Abstract
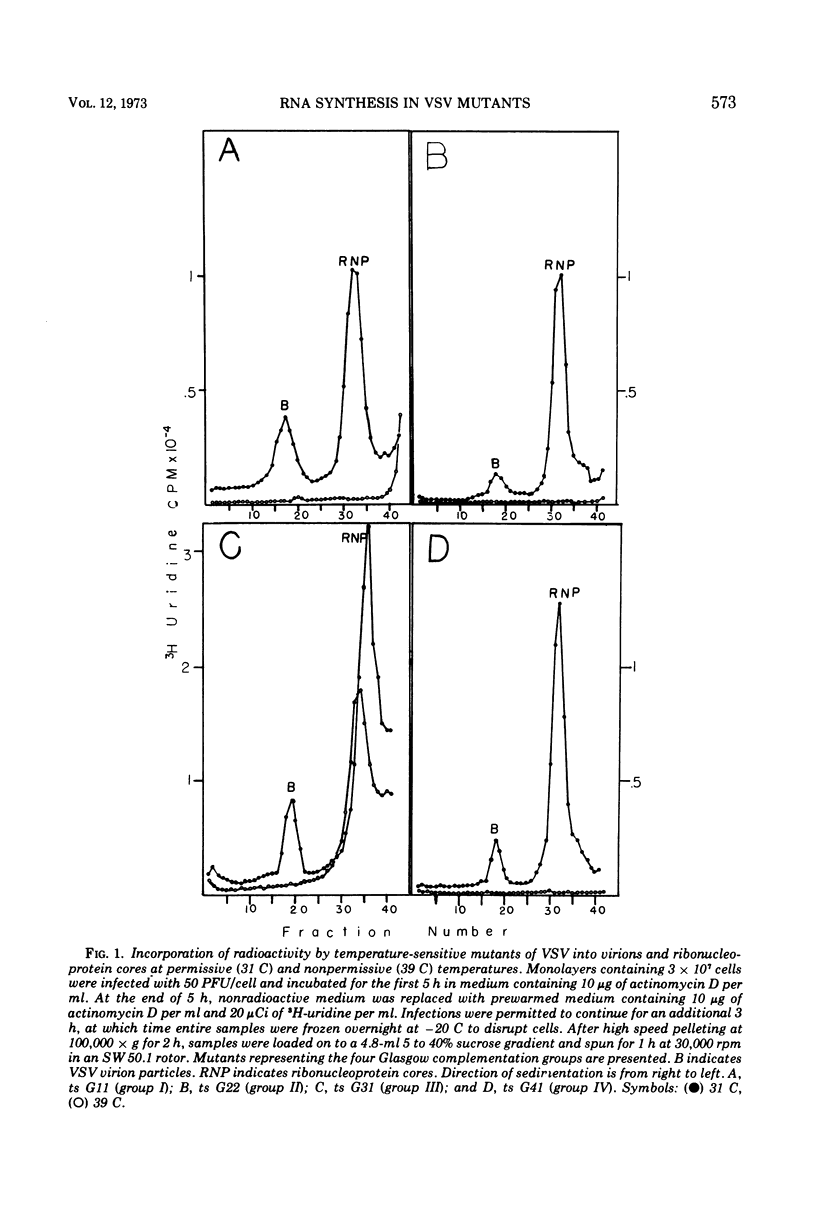
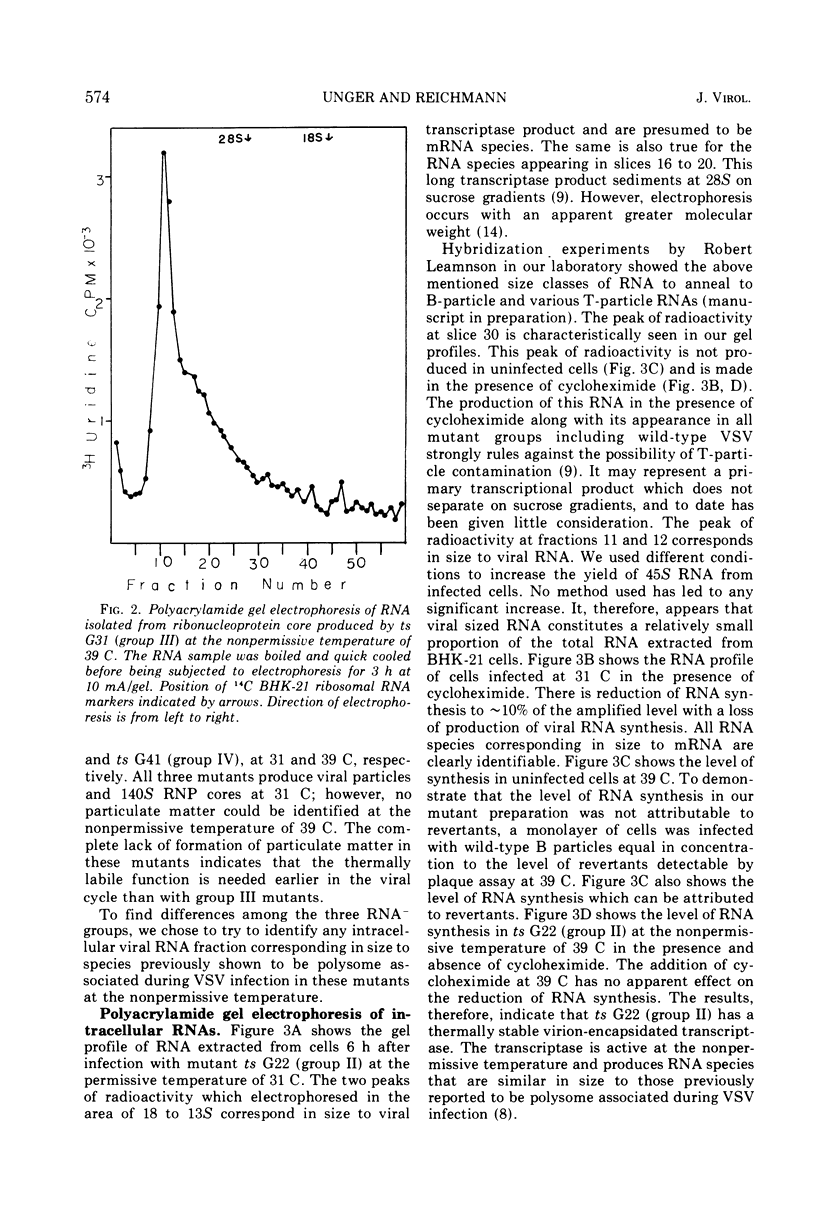
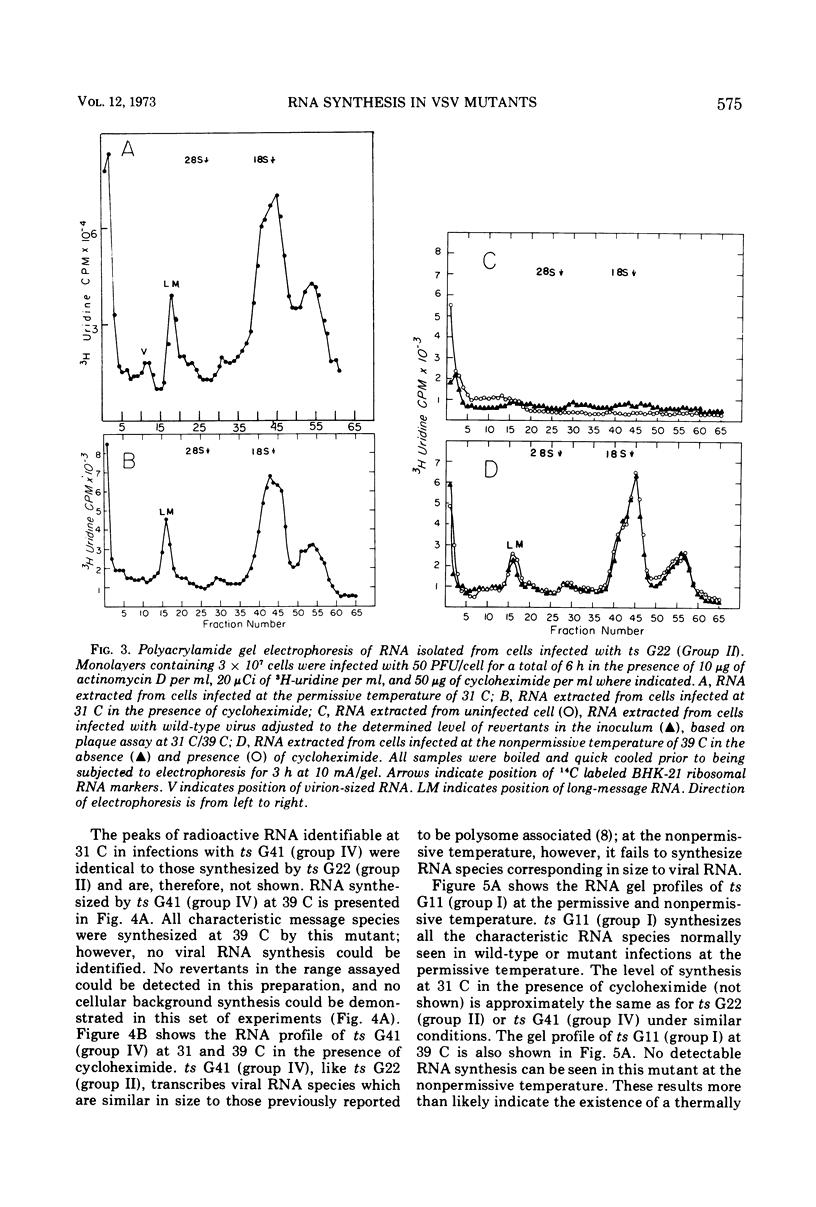
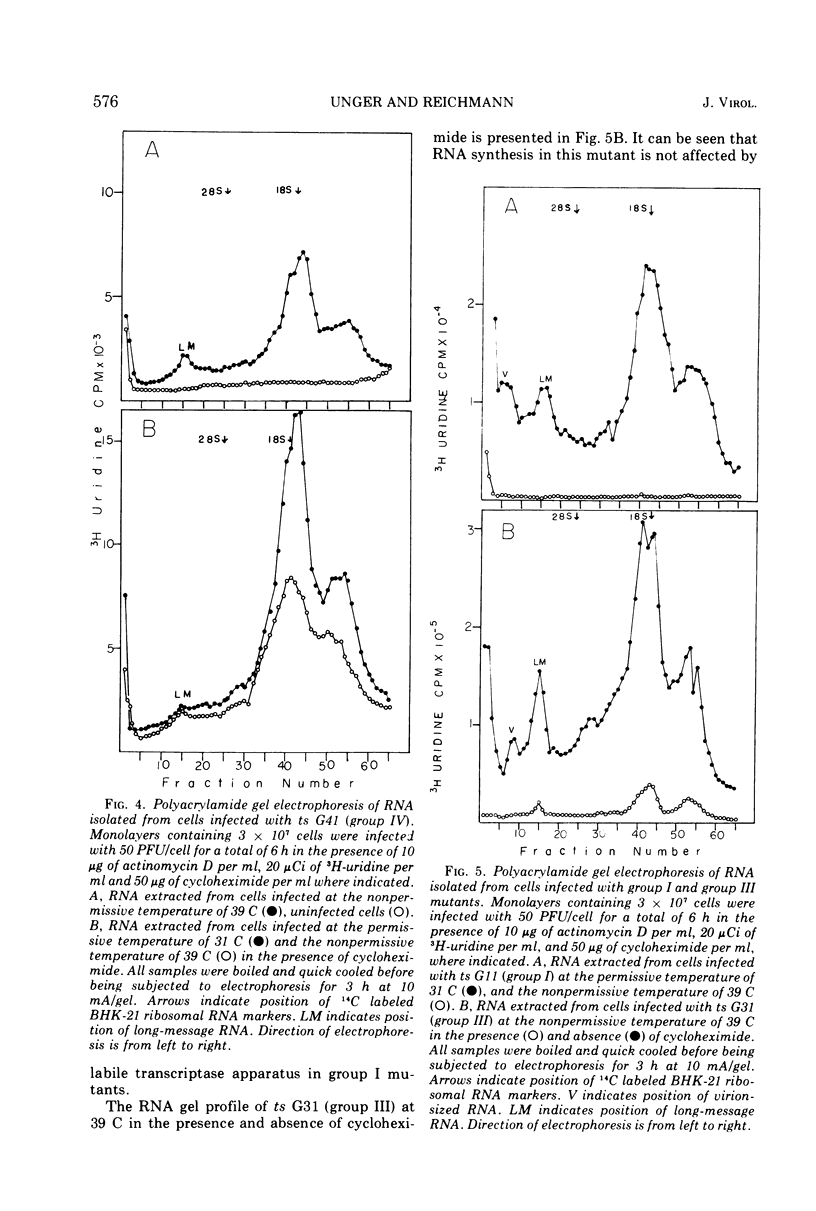
T-particle-free stocks of temperature-sensitive mutants representing the four Glasgow complementation groups of the Indiana serotype of vesicular stomatitis virus were used to study RNA synthesis at the permissive and nonpermissive temperatures of 31 and 39 C, respectively. Mutants selected from the four Glasgow complementation groups were characterized on the basis of particle and ribonucleoprotein formation. Intracellular RNAs were further characterized by polyacrylamide gel electrophoresis. ts G22 (group II) and ts G41 (group IV), previously characterized as RNA negative at the nonpermissive temperature, synthesized low levels of RNA which could not be attributed to contaminating levels of revertants. Furthermore, the levels of synthesis could not be reduced by the addition of cycloheximide. These data suggest that ts G22 (group II) and ts G41 (group IV) contain a thermally stable, virion-encapsidated transcriptase, but fail to amplify RNA synthesis due to a thermally labile function presumably necessary for the synthesis of viral RNA. ts G31, a group III mutant, synthesized intracellular RNA at amplified levels at the nonpermissive temperature. Intracellular ribonucleoprotein complexes were isolated in copious amounts; however, no particles corresponding in size to finished virions were observed. These data suggest a thermally labile maturation factor or envelope associated structural protein to be defective in ts G31 (group III). ts G11 (group 1) showed no detectable RNA synthesis at the nonpermissive temperature. These data suggest ts G11 (group I) contains a thermally labile component involved in early transcription. This group may contain a number of mutants defective in different components of the transcription apparatus, which may not complement in vivo because of the physical improbability of subunit exchange between virion particles of the incoming inoculum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Flamand A. Etude des mutants thermosensibles du virus de la stomatite vésiculaire. Mise au point d'un test de complémentation. C R Acad Sci Hebd Seances Acad Sci D. 1969 May 5;268(18):2305–2308. [PubMed] [Google Scholar]
- Flamand A. Etude génétique du virus de la stomatite vésiculaire: classement de mutants thermosensibles spontanés en groupes de complémentation. J Gen Virol. 1970 Sep;8(3):187–195. doi: 10.1099/0022-1317-8-3-187. [DOI] [PubMed] [Google Scholar]
- Flamand A., Pringle C. R. The homologies of spontaneous and induced temperature-sensitive mutants of vesicular stomatitis virus isolated in chick embryo and BHK 21 cells. J Gen Virol. 1971 May;11(2):81–85. doi: 10.1099/0022-1317-11-2-81. [DOI] [PubMed] [Google Scholar]
- Holloway A. F., Wong P. K., Cormack D. V. Isolation and characterization of temperature-sensitive mutants of vesicular stomatitis virus. Virology. 1970 Dec;42(4):917–926. doi: 10.1016/0042-6822(70)90340-5. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus. 3. Multiple complementary messenger RNA molecules. Virology. 1970 Dec;42(4):946–957. doi: 10.1016/0042-6822(70)90343-0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Manders E. K. Ribonucleic acid synthesis of vesicular stomatitis virus. IV. Transcription by standard virus in the presence of defective interfering particles. J Virol. 1972 Jun;9(6):909–916. doi: 10.1128/jvi.9.6.909-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley M. P., Wagner R. R. Ribonucleic acid species of intracellular nucleocapsids and released virions of vesicular stomatitis virus. J Virol. 1972 Aug;10(2):244–255. doi: 10.1128/jvi.10.2.244-255.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay F. Etude des fonctions du virus de la stomatite vésiculaire altérées par une mutation thermosensible: mise en evidence dela protéine structurale affectée par la mutation ts 23. J Gen Virol. 1971 Dec;13(3):449–453. doi: 10.1099/0022-1317-13-3-449. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Engelhardt D. L., Hunt J. M., Sekellick M. J. Interferon action: inhibition of vesicular stomatitis virus RNA synthesis induced by virion-bound polymerase. Science. 1971 Nov 5;174(4009):593–598. doi: 10.1126/science.174.4009.593. [DOI] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Polysomal ribonucleic acid of vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Dec;42(4):958–968. doi: 10.1016/0042-6822(70)90344-2. [DOI] [PubMed] [Google Scholar]
- Perrault J., Holland J. Variability of vesicular stomatitis virus autointerference with different host cells and virus serotypes. Virology. 1972 Oct;50(1):148–158. doi: 10.1016/0042-6822(72)90355-8. [DOI] [PubMed] [Google Scholar]
- Pringle C. R., Duncan I. B. Preliminary physiological characterization of temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Jul;8(1):56–61. doi: 10.1128/jvi.8.1.56-61.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Printz-Ané C., Combard A., Martinet C. Study of the transcription and the replication of vesicular stomatitis virus by using temperature-sensitive mutants. J Virol. 1972 Nov;10(5):889–895. doi: 10.1128/jvi.10.5.889-895.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Printz P., Wagner R. R. Temperature-sensitive mutants of vesicular stomatitis virus: synthesis of virus-specific proteins. J Virol. 1971 May;7(5):651–662. doi: 10.1128/jvi.7.5.651-662.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann M. E., Pringle C. R., Follett E. A. Defective particles in BHK cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Aug;8(2):154–160. doi: 10.1128/jvi.8.2.154-160.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schincariol A. L., Howatson A. F. Replication of vesicular stomatitis virus. I. Viral specific RNA and nucleoprotein in infected L cells. Virology. 1970 Nov;42(3):732–743. doi: 10.1016/0042-6822(70)90319-3. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Absence of interference during high-multiplicity infection by clonally purified vesicular stomatitis virus. J Virol. 1971 Mar;7(3):409–411. doi: 10.1128/jvi.7.3.409-411.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., Holloway A. F., Cormack D. V. Characterization of three complementation groups of vesicular stomatitis virus. Virology. 1972 Dec;50(3):829–840. doi: 10.1016/0042-6822(72)90437-0. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Pringle C. R. Protein synthesis in BHK21 cells infected with vesicular stomatitis virus. I. ts Mutants of the Indiana serotype. Virology. 1972 Apr;48(1):104–111. doi: 10.1016/0042-6822(72)90118-3. [DOI] [PubMed] [Google Scholar]



