammatory cells fail to localize and control chlamydial infection. Eur J Immunol. 1999;29(11):3782–92. doi: 10.1002/(SICI)1521-4141(199911)29:11<3782::AID-IMMU3782>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- [32].Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infection and immunity. 1997;65(3):1032–44. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hawkins RA, Rank RG, Kelly KA. A Chlamydia trachomatis-specific Th2 clone does not provide protection against a genital infection and displays reduced trafficking to the infected genital mucosa. Infection and immunity. 2002;70(9):5132–9. doi: 10.1128/IAI.70.9.5132-5139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Perry LL, Feilzer K, Hughes S, Caldwell HD. Clearance of Chlamydia trachomatis from the murine genital mucosa does not require perforin-mediated cytolysis or Fas-mediated apoptosis. Infection and immunity. 1999;67(3):1379–85. doi: 10.1128/iai.67.3.1379-1385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kim SK, Devine L, Angevine M, DeMars R, Kavathas PB. Direct detection and magnetic isolation of Chlamydia trachomatis major outer membrane protein-specific CD8+ CTLs with HLA class I tetramers. J Immunol. 2000;165(12):7285–92. doi: 10.4049/jimmunol.165.12.7285. [DOI] [PubMed] [Google Scholar]
- [36].Kim SK, Angevine M, Demick K, Ortiz L, Rudersdorf R, Watkins D, et al. Induction of HLA class I-restricted CD8+ CTLs specific for the major outer membrane protein of Chlamydia trachomatis in human genital tract infections. J Immunol. 1999;162(11):6855–66. [PubMed] [Google Scholar]
- [37].Roan NR, Starnbach MN. Antigen-specific CD8+ T cells respond to Chlamydia trachomatis in the genital mucosa. J Immunol. 2006;177(11):7974–9. doi: 10.4049/jimmunol.177.11.7974. [DOI] [PubMed] [Google Scholar]
- [38].Holland MJ, Conway DJ, Blanchard TJ, Mahdi OM, Bailey RL, Whittle HC, et al. Synthetic peptides based on Chlamydia trachomatis antigens identify cytotoxic T lymphocyte responses in subjects from a trachoma-endemic population. Clinical and experimental immunology. 1997;107(1):44–9. doi: 10.1046/j.1365-2249.1997.2511129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Starnbach MN, Bevan MJ, Lampe MF. Murine cytotoxic T lymphocytes induced following Chlamydia trachomatis intraperitoneal or genital tract infection respond to cells infected with multiple serovars. Infection and immunity. 1995;63(9):3527–30. doi: 10.1128/iai.63.9.3527-3530.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Starnbach MN, Bevan MJ, Lampe MF. Protective cytotoxic T lymphocytes are induced during murine infection with Chlamydia trachomatis. J Immunol. 1994;153(11):5183–9. [PubMed] [Google Scholar]
- [41].Igietseme JU, Black CM, Caldwell HD. Chlamydia vaccines: strategies and status. BioDrugs. 2002;16(1):19–35. doi: 10.2165/00063030-200216010-00003. [DOI] [PubMed] [Google Scholar]
- [42].Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infection and immunity. 2002;70(6):2741–51. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Coutelier JP, van der Logt JT, Heessen FW, Warnier G, Van Snick J. IgG2a restriction of murine antibodies elicited by viral infections. The Journal of experimental medicine. 1987;165(1):64–9. doi: 10.1084/jem.165.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mosmann TRaC RL. Th1 and Th2 cells: Different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- [45].Pal S, Peterson EM, de la Maza LM. Vaccination of newborn mice induces a strong protective immune response against respiratory and genital challenges with Chlamydia trachomatis. Vaccine. 2005;23(46-47):5351–8. doi: 10.1016/j.vaccine.2005.06.026. [DOI] [PubMed] [Google Scholar]
- [46].Punnonen R, Terho P, Nikkanen V, Meurman O. Chlamydial serology in infertile women by immunofluorescence. Fertil Steril. 1979;31(6):656–9. doi: 10.1016/s0015-0282(16)44056-2. [DOI] [PubMed] [Google Scholar]
- [47].Su H, Feilzer K, Caldwell HD, Morrison RP. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infection and immunity. 1997;65(6):1993–9. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Morrison SG, Morrison RP. The protective effect of antibody in immunity to murine chlamydial genital tract reinfection is independent of immunoglobulin A. Infection and immunity. 2005;73(9):6183–6. doi: 10.1128/IAI.73.9.6183-6186.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pal S, Bravo J, Peterson EM, de la Maza LM. Protection of wild-type and severe combined immunodeficiency mice against an intranasal challenge by passive immunization with monoclonal antibodies to the Chlamydia trachomatis mouse pneumonitis major outer membrane protein. Infection and immunity. 2008;76(12):5581–7. doi: 10.1128/IAI.00574-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pal S, Theodor I, Peterson EM, de la Maza LM. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine. 1997;15(5):575–82. doi: 10.1016/s0264-410x(97)00206-5. [DOI] [PubMed] [Google Scholar]
- [51].Brunham RC, Kuo CC, Cles L, Holmes KK. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infection and immunity. 1983;39(3):1491–4. doi: 10.1128/iai.39.3.1491-1494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]