Abstract
Chronic granulomatous disease (CGD) is an inherited disorder characterized by recurrent life-threatening bacterial and fungal infections. CGD results from defective production of reactive oxygen species (ROS) by phagocytes caused by mutations in genes encoding the NADPH oxidase 2 (NOX2) complex subunits. Mice with a spontaneous mutation in Ncf1, which encodes the NCF1 (p47phox) subunit of NOX2, have defective phagocyte NOX2 activity. These mice occasionally develop local spontaneous infections by Staphylococcus xylosus or by the common CGD pathogen S. aureus. Ncf1 mutant mice were more susceptible to systemic challenge with these bacteria than wild type mice. Transgenic Ncf1 mutant mice harboring wild type Ncf1 gene under the human CD68 promoter (MN+ mice) gained the expression of NCF1 and functional NOX2 activity specifically in monocyte/macrophages, although minimal NOX2 activity was detected also in some CD11b+Ly6G+ cells defined as neutrophils. MN+ mice did not develop spontaneous infection and were more resistant to administered staphylococcal infections compared to MN− mice. Most strikingly, MN+ mice survived after administered Burkholderia cepacia, an opportunistic pathogen in CGD patients, whereas MN− mice died. Thus, monocyte/macrophage expression of functional NCF1 protected against spontaneous and administered bacterial infections.
Introduction
Chronic granulomatous disease (CGD) is an inherited disorder characterized by recurrent life-threatening bacterial and fungal infections and persistent inflammation (1). The pathogens vary according to the geographical area. In North America and Europe the majority of the infections are caused by the following pathogens: Staphylococcus aureus, Burkholderia cepacia, Serratia marcescens, Nocardia species, Salmonella species, Aspergillus species and other moulds (reviewed by Grimm et al. (2) and Holland (3) and (4)). The impaired host defense is due to defective production of reactive oxygen species (ROS) by phagocytes caused by mutations in genes encoding the phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 complex (NOX2) protein subunits (5, 6). CGD can result from recessive mutations in any of the 5 genes encoding subunits of the NOX2 complex. (4, 7). X-linked CGD results from mutations in the cytochrome b-245 heavy chain catalytic subunit (also known as gp91phox or NOX2 and coded by CYBB), and accounts for approximately 70% of CGD cases, The most common form of autosomal recessive CGD results from mutations in the regulatory protein NCF1 (coded by NCF1 and also known as p47phox), and accounts for 20% of CGD cases (4). Knocking out Cybb or Ncf1 (the latter referred to as Ncf1−/−) generated mouse models of CGD that showed increased susceptibility to bacterial and fungal infections compared to wild type (wt) mice (8–11). Spontaneous bacterial and fungal infections occurred in Ncf1−/− mice, with soft tissue infections by Staphylococcus xylosus being the most common (11).
We have previously reported that a spontaneous single nucleotide mutation in the Ncf1 gene (referred to as Ncf1*/*) results in impaired phagocyte NADPH oxidase activity and enhanced inflammatory responses in autoimmune chronic inflammatory diseases, such as collagen induced arthritis (CIA) and experimental autoimmune encephalomyelitis (12). As human CGD is most commonly caused by single mutations, we investigated whether Ncf1*/* mice exhibit the CGD phenotype regarding increased susceptibility to infections. We observed that Ncf1*/* mice in separate mouse facilities occasionally developed spontaneous soft tissue infections with similar features as the ones reported in the Ncf1−/− mice. The most common cultured isolates were Staphylococcus xylosus the same strain previously isolated in Ncf1−/− mice (11), but also Staphylococcus aureus, a frequent pathogen in CGD patients. Taken together, these results show that the Ncf1*/* mice manifest both increased susceptibility to spontaneous infections and a more severe inflammatory phenotype in experimental models of autoimmune chronic inflammation. The NOX2 complex is functional in both neutrophils and macrophages. While the critical role of neutrophil NOX2 in host defense is well established (13), the role of NOX2 in macrophages is less clear. Since in both CGD patients and mouse models, the NOX2 complex is deficient in all myeloid cells, it was not possible to delineate the specific contribution of macrophage NOX2 to host defense in vivo. Now, for the first time, we show that monocyte expression of functional NCF1 under the control of the human CD68 promoter protected mice from lethal infection by S. xylosus and Burkholderia cepacia. In conclusion, a natural occurring mutation in Ncf1, which impairs the protein function, limits the anti-bacterial defense in mice similarly to the human CGD condition. Transgenic mice where functional NCF1 is targeted to monocytes are protected from lethal bacterial infections. These findings are important in the dissection of the mechanisms of impaired host defense in CGD and for the development of targeted cures.
Material and Methods
Ethics statement
All procedures performed on mice were approved at the respective institutes and complied with federal guidelines. The experiments conducted at Roswell Park Cancer Institute followed the federal Animal Welfare Act and the NIH guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee under the number 1135M. Experiments carried out in Finland were approved by the Regional state administrative agency in Southern Finland under the number ESAVI-0000497/041003/2011. The ones performed in Sweden followed the Animal Protection Law (Djurskyddslag) and were approved by the Gothenburg committee with number 106-2009 and the Northern Stockholm committee under number N169/10.
Animals
All mice used were genetically controlled and shared the C57Black background. The C57BL/10.Q/rhd background was confirmed to be homozygous apart from the previously described (Ncf1m1J/Ncf1*) mutation in the Ncf1 gene using a 10k SNP chip (12, 14). The MHC class II congenic C57BL/10.Q/rhd (B10.Q) mice express MHC class II H2-Aq that is encoded by a fragment from the DBA/1 strain. The Ncf1 mutation impairs the function of NCF1 as described earlier (12). The B10.P.MBQ mice have been described previously (15): they are transgenic mice where H-2Aq is expressed under the hCD68 promoter. The B10.Q.Bko mice were previously called B10.Q.µMT mice: they were obtained from backcrossing the original µMT founder, kindly provided by Dr. Werner Müller (Institute of Genetics, Cologne, Germany), to B10.Q for more than 10 generations (16). TCRko animals were bought form Jackson laboratories (Tcrbtm1Mom) and then backcrossed for more than 10 generations to B10.Q. The B10.Q.COMP ko mice were previously described and backcrossed to B10.Q for more than 10 generations. (17). B10.Q.MN mice were described previously (18): the MN transgene encodes the functional NCF1 under the hCD68 promoter. Ncf1−/− (also denoted p47phox−/−) mice generated as previously described (11) were backcrossed to N14 in C57BL/6, and maintained at Roswell Park Cancer Institute, Buffalo, NY. Mice were housed under SPF conditions.
Bacterial isolates
Bacterial growth of isolated samples from infected animals was assessed on various media under different incubation conditions. All identified isolates could be cultured on hematin agar at 37°C under aerobic conditions. Species identification was based on 16S rDNA sequence analysis and phenotypic tests. Cultured colonies was suspended in 100 µl H2O and heat treated for 15 minutes at 95°C. PCR for 16S analysis was performed without further extraction of DNA and amplification was carried out in a 50-µl reaction mixture containing 1× PCR buffer (Qiagen), 3 mM MgCl2, 200 µM each deoxynucleoside triphosphate, 1.0 U of HotStarTaq DNA polymerase (Qiagen), 10 pmol of each primer, and 5 µl of DNA template. P8F (5´- AGA GTT TGA TCM TGG CTC AG-3′) (19) and P911r (5′-CCC GTC AAT TCH TTT GAG T-3′) (20) were used as PCR and sequencing primers. A pre-PCR step of 15 min at 95°C was followed by 40 cycles of 93°C for 50 s, 52°C for 50 s, and 72°C for 50 s. A final step of 5 min at 72°C terminated the amplification. Tubes with no target DNA and Escherichia coli DNA were included as negative and positive controls, respectively. Both strands of the approximately 800-bp PCR product were sequenced using the BigDye Terminator Cycle Sequencing kit (Applied Biosystems Inc., Foster City, CA) and analyzed on an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems Inc.) by BMLabbet (Furulund, Sweden). The sequences of the isolates were identical to the 16S rRNA genes either of Staphylococcus aureus, Staphylococcus saprophyticus/xylosus or Escherichia coli available at the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov). Strains determined to belong to the family of S. saprophyticus and S. xylosus with 16S were differentiated and identified to be S. xylosus by their property to ferment xylose and mannose in oxidative-fermentative medium (21).
Strain typing
The S. xylosus isolates were typed using automated repetitive PCR (rep-PCR) (DiversiLab) as recommended by the manufacturer. DNA was extracted using the UltraClean Microbial DNA isolation kit (MO BIO Laboratories, Carlsbad, CA) and a minimum of 50 ng DNA was then amplified using the Staphylococcus fingerprinting kit (Diversi- Lab; bioMérieux) according to the manufacturer's instructions. PCR products were separated on DiversiLab LabChips (bioMérieux) utilizing a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) operating the DiversiLab v1.4 assay. Bioanalyzer data were exported to a DiversiLab website and analyzed with DiversiLab v3.4 software. Data analysis was performed with the web-based software using the Pearson coefficients to determine distance matrices and the unweighted pair group method with arithmetic mean to create dendrograms. The DiversiLab data generated for each organism included a dendrogram with virtual gel images, a graph of fluorescence intensity that corresponds to the organism's banding pattern, and a similarity matrix. Cluster analysis was done by comparing the automated rep-PCR pattern for each isolate to a representative rep-PCR pattern obtained for each of the reference strains. Strain relatedness for the replicate studies was defined as a minimum of 90% similarity with a difference of up to 3 bands.
Systemic administration of bacteria
S. xylosus strains 720 and 1056B and S. aureus LS-1 and 1056A were inoculated intravenously (i.v.) in the tail vein in a total volume of 200 µl phosphate-buffered saline (PBS)/mouse. Viable counts were performed to determine the number of bacteria injected. The mice received the following number of bacteria: S. xylosus 1056B 0.8–1.2×108 bacteria/mouse, S. xylosus 720 1.4×108 bacteria/mouse, S. aureus LS-1 5.7×107 bacteria/mouse and S. aureus 1056A 0.8×108 bacteria/mouse. We used a strain of Burkholderia cepacia isolated from a CGD patient, as previously described (22). Mice were administered 4.5×105 CFU intraperitoneal (i.p.), an inoculum previously known to be lethal in p47phox−/− mice, but not in wildtype mice (22).
Bacterial counts in organs
Organs were aseptically dissected, homogenized, serially diluted in PBS and spread on agar plates. In B. cepacia experiments, peritoneal lavage was performed with 10 ml of DPBS. The number of colony forming units (CFUs) per site was determined after 24–48 h of incubation at 37°C.
Arthritis scoring
All mice were followed up individually and checked daily. Mice were graded blindly for arthritis severity and frequency. Finger/toe and ankle/wrist joints were inspected and arthritis was defined as visible erythema and or swelling. To evaluate the intensity of arthritis, a clinical scoring (arthritic index) was carried out using a system where macroscopic inspection yielded a score of 0–3 points for each limb (0, neither swelling nor erythema; 1, mild swelling and/or erythema; 2, moderate swelling and erythema; 3, marked swelling and erythema). The total score was calculated by adding up all the scores within each animal tested.
The overall condition of each mouse was also examined daily by assessing signs of systemic inflammation, i.e., weight decrease, reduced alertness, and ruffled coat. In cases of severe systemic infection, when a mouse was judged too ill to survive another 24 h, it was culled and defined as dead due to sepsis.
Enrichment of neutrophils from bone marrow with Percoll gradient
Bone marrow from femurs and tibiae were extracted by flushing the bones with PBS using a 26G needle. Red blood cells were lysed with ACK buffer and the cells washed with PBS before resuspending them in 3 ml 45% Percoll solution. A Percoll gradient was formed in a 15 ml tube, loading 3 ml of 66% Percoll followed by 2 ml of 60%, 2 ml of 55% and finally adding the cells. After 30 min centrifugation at 3000 rpm with brakes off, the 66-60% interface layer of cells was recovered. All solutions and operations were at room temperature. This method leads to a myeloid preparation consisting of ~ 90% neutrophils based on cytology.
Antibodies
Pacific Blue labeled anti-CD11b (Mac-1, M1/70) antibody and PE-Cy7 or PE labeled anti-Ly6G (1A8) (from BD Biosciences) and anti-FcR (24G2, generated in our laboratory) were used to stain the blood samples. Intracellular staining for Ncf1 was performed with antibody mouse anti human Ncf1 D10 (Santa Cruz) and detected with anti-mouse IgG1-APC (BD Biosciences).
Oxidative burst assay and flow cytometry
20 µl of freshly drawn blood were stained for ROS production as described previously (23). FcR interaction was blocked with anti-FcR blocker 24G2 before incubation with the surface markers antibodies, Pacific Blue anti-CD11b (Mac-1) antibody and PE-cy7 anti-Ly6G (BD Biosciences). DHR123 (Sigma) was added to the cells at a concentration of 3 nM and the cells were stimulated with phorbol 12-myristate 13-acetate (PMA) at a concentration of 200 ng/ml. After washing, 2×105 cells were acquired with a BD LSRII flow cytometer and the data analyzed with FlowJo version 8.8.6.
For NCF1 intracellular staining, after incubation with surface marker antibodies and washing in PBS, the cells were fixed and permeabilized with Cytofix/CytoPerm (BD Biosciences) and subsequently stained with anti-NCF1 antibody and anti-mouse IgG1-APC according to manufacturer instructions. NCF1 expression was measured as difference between the Geo mean of cells stained with anti-NCF1 antibody and the anti-mouse IgG1-APC and the geo mean of cells stained with only the anti-mouse IgG1-APC: the Δ Geo mean is represented.
In vivo ROS detection
Naïve mice were anaesthetized with isofluorane and injected i.p. with 20 mg/kg L-012 (8-amino-5-chloro-7-phenylpyrido[3,4-d]pyridazine-1,4(2H,3H)dione) probe (Wako Chemicals) dissolved in physiological saline (24). The luminescent signal was detected with IVIS 50 bioluminescent system (Xenogen), consisting of an anesthesia unit in a light tight chamber with a CCD camera IVIS 132323, DW434. Image acquisition and analysis were performed with Living Image software (Xenogen).
Histology
Infected and healthy paws were collected and fixed in 4% paraformaldehyde buffered solution. The skin was removed and the paws were decalcified in a solution of 4% paraformaldehyde and formic acid (1:1) for 48 hr. After dehydration, paws were embedded in paraffin and sectioned (10 or 4 µm) before staining with hematoxylin-eosin or Gram staining.
Statistical methods
Mann-Whitney U test was used to compare two groups. For comparison of three groups, Kruskal-Wallis with Dunn’s comparison post-test was used. For incidence comparisons, Fisher’s exact test was used. Survival curves were displayed by Kaplan-Meier and intergroup comparisons assessed by the log-rank method. GraphPad Prism, version 5.0c, was used for statistical analysis. P < 0.05 was considered statistically significant.
Results
Spontaneous inflammation in soft tissue of Ncf1 mutant mice
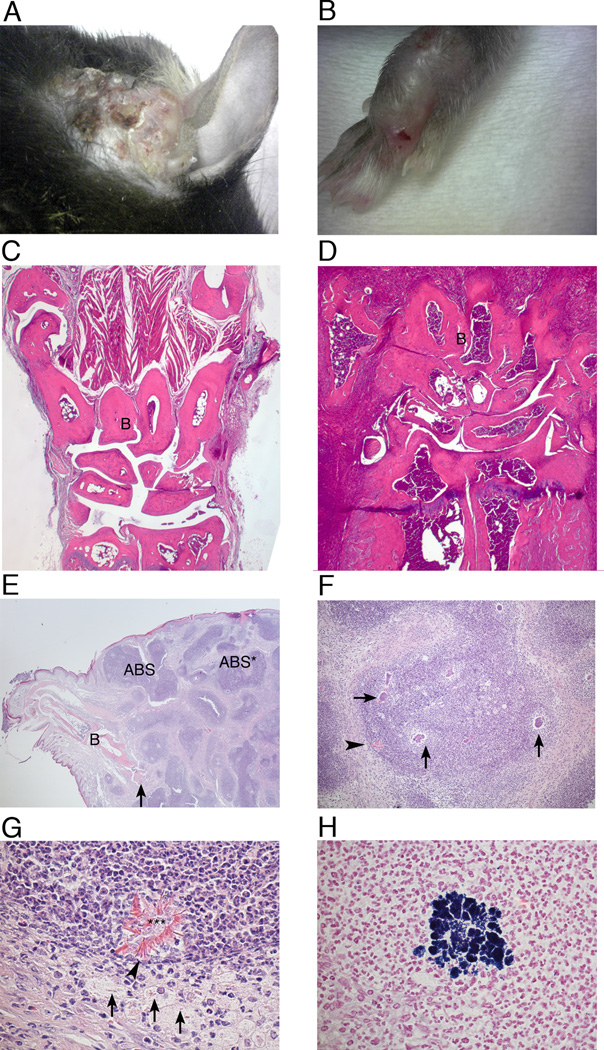
We observed local spontaneous infections in Ncf1 mutant (Ncf1*/*) but not in Ncf1 wt mice in the our mouse colonies in two different animal facilities, one being a conventional facility in Lund and one being a SPF, Felasa II facility in Stockholm. All the mice shared the C57Bl/10.Q.rhd background and carried the Ncf1 mutation, but their genotype varied with respect to other congenic fragments and transgenes (table I). The frequency of observed infections averaged 2% of 2600 B10.Q.Ncf1*/* mice. Infections occurred most commonly in the paws, but were also found in the tail, limbs, head and neck soft tissue. The inflamed tissue was swollen, red and infiltrated with pus, and was generally associated with scarring (fig 1 A and B). Histology revealed massive cell infiltration in the skin and bone, sometimes associated with bone necrosis (fig 1 C–E). In the abscesses, several bacterial colonies were present (fig 1 F), where Gram positive cocci were visible (fig 1 H), together with a large number of neutrophils and macrophages (fig 1 G). Splendore-Hoeppli material was observed around necrotic leukocytes and cell debris and inside necrotic macrophages (fig 1 G). Infections were more common in males than females.
Table I.
Number and frequency of observation of local infections in Ncf1 mutant mice in the Stockholm mouse cohorti
| Genotype | N° of observations | Total number of miceb |
Frequency (%) |
||
|---|---|---|---|---|---|
| Total | Males | Femalesa | |||
| B10.Q.Ncf1*/* | 27 | 21 | 6** | ≈ 2000 | ≈ 1.3 |
| B10.P.Ncf1*/*.MBQ | 4 | 3 | 1 | 154 | 3 |
| B10.Q.Ncf1*/*.uMT | 13 | 9 | 4* | 220 | 6 |
| B10.Q.Ncf1*/*.COMPko | 4 | 2 | 2 | 125 | 3 |
| B10.Q.Ncf1*/*.TCRko | 5 | 5 | 0 | 268 | 2 |
| Total B10.Q.Ncf1*/* background | 53 | 40 | 13*** | ≈ 2767 | ≈ 2 |
Statistical significance for the gender difference was calculated with Chi-square test: * P<0.05, ** P<0.001, *** P<0.0001.
The number of B10.Q.Ncf1*/* mice could only be estimated. For the other strains the total number of mice, males and females, is shown.
Number of Ncf1*/* mice from different lines with infections (most commonly in paws, nose, neck and ear) during 2009 and 2010. No infections were seen in any other mice, including the B10.Q.Ncf1+/+ (wt) controls or in heterozygous B10.Q.Ncf1*/+ mice. The frequency is calculated dividing the number of observed cases with the total number of Ncf1*/* mice. B10.Q.Ncf1*/*.TCRko are lacking T cells (deficient in TCRb), B10.Q.Ncf1*/*.Bko are lacking B cells (deficient in the membrane part of IgM), B10.Q.Ncf1*/*.COMPko are deficient in COMP and B10.P.Ncf1*/*.MBQ express H2-Aq specifically on macrophages. They are all on the B10.Q.Ncf1*/* background and have been previously been described (15–17). Statistical significance for the gender difference was calculated with Chi-square test: * P<0.05, ** P<0.01, *** P<0.001.
Figure 1. Spontaneous infections in soft tissue of Ncf1 mutant mice.
(A and B) Photograph of inflamed ear and paw of Ncf1 mutant mice with spontaneous infection. (C–H) Histology of inflamed and healthy front paws. After fixation in paraformaldehyde and decalcification, the paws were sectioned and stained with hematoxylin and eosin. Front paw from a healthy mouse (C) and from an infected one (D), 2.5X magnification. (E) Markedly enlarged, inflamed paw from an infected mouse shows profound, chronic inflammation with numerous abscesses (ABS) and cellulitis. Bones (B) and also necrosis of bone is visible (arrow). The abscess marked ABS* is shown with more detail in figure F. 2× magnification. (F) Detail of the abscess marked ABS* in the figure E. The arrows point at some of the bacterial colonies. The structure at the periphery of the abscess (arrow-head) is shown in more detail in figure G.10× magnification. (G) Close view of the structure marked with an arrow-head in figure F. It exhibits necrotic leukocytes, bacteria and cell debris (***) surrounded by brightly eosinophilic, radial Splendore-Hoepli material (arrow-heads). Part of this material also appears intracellularly in degenerate and necrotic macrophages (arrows) positioned at the periphery of the abscess. The vast majority of cells visible in this figure are neutrophils. 60× magnification. (H) Detail of abscess in paw shows a colony of Gram-positive cocci immersed in exudate of neutrophils and also some macrophages. Gram stain. 60× magnification.
Usually, several, if not all, mice in a cage developed infection, raising the possibility of transmitted infection. Interestingly, we also observed the same infection frequency in B10.Q.Ncf1*/* mice (but not in NOX2-competent mice) with specific engineered cell or protein deficiencies, including deficiency in B cells or T cells, deletion of cartilage oligomeric matrix protein (COMP) (17) or deficient expression of specific MHC class II alleles on macrophages (15), all on the same B10.Q genetic background.
To characterize the bacterial strains responsible for the infections, samples from infected animals were assessed. All isolates could be grown on hematin agar at 37°C under aerobic conditions. Species identification was based on 16S rDNA sequence analysis and phenotypic tests. The sequences of the isolates were identical to the 16S rRNA genes either of Staphylococcus aureus or Staphylococcus saprophyticus/xylosus. The S. aureus strain was denominated 1056A. Strains determined to belong to the family of S. saprophyticus and S. xylosus with 16S were differentiated and identified to be S. xylosus by their property to ferment xylose and mannose in oxidative-fermentative medium (21). Two S. xylosus strains were further characterized using the DiversiLab platform and were denominated S. xylosus 720 and S. xylosus 1056B. Interestingly all investigated samples from the animal house in Lund carried the S. xylosus 720 strain, whereas all samples from the Stockholm facility were either the S. xylosus 1056B or the S aureus 1056A strains. Thus, B10.Q.Ncf1*/* mice exhibited increased susceptibility to spontaneous staphylocococcal infections, with the mouse facility influencing the acquisition of specific bacterial strains.
Administered Staphylococcus xylosus and Staphylococcus aureus infections induced higher mortality in Ncf1 mutant mice compared with Ncf1 wt mice
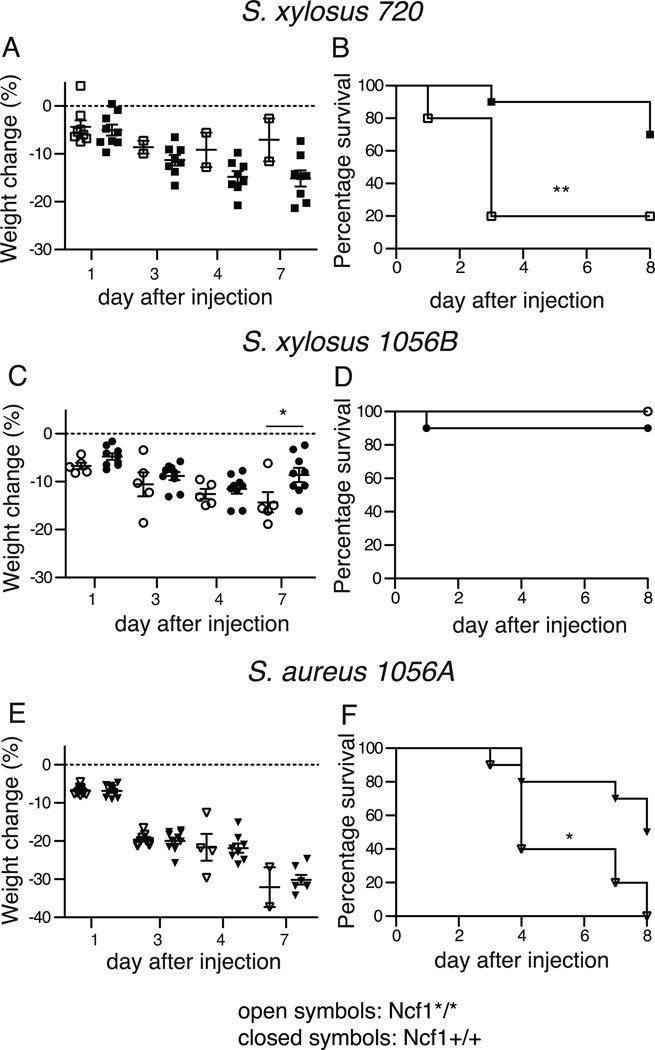
Based on our observations of spontaneous infections in Ncf1*/* mice, we evaluated susceptibility of Ncf1*/* versus Ncf1 wt (Ncf1+/+) mice to systemic infection. About 1×108 CFU of the isolated staphylococcal strains S. xylosus 720 and 1056B or S. aureus 1056A were injected intravenously (i.v.) in Ncf1*/* and Ncf1+/+ mice. The Ncf1*/* mice were more sensitive to infection than Ncf1+/+ mice based on mortality (fig 2 B, D and F). The bacteria differed greatly in virulence: S. xylosus 720 and the S. aureus 1056A caused lethal infection while the S. xylosus 1056B did not result in lethality in Ncf1*/* mice (figure 2 B, D and F).
Figure 2. Ncf1 mutant mice have increased susceptibility to systemic Staphylococcus xylosus and Staphylococcus aureus infections.
0.8–1.4×108 bacteria of the two different strains of S. xylosus (720 and 1056 B) and the S. aureus 1056A isolated from infected paws of Ncf1 mutant mice were injected i.v. into Ncf1 mutant (Ncf1*/*) mice (N=10 for S. aureus and S. xylosus 720 and N=5 for S. xylosus 1056 A) and into wt (Ncf1+/+) mice (N=10). Mean and SEM is shown.
Weight loss (A, C and E) and survival (B, D, and F) were measured up to 7 days after bacterial challenge. Survival curves were displayed by Kaplan-Meier and intergroup comparisons assessed by the log-rank method. * P<0.05, ** P<0.01
Weight loss observed during the infection was comparable in the Ncf1*/* and Ncf1+/+ mice (fig 2 A, C, E). The analysis of this parameter is however heavily affected by the high mortality in the Ncf1*/* group as only few surviving Ncf1*/* mice could be evaluated for weight loss. These data indicate that the Ncf1*/* mice are more susceptible to systemic S. xylosus (with variability in strain virulence) and S. aureus infection than Ncf1 +/+ mice.
Staphylococcus aureus-induced arthritis was less severe in Ncf1 mutant as compared with Ncf1 wt mice
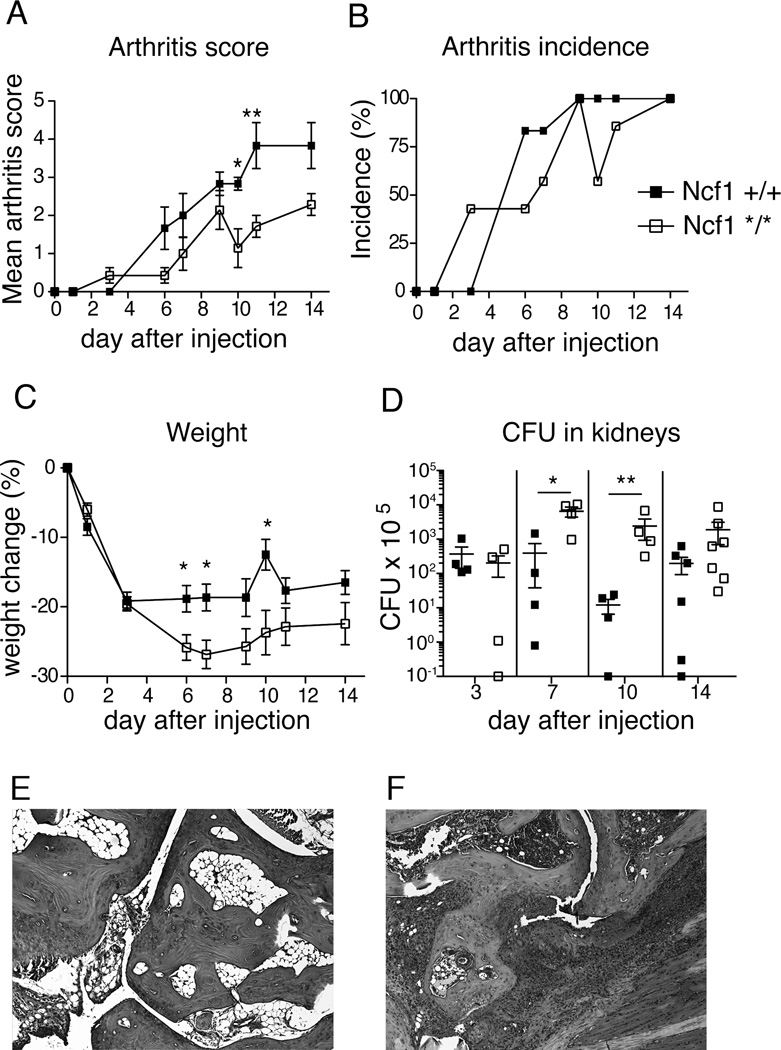
We have previously shown that experimental autoimmune arthritis is more severe in Ncf1*/* compared with Ncf1+/+ mice (12). To investigate whether this is also the case with septic arthritis we injected 5.7×107 S. aureus LS-1 bacteria i.v. into Ncf1*/* and Ncf1+/+ mice. The LS-1 strain of S. aureus was selected because of its propensity to cause septic arthritis in mice (25). Both groups developed arthritis with a similar frequency, but Ncf1+/+ mice had significantly greater arthritic severity (fig 3 A and B). Ncf1*/* mice lost more weight compared to Ncf1+/+ littermates (fig 3 C), and had a higher bacterial count in the kidneys after bacterial injection (fig 3 D). No significant difference between the genotypes was observed in bacterial counts from blood at day 1 and 3 after infection (data not shown). Histology showed infiltration of cells into the joints and tissue and bone destruction (fig 3 E–F). These data indicate that Ncf1*/* mice have impaired clearance of bacteria, but a milder septic arthritis than Ncf1+/+ mice. Since Ncf1*/* mice are prone to more severe autoimmune chronic arthritis (12), our results suggest that NOX2 has distinct roles in modulating the inflammatory response in autoimmune and septic arthritis.
Figure 3. Weight loss and arthritis development after S. aureus LS-1 i.v. injection in Ncf1 mutant and wt mice.
5.7×107 S. aureus LS-1 was injected i.v. in Ncf1 wt and mutant mice (n=19 mice per group). Arthritis severity and incidence (A and B) and weight (C) were evaluated over 14 days. (D) Four mice per group were sacrificed on day 3, 7, and 10, and the remaining 7 mice per group were sacrificed on day 14 after infection, and quantitative bacterial cultures were performed on kidneys. Mean and SEM is shown, except for incidence. * P<0.05, ** P<0.01. Statistics was calculated with Mann-Whitney for arthritis score and weight and with Kruskal-Wallis with Dunn’s comparison post-test for bacterial cultures. On fig 3 A–C only the 7 mice/genotype that were kept alive until day 14 are illustrated. (E–F) Histology of healthy and affected paw after injection of S. aureus LS-1, respectively. 5× magnification.
Expression of Ncf1 in monocytes protects from infection after Staphylococcus aureus and Staphylococcus xylosus infection
Previously, we generated transgenic mice (transgene designated, MN) on the B10.Q.Ncf1*/* background (Ncf1*/* MN+/−) that express the functional form of NCF1 through the human CD68 promoter, leading to restricted expression of NCF1 to monocytes and macrophages. Ncf1*/* MN+/− mice were partially protected from autoimmune arthritis compared to Ncf1*/* mice (18). We observed that Ncf1*/* MN+/− mice did not develop spontaneous infections, and thus it became relevant to investigate whether Ncf1*/* MN+/− mice were protected from administered infections.
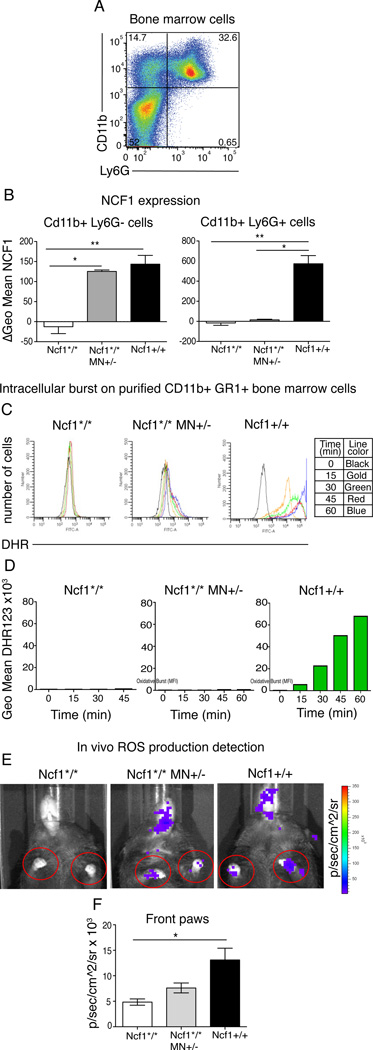
We previously reported that in Ncf1*/* MN+/− mice, NCF1 is specifically expressed on CD68 positive macrophages and not on other cells such as B cells and T cells (18), and we have now confirmed that it is not expressed in neutrophils, defined as CD11b+Ly6G+ expressing cells (26) (fig 4 A and supplementary fig 1 A and D). In Ncf1*/* MN+/− mice, there was full expression of NCF1 in monocyte/macrophages (CD11b+Ly6G− cells) comparable to the levels in wild type monocytes/macrophages, but no expression in neutrophils (CD11b+Ly6G+ cells), as investigated in cells from bone marrow, blood and spleen (fig 4 B and supplementary fig 1 B and E). Intracellular ROS production was measured by flow cytometry using the probe dihydrorhodamine 123 (DHR123) (27), which upon oxidation emits a fluorescent signal. Phorbol 12-myristate 13-acetate (PMA) was used as activator of the NOX2 complex. Blood monocytes (CD11b+Ly6G−) from Ncf1*/* MN+/− mice showed about the half of the DHR staining of monocytes from Ncf1+/+ mice (supplementary fig 1 C). Blood neutrophils (CD11b+Ly6G+) from Ncf1*/* MN+/− mice had approximately 15% of the DHR staining as neutrophils from Ncf1+/+ mice (supplementary fig 1 C). This observation was confirmed in splenic and thioglycollate-elicited peritoneal neutrophils (supplementary fig 1 F and data not shown). The discrepancy between the lack of NCF1 expression and the detectable DHR staining in neutrophils could be explained by leakage of ROS or other DHR stained products from macrophages to neutrophils after PMA stimulation in vitro. To examine this hypothesis, neutrophils from bone marrow were purified with a Percoll gradient, stained for CD11b and Ly6G and analyzed via flow cytometry for their capacity to oxidize DHR. PMA-stimulated ROS production from CD11b+Ly6G+ cells from Ncf1*/* MN+ mice was minimal (< 1% of Ncf1+/+ neutrophils) but distinguishable from ROS production in CD11b+Ly6G+ cells from Ncf1*/* mice (fig 4 C and D). In order to confirm that the monocytes ROS production in Ncf1*/* MN+/− mice was detectable also in vivo whole body imaging of ROS production was performed using the probe L-012 (24). L-012 is a sensitive luminol derivative that detects ROS in biological samples, including NOX2 derived superoxide (28). L-012 was injected i.p. in sedated naïve mice and chemiluminescent signal was recorded over time with a CCD camera. Apart from the peritoneal area where the probe was injected, the luminescent signal was best detectable in paws where fur is absent. Therefore the signal was quantified around the front paws as represented in fig 4E In vivo oxidative burst was higher in paws from naïve Ncf1+/+ and Ncf1*/* MN+/− mice compared to Ncf1*/* mice. The signal from Ncf1*/* MN+/− mice was reduced compared to wild type, as expected since in healthy joints different ROS-producing cells other than macrophages are present (fig 4F). This observation illustrates the presence of detectable NOX2 dependent ROS even in naïve animals and the crucial role of the Ncf1 mutation in abolishing it.
Figure 4. NCF1 expression and ROS quantification in bone marrow neutrophils from Ncf1*/* MN +/− mice.
(A) Bone marrow cells were stained for surface markers CD11b (Mac-1, M1/70) and Ly6G (1A8) and gated accordingly. CD11b+ Ly6G− cells are considered as monocytes and CD11b+ Ly6G+ cells as neutrophils. (B) Intracellular NCF1 expression was measured in both cell populations. Mean and SEM of 5 animals per group is shown. (C) Intracellular ROS production was measured in purified on neutrophils from pooled bone marrows. After enrichment for neutrophils with Percoll gradient, cells were identified as neutrophils according to their scatter and CD11b and Ly6G expression by flow cytometry. DHR fluorescence was measured at different time points after PMA stimulation. Histograms with different colors represent different time points: black 0 min, gold 15 min, green 30 min, red 45 min and blue 60 min. (D) Bar chart of the geo mean of DHR fluorescence at different time points after PMA stimulation. (E) In vivo oxidative burst imagining: luminescent probe L-012 was injected i.p. in naïve anesthetized mice and the luminescent signal was detected over 35 min by a CCD camera. Images illustrate a representative example for each genotype. (F) To quantify the in vivo oxidative burst, the luminescent signal was quantified at 15 min as photons/second/cm2/steradian in the circled areas surrounding the front paws and the mean of the two front paws measurements was used. Mean and SEM of 5 mice per group. Statistical comparison is performed with Kruskal-Wallis with Dunn’s comparison post-test.
Statistical significance between the groups indicated by a bar: * P<0.05, ** P<0.01, *** P<0.001.
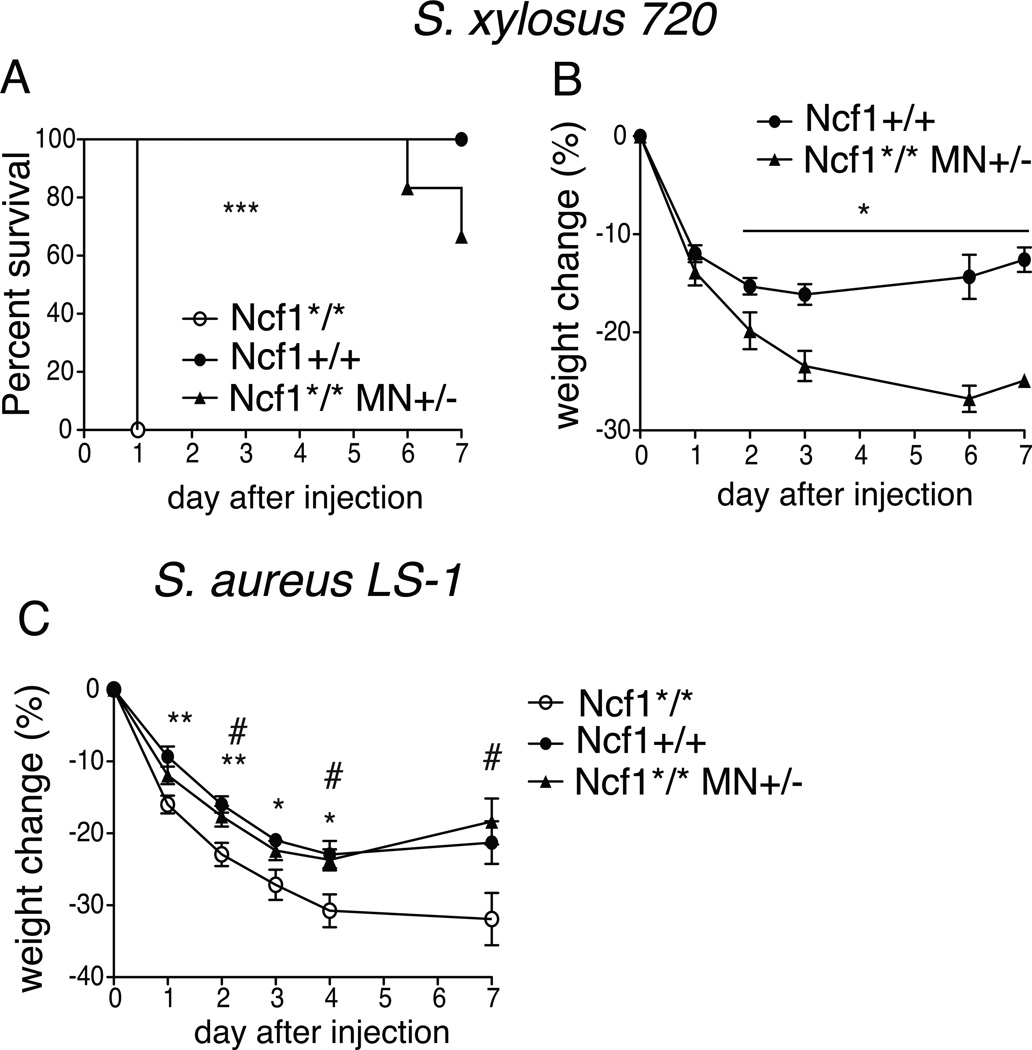
We next evaluated susceptibility to administered infection in transgenic Ncf1*/* MN+/− mice compared to littermates negative for the transgene (Ncf1*/*) and wt Ncf1+/+ mice. Mice were injected with S. xylosus (1×108 CFU i.v), and monitored for 7-day survival. Ncf1*/* mice died within 24 hours, whereas all Ncf1+/+ survived. Mortality was observed in 30 % of Ncf1*/* MN+/− on day 6 and 7 (fig. 5A). Between the surviving groups, Ncf1*/* MN+/− lost more weight than Ncf1+/+ (fig 5B). Together, these data indicate that a functional NOX2 complex expressed in monocytes protects from S. xylosus challenge, although the transgene may not confer full protection as compared to fully NOX2-competent Ncf1+/+ mice.
Figure 5. NCF1 expression in monocytes protects from Staphylococci.
0.8×108 bacteria of S. xylosus 720 isolated from infected paws of Ncf1 mutant mice were injected i.v. into Ncf1*/* MN+/− and non transgenic littermates Ncf1*/* mice as well as Ncf1 wt (Ncf1+/+) mice (N=6–9). Survival (A) and weight change (B) were monitored over 7 days. Survival curves were displayed by Kaplan-Meier and intergroup comparisons assessed by the log-rank method: *** P<0.001. For weight changes, mean and SEM is shown and genotypes were compared using Mann-Whitney *P<0.05.
(C) Male Ncf1*/* MN+/− mice (N=9), as well as littermates negative for the transgene (Ncf1*/*, N=10) and Ncf1 wt (Ncf1+/+) (N=7) mice were injected i.v. with 5.7 × 10^7 S. aureus LS-1. Weight was monitored for 7 days. Mean and SEM is shown. * P<0.05 and ** P<0.01 between Ncf1*/* and Ncf1 +/+ mice, # P<0.05 between Ncf1*/* MN+/− and Ncf1*/* calculated with Kruskal-Wallis with Dunn’s comparison post-test.
Transgenic Ncf1*/* MN+/− mice and littermates negative for the transgene (Ncf1*/*) and Ncf1+/+ mice were next evaluated for their response to the arthritogenic S. aureus LS-1 strain. Weight and arthritis were monitored for 7 days following bacterial administration. Ncf1*/* male mice lost significantly more weight than Ncf1+/+ and Ncf1*/* MN+/− mice. (fig 5C). No difference in lethality between the groups was observed (data not shown). Bacterial load in the kidney at day 2, 4, and 7, in synovial fluid at day 7, and in blood at day 1 did not differ among the genotypes (data not shown). Arthritis developed in all groups but with very low scores (data not shown).
Ncf1 expression in monocytes rescued mice from lethal Burkholderia cepacia infection
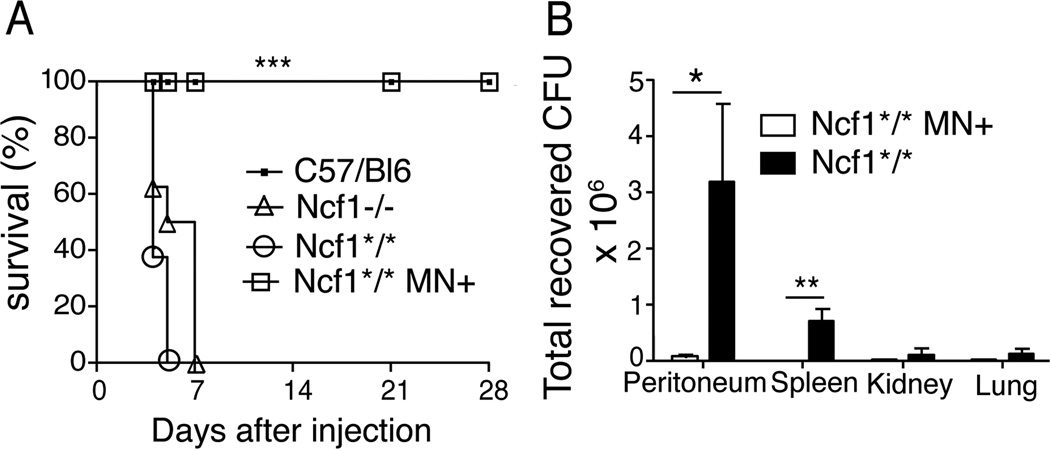
Burkholderia cepacia is a Gram-negative rod that can cause severe infections in CGD patients (29, 30). Studies in isolated human neutrophils showed the key role of NOX2 in killing B. cepacia (31). Genetically engineered Ncf1 knock out (Ncf1−/− or p47phox−/−) mice had increased susceptibility to B. cepacia compared to wt mice (32). To evaluate the specific contribution of monocyte NOX2 expression in the defense against B. cepacia we evaluated survival and bacterial clearance in Ncf1*/* MN+ mice as compared with the Ncf1*/* MN− mice. Mice were challenged i.p. with a B. cepacia strain isolated from a CGD patient. All Ncf1*/* mice died within 6 days after infection, while Ncf1*/* MN+ mice had uniform survival (Figure 6A). Engineered Ncf1−/− mice in the C57BL/6 lineage were tested in parallel to determine whether knockout and spontaneous mutant mice differed in response to bacterial infection. Ncf1−/− mice died with a similar time course as Ncf1*/* mice, while wt C57BL/6 mice survived. Thus, spontaneous and engineered NOX2-deficient mice were highly susceptible to B. cepacia whereas transgenic mice with monocyte NADPH expression were protected.
Figure 6. NCF1 expression in monocytes protects Ncf1*/* mice from lethal B. cepacia infection.
B10.Q.Ncf1*/* MN+ and B10.Q.Ncf1*/* littermates negative for the transgene, Ncf1−/− and C57Bl/6 mice (N=10) were challenged i.p. with 4.5×105 B. cepacia. (A) Survival curves are displayed by Kaplan-Meier and intergroup comparisons between globally NADPH oxidase-deficient mice (Ncf1−/− and Ncf1*/*) and Ncf1*/* MN+ mice assessed by the log-rank method: *** P<0.001. (B) In separate studies, mice were administered i.p. 4 × 105 B. cepacia and bacteria load in peritoneal lavage, spleen, kidneys and lungs cell suspensions were quantified at 24h. Data are the combined results of two independent experiments; n= 8 mice per genotype. Mean and SEM is shown. * P< 0.004 using Mann Whitney test; ** p< 0.04 using Wilcoxon Signed Rank Test, which was used when bacterial cultures from a group were negative.
We next compared clearance of B. cepacia at a fixed time point after bacterial challenge in Ncf1*/* MN− and Ncf1*/* MN+ mice. Mice administered the same inoculum were sacrificed at 24h, a time that precedes the onset of clinical morbidity. Higher numbers of live bacteria were recovered from the peritoneum and spleens of Ncf1*/* MN− mice compared to Ncf1*/* MN+ (Fig. 6B). No difference between the genotypes was observed in bacterial counts in kidneys and lungs (data not shown). The majority of blood cultures showed no growth, and no significant difference was observed between genotypes (not shown). Taken together, the survival and bacterial clearance data demonstrate protection associated with monocyte NOX2 expression against B. cepacia. We conclude that NCF1 expression in monocytes plays an essential role in the systemic response to both spontaneous and induced bacterial infections.
Discussion
Our results show that monocyte-specific expression of the NOX2 regulatory component, NCF1, protected mice from lethal infections with staphylococci and Burkholderia cepacia, pathogens that commonly affect CGD patients. Interestingly, a similar phenomenon has been recently described in humans regarding susceptibility to tuberculosis. A macrophage specific impairment of the core subunit of the NOX2 complex, gp91phox resulted in susceptibility to tuberculous mycobacterial disease (33). These observations suggest that a functional NOX2 complex in monocytes is important for clearance of specific intra- and extracellular bacteria.
Transgenic mice in which a gene of interest is expressed under the control of the human CD68 promoter has been widely used to achieve monocyte/macrophage-restricted expression (34, 35) based on the monocyte predominance of CD68 expression. Using the same promoter we could confirm a monocyte/macrophage specific NCF1 expression in Ncf1*/*MN+/− versus Ncf1*/*. A previous publication from our group shows that in Ncf1*/*MN+/− mice the expression of Ncf1 was undetectable in dendritic cells (DCs) (18). A more sensitive analysis revealed expression of functional NCF1 protein in DCs from Ncf1*/*MN+/− transgenic mice (Pizzolla A. and Holmdahl R., unpublished results). Since myeloid DCs and monocytes have a shared lineage, it is not unexpected that CD68 promoter activity would be present in both cells, as reported recently (36). NOX2 is expressed in DCs and could have a role in antigen display and priming T cell responses (37). However, the role of DCs in controlling acute bacterial infection, if any, is unclear. Ncf1*/*MN+/− mice showed a low but significant DHR staining indicating presence of ROS in neutrophils after activation in vitro. Here neutrophils are defined as CD11b+Ly6G+ cells. Even though it is difficult to formally exclude any NCF1 expression in neutrophils, we could not detect the protein by flow cytometry in blood CD11b+Ly6G+ cells. It is possible that a fraction of those cells transcribes the human CD68 promoter or that NCF1 is expressed in vivo due to promoter-independent expression. Kuhns et al. (38) recently showed that among CGD patients, even very low levels of neutrophil NOX2 was associated with better outcomes compared to patients with complete NOX2 deficiency. Studies of isolated neutrophils have shown that hydrogen peroxide generated by NOX2-competent cells can diffuse into intracellular compartment of bystander NOX2-deficient neutrophils (39, 40). These observations indicate how the function of ROS may not be restricted to the cell that produces it, raising the possibility that also in vivo, in our transgenic mice, ROS produced by monocytes could penetrate into low bursting neutrophils, increasing their antimicrobial activity.
The importance of neutrophil ROS production in antimicrobial defense is well established (13). The critical amount of ROS-producing neutrophils necessary to protect the host is however, difficult to determine. Studies on CGD carriers and genetically corrected CGD mouse models showed that a small fraction of ROS-producing neutrophils, about 10% of the total, could efficiently protect from bacterial and fungal infection (41, 42). Nevertheless, in these studies, all phagocytes, both neutrophils and monocytes, expressed the functional NOX2 complex, and, most commonly, only the neutrophils ROS production was determined. Therefore the immunological relevance of neutrophils and monocytes separately could not be assessed.
The NOX2 complex accounts for most of the ROS production that is essential for anti-microbial defense, since mutations in any of the genes coding for the subunits of the complex leads to a CGD phenotype in humans (4, 7). The NOX2 complex also generates the majority of the ROS observed in vivo since the mutation in Ncf1 abolishes the visible ROS production in both naïve (fig 4 E and F) and arthritic mice (24, 43). In addition, ROS produced by other sources such as the mitochondrial respiratory chain, have been described to have bactericidal activity in macrophage cell lines (44).
Similar to the situation in CGD patients, in mice a naturally occurring mutation in Ncf1 increases the risk of uncontrolled bacterial infections. The infections observed in Ncf1 mutant mice were caused by S. aureus and S. xylosus. S. aureus is a common pathogen in CGD patients (3) and is commonly found on the skin of healthy individuals, but never so far reported in mouse models of CGD. S. xylosus was found to cause abscesses in soft tissue of Ncf1−/− mice with similar characteristics to the abscesses observed in Ncf1*/* mice (8). Environmental factors could be relevant in determining the type and frequency of infections. The conditions under which the mice were maintained and the bacterial flora carried by the handlers could explain the difference in infectious strains observed in various colonies of Ncf1*/* and Ncf1−/− mice. Staphylococci are normally non-pathogenic bacteria for mice, since wt mice have not developed any infection. The infection rapidly spreads in the cage between homozygous Ncf1*/* offspring of Ncf1*/* parents. Males are affected more often than females. This could be due to the fact that males fight more than females resulting in wounds. A wound was always observed in the infected area and it is likely to be the access for bacteria into the soft tissue. Recently it was reported that NOX2 deficient mice develop spontaneous inflammation in their paws (45), which phenotypically resembled the infections observed in Ncf1−/− (11) and Ncf1*/* animals (fig. 1A).
The involvement of cells other than phagocytes in the NOX2-dependent clearance of pathogens does not seem to play a role in spontaneous infections. The absence of B cells or T cells did not dramatically alter the frequency of spontaneous infection by staphylococci. In both mice and humans it is known that an effective immunological memory is not built after S. aureus infections (mechanisms reviewed by Foster (46). In addition to antimicrobial host defense, NOX2 is also a critical modulator of inflammation. Indeed, one of the characteristics of CGD is excessive inflammation, including inflammatory bowel disease, granulomatous cystitis, and pneumonitis. Macrophage NOX2 may be important in controlling inflammation. We have previously shown that monocytes/macrophage expression of NCF1 protects against autoimmune chronic inflammatory disease (18). There are a number of mechanisms by which macrophage NOX2 may limit inflammation, including internalization of apoptotic neutrophils (47–49), modulation of transcriptional factors, such as NF-κB and Nrf2 (50), modifying antigen-processing (43, 51) or regulating T cells during antigen presentation (52) and reviewed by Sareila et al. (43). A longstanding question has been whether this is secondary to the deficient protection against infections or whether lack of oxidative burst in fact promotes inflammatory disorders. In mice, arthritis directly caused by S. aureus infection is in fact decreased in the ROS-deficient mice in contrast to autoimmune arthritis where we previously found that inflammation was increased (12).
In conclusion, monocyte NOX2 expression is associated with protection from spontaneous and induced bacterial infections. Further characterization of cell specific molecular mechanisms of bacterial clearance is important for elucidating the pathways involved in microbial defense and for the development of targeted cures for CGD.
Supplementary Material
Acknowledgements
The authors would like to thank Emma Mondoc and Dr. Ricardo Feinstein at SVA, Uppsala, for histology, Carlos and Kristina Palestro and Tomasz Klaczkowski for care of the animals.
Footnotes
Authorships contributions
A.P. performed research, collected data, analyzed and interpreted data and wrote the manuscript. M.H. provided animals, analyzed and interpreted data. B.N., M.J.G., T.E., I-M. J., M.V. and T.K. performed research, collected data, analyzed and interpreted data. I.G., B.H.S. and R.H. designed research and wrote the manuscript.
This work was supported by the Swedish Research Council, the Swedish Strategic Science Foundation, the Academy of Finland, the European Union grants BeTheCure and Masterswitch (HEALTH-F2-2008-223404), the NIAID R01AI079253 (BHS), NCI Cancer Center and support Grant to Roswell Park Cancer Institute (CA016056).
Abbreviations:
CGD: Chronic granulomatous disease
DHR: dehydrorhodamine
NCF1: neutrophils cytosolic factor 1
NOX2: NADPH oxidase 2 complex
ROS: reactive oxygen species
DC: Dendritic cell
References
- 1.Bridges RA, Berendes H, Good RA. A fatal granulomatous disease of childhood; the clinical, pathological, and laboratory features of a new syndrome. AMA J Dis Child. 1959;97:387–408. [PubMed] [Google Scholar]
- 2.Grimm MJ, Vethanayagam RR, Almyroudis NG, Lewandowski D, Rall N, Blackwell TS, Segal BH. Role of NADPH oxidase in host defense against aspergillosis. Med Mycol. 2011;49(Suppl 1):S144–S149. doi: 10.3109/13693786.2010.487077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holland MS. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38:3–10. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- 4.van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, Espanol T, Fischer A, Kurenko-Deptuch M, Mouy R, Petropoulou T, Roesler J, Seger R, Stasia MJ, Valerius NH, Weening RS, Wolach B, Roos D, Kuijpers TW. Chronic granulomatous disease: the European experience. PLoS One. 2009;4:e5234. doi: 10.1371/journal.pone.0005234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal AW, Jones OT, Webster D, Allison AC. Absence of a newly described cytochrome b from neutrophils of patients with chronic granulomatous disease. Lancet. 1978;2:446–449. doi: 10.1016/s0140-6736(78)91445-9. [DOI] [PubMed] [Google Scholar]
- 6.Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Matute JD, Arias AA, Wright NA, Wrobel I, Waterhouse CC, Li XJ, Marchal CC, Stull ND, Lewis DB, Steele M, Kellner JD, Yu W, Meroueh SO, Nauseef WM, Dinauer MC. A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood. 2009;114:3309–3315. doi: 10.1182/blood-2009-07-231498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 9.Ju JY, Polhamus C, Marr KA, Holland SM, Bennett JE. Efficacies of fluconazole, caspofungin, and amphotericin B in Candida glabrata-infected p47phox−/− knockout mice. Antimicrob Agents Chemother. 2002;46:1240–1245. doi: 10.1128/AAC.46.5.1240-1245.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mardiney M, 3rd, Jackson SH, Spratt SK, Li F, Holland SM, Malech HL. Enhanced host defense after gene transfer in the murine p47phox-deficient model of chronic granulomatous disease. Blood. 1997;89:2268–2275. [PubMed] [Google Scholar]
- 11.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hultqvist M, Olofsson P, Holmberg J, Bäckström BT, Tordsson J, Holmdahl R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci U S A. 2004;101:12646–12651. doi: 10.1073/pnas.0403831101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quie PG, White JG, Holmes B, Good RA. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest. 1967;46:668–679. doi: 10.1172/JCI105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang CK, Zhan L, Hannigan MO, Ai Y, Leto TL. P47(phox)-deficient NADPH oxidase defect in neutrophils of diabetic mouse strains, C57BL/6J-m db/db and db/+ J Leukoc Biol. 2000;67:210–215. doi: 10.1002/jlb.67.2.210. [DOI] [PubMed] [Google Scholar]
- 15.Pizzolla A, Gelderman KA, Hultqvist M, Vestberg M, Gustafsson K, Mattsson R, Holmdahl R. CD68-expressing cells can prime T cells and initiate autoimmune arthritis in the absence of reactive oxygen species. Eur J Immunol. 2011;41:403–412. doi: 10.1002/eji.201040598. [DOI] [PubMed] [Google Scholar]
- 16.Svensson L, Jirholt J, Holmdahl R, Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA) Clin Exp Immunol. 1998;111:521–526. doi: 10.1046/j.1365-2249.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng H, Carlsen S, Nandakumar KS, Holmdahl R, Aspberg A, Oldberg A, Mattsson R. Cartilage oligomeric matrix protein deficiency promotes early onset and the chronic development of collagen-induced arthritis. Arthritis Res Ther. 2008;10:R134. doi: 10.1186/ar2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelderman KA, Hultqvist M, Pizzolla A, Zhao M, Nandakumar KS, Mattsson R, Holmdahl R. Macrophages suppress T cell responses and arthritis development by producing Reactive Oxygen Species. J Clin Invest. 2007;117:3020–3028. doi: 10.1172/JCI31935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldenberger D, Schmidheini T, Altwegg M. Detection of Bartonella henselae and Bartonella quintana by a simple and rapid procedure using broad-range PCR amplification and direct single-strand sequencing of part of the 16S rRNA gene. Clin Microbiol Infect. 1997;3:240–245. doi: 10.1111/j.1469-0691.1997.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 20.Fredricks ND, Relman DA. Improved amplification of microbial DNA from blood cultures by removal of the PCR inhibitor sodium polyanetholesulfonate. J Clin Microbiol. 1998;36:2810–2816. doi: 10.1128/jcm.36.10.2810-2816.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bannerman TL. Staphylococcus, Micrococcus, and other catalase-positive cocci that grow aerobically. In: Murray EJOBPR, Jorgensen JH, Pfaller MA, Yolken RH, editors. Manual of clinical microbiology. 8th ed. Washington DC: ASM Press; 2003. pp. 719–728. [Google Scholar]
- 22.Segal BH, Ding L, Holland SM. Phagocyte NADPH oxidase, but not inducible nitric oxide synthase, is essential for early control of Burkholderia cepacia and chromobacterium violaceum infection in mice. Infect Immun. 2003;71:205–210. doi: 10.1128/IAI.71.1.205-210.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hultqvist M, Olofsson P, Gelderman KA, Holmberg J, Holmdahl R. A new arthritis therapy with oxidative burst inducers. PLoS Med. 2006;3:e348. doi: 10.1371/journal.pmed.0030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kielland A, Blom T, Nandakumar KS, Holmdahl R, Blomhoff R, Carlsen H. In vivo imaging of reactive oxygen and nitrogen species in inflammation using the luminescent probe L-012. Free Radic Biol Med. 2009;47:760–766. doi: 10.1016/j.freeradbiomed.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Bremell T, Abdelnour A, Tarkowski A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect Immun. 1992;60:2976–2985. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 27.Rothe G, Oser A, Valet G. Dihydrorhodamine 123: a new flow cytometric indicator for respiratory burst activity in neutrophil granulocytes. Naturwissenschaften. 1988;75:354–355. doi: 10.1007/BF00368326. [DOI] [PubMed] [Google Scholar]
- 28.Daiber A, August M, Baldus S, Wendt M, Oelze M, Sydow K, Kleschyov AL, Munzel T. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic Biol Med. 2004;36:101–111. doi: 10.1016/j.freeradbiomed.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Winkelstein JA, Marino MC, Johnston RB, Jr, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, Buckley RH, Foster CB, Chanock SJ, Dickler H. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Sieber OF, Jr, Fulginiti VA. Pseudomonas cepacia pneumonia in a child with chronic granulomatous disease and selective IgA deficiency. Acta Paediatr Scand. 1976;65:519–520. doi: 10.1111/j.1651-2227.1976.tb04924.x. [DOI] [PubMed] [Google Scholar]
- 31.Speert DP, Bond M, Woodman RC, Curnutte JT. Infection with Pseudomonas cepacia in chronic granulomatous disease: role of nonoxidative killing by neutrophils in host defense. J Infect Dis. 1994;170:1524–1531. doi: 10.1093/infdis/170.6.1524. [DOI] [PubMed] [Google Scholar]
- 32.Segal BH, Sakamoto N, Patel M, Maemura K, Klein AS, Holland SM, Bulkley GB. Xanthine oxidase contributes to host defense against Burkholderia cepacia in the p47(phox−/−) mouse model of chronic granulomatous disease. Infect Immun. 2000;68:2374–2378. doi: 10.1128/iai.68.4.2374-2378.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bustamante J, Arias AA, Vogt G, Picard C, Galicia LB, Prando C, Grant AV, Marchal CC, Hubeau M, Chapgier A, de Beaucoudrey L, Puel A, Feinberg J, Valinetz E, Janniere L, Besse C, Boland A, Brisseau JM, Blanche S, Lortholary O, Fieschi C, Emile JF, Boisson-Dupuis S, Al-Muhsen S, Woda B, Newburger PE, Condino-Neto A, Dinauer MC, Abel L, Casanova JL. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol. 2011;12:213–221. doi: 10.1038/ni.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gough PJ, Gordon S, Greaves DR. The use of human CD68 transcriptional regulatory sequences to direct high-level expression of class A scavenger receptor in macrophages in vitro and in vivo. Immunology. 2001;103:351–361. doi: 10.1046/j.1365-2567.2001.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang R, Rutschman RL, Greaves DR, Murray PJ. Autocrine deactivation of macrophages in transgenic mice constitutively overexpressing IL-10 under control of the human CD68 promoter. J Immunol. 2002;168:3402–3411. doi: 10.4049/jimmunol.168.7.3402. [DOI] [PubMed] [Google Scholar]
- 36.Lykens JE, Terrell CE, Zoller EE, Divanovic S, Trompette A, Karp CL, Aliberti J, Flick MJ, Jordan MB. Mice with a selective impairment of IFN-gamma signaling in macrophage lineage cells demonstrate the critical role of IFN-gamma-activated macrophages for the control of protozoan parasitic infections in vivo. J Immunol. 184:877–885. doi: 10.4049/jimmunol.0902346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, Amigorena S. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 38.Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, Uzel G, DeRavin SS, Priel DA, Soule BP, Zarember KA, Malech HL, Holland SM, Gallin JI. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363:2600–2610. doi: 10.1056/NEJMoa1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohno Y, Gallin JI. Diffusion of extracellular hydrogen peroxide into intracellular compartments of human neutrophils. Studies utilizing the inactivation of myeloperoxidase by hydrogen peroxide and azide. J Biol Chem. 1985;260:8438–8446. [PubMed] [Google Scholar]
- 40.Rex JH, Bennett JE, Gallin JI, Malech HL, Melnick DA. Normal and deficient neutrophils can cooperate to damage Aspergillus fumigatus hyphae. J Infect Dis. 1990;162:523–528. doi: 10.1093/infdis/162.2.523. [DOI] [PubMed] [Google Scholar]
- 41.Roos D, de Boer M, Kuribayashi F, Meischl C, Weening RS, Segal AW, Ahlin A, Nemet K, Hossle JP, Bernatowska-Matuszkiewicz E, Middleton-Price H. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood. 1996;87:1663–1681. [PubMed] [Google Scholar]
- 42.Bjorgvinsdottir H, Ding C, Pech N, Gifford MA, Li LL, Dinauer MC. Retroviral-mediated gene transfer of gp91phox into bone marrow cells rescues defect in host defense against Aspergillus fumigatus in murine X-linked chronic granulomatous disease. Blood. 1997;89:41–48. [PubMed] [Google Scholar]
- 43.Sareila O, Kelkka T, Pizzolla A, Hultqvist M, Holmdahl R. NOX2 Complex-derived ROS as Immune Regulators. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2010.3635. [DOI] [PubMed] [Google Scholar]
- 44.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee K, Won HY, Bae MA, Hong JH, Hwang ES. Spontaneous and aging-dependent development of arthritis in NADPH oxidase 2 deficiency through altered differentiation of CD11b+ and Th/Treg cells. Proc Natl Acad Sci U S A. 2011;108:9548–9553. doi: 10.1073/pnas.1012645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 47.Sanmun D, Witasp E, Jitkaew S, Tyurina YY, Kagan VE, Ahlin A, Palmblad J, Fadeel B. Involvement of a functional NADPH oxidase in neutrophils and macrophages during programmed cell clearance: implications for chronic granulomatous disease. Am J Physiol Cell Physiol. 2009;297:C621–C631. doi: 10.1152/ajpcell.00651.2008. [DOI] [PubMed] [Google Scholar]
- 48.Hampton MB, Vissers MC, Keenan JI, Winterbourn CC. Oxidant-mediated phosphatidylserine exposure and macrophage uptake of activated neutrophils: possible impairment in chronic granulomatous disease. J Leukoc Biol. 2002;71:775–781. [PubMed] [Google Scholar]
- 49.Fernandez-Boyanapalli RF, Frasch SC, McPhillips K, Vandivier RW, Harry BL, Riches DW, Henson PM, Bratton DL. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood. 2009;113:2047–2055. doi: 10.1182/blood-2008-05-160564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segal BH, Han W, Bushey JJ, Joo M, Bhatti Z, Feminella J, Dennis CG, Vethanayagam RR, Yull FE, Capitano M, Wallace PK, Minderman H, Christman JW, Sporn MB, Chan J, Vinh DC, Holland SM, Romani LR, Gaffen SL, Freeman ML, Blackwell TS. NADPH oxidase limits innate immune responses in the lungs in mice. PLoS One. 5:e9631. doi: 10.1371/journal.pone.0009631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mantegazza AR, Savina A, Vermeulen M, Perez L, Geffner J, Hermine O, Rosenzweig SD, Faure F, Amigorena S. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112:4712–4722. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, Segal BH, Puccetti P. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.