Abstract
Background
Docosahexaenoic acid (DHA) is a long-chain polyunsaturated fatty acid important for neonatal neurodevelopment and immune homeostasis. Preterm infants fed donor milk from a Midwestern source receive only 20% of the intrauterine accretion of DHA. We tested the hypothesis that DHA supplementation of donor mothers would provide preterm infants with DHA intake equivalent to fetal accretion.
Subjects and Methods
After Institutional Review Board approval and informed consent, human milk donors to the Mother's Milk Bank of Ohio were randomized to receive 1 g of DHA (Martek® [now DSM Nutritional Lipids, Columbia, MD]) or placebo soy oil. Dietary intake data were collected and analyzed by a registered dietitian. Fatty acids were measured by gas chromatography/flame ionization detection. Statistical analysis used linear mixed models.
Results
Twenty-one mothers were randomly assigned to either the DHA group (n=10) or the placebo group (n=11). Donor age was a median of 31 years in both groups with a mean lactational stage of 19 weeks. Dietary intake of DHA at baseline in both groups was a median of 23 mg/day (range, 0–194 mg), significantly (p<0.0001) less than the minimum recommended intake of 200 mg/day. The DHA content of milk increased in the DHA-supplemented group (p<0.05).
Conclusions
The women enrolled in this study had low dietary DHA intake. Supplementation with preformed DHA at 1 g/day resulted in increased DHA concentrations in the donor milk with no adverse outcomes. Infants fed donor milk from supplemented women receive dietary DHA levels that closely mimic normal intrauterine accretion during the third trimester.
Introduction
Preterm infants miss the last trimester of nutrient accretion and may be deficient in essential molecules.1,2 These deficiencies may contribute to increased rates of infection and make these infants more vulnerable to disease3 and poor developmental outcomes.4 Long-chain ω-3 fatty acids, in particular docosahexaenoic acid (DHA), are preferentially transported across the placenta5 and provide important components of membrane phospholipids that have been associated with improved markers of brain and retinal development.6,7 It is currently estimated that DHA is accumulated at 67–75 mg/day in utero during the last trimester5,6; therefore premature infants do not benefit from this increase in DHA delivery. The preterm infant depends on the concentration of DHA in the milk provided in the neonatal intensive care unit because the only Food and Drug Administration–approved intravenous fat emulsion provided to babies in the United States does not contain DHA. In adults, DHA can be synthesized from its precursor, the essential fatty acid 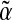 linolenic acid, through a series of desaturase and elongase enzyme activities that may be limited in the preterm infant.8,9 Preterm infants may utilize the majority of fat sources for fuel sources.9,10
linolenic acid, through a series of desaturase and elongase enzyme activities that may be limited in the preterm infant.8,9 Preterm infants may utilize the majority of fat sources for fuel sources.9,10
Depending on the population examined, milk concentrations have ranged from 0.1% to 2%.11 Our data indicate that women donating to human milk banks fall in the lower portion of this range,12 and the human donor milk fed to preterm infants is deficient in DHA content. Currently, the American Academy of Pediatrics recommends that the preterm infant feeding plans mimic intrauterine nutrient intake as the most logical approach for appropriate nutritional levels.13 Consequently, a better understanding of the influence of the maternal diet and the need for DHA supplementation, specifically among women donating milk, is warranted to maximize nutrition for the high-risk neonate. Our hypothesis was that an intake of 1 g/day would be required in the maternal diet to produce a milk composition to mimic intrauterine intake.
Subjects and Methods
After Institutional Review Board approval and informed consent we enrolled mothers donating milk to the Mothers' Milk Bank of Ohio, Grant Hospital, Columbus, OH, in a pilot feasibility trial of DHA compared with placebo supplementation. All women donating to the milk bank were included. The only exclusion was a bereaved mother who lacked milk volume. The mothers selected into the study were the first 21 women donating milk in that time period. The power analysis was done using previous work of Makrides et al.14 on supplementation and milk concentration, and the predefined outcome was to increase maternal milk concentrations of DHA to 0.8% mol weight.
The randomization schedule was developed by the statistician in the study, and assignment was made by the pharmacist. DHA (single-cell algal oil) and placebo (soy oil) capsules were packaged by Martek® (now DSM Nutritional Lipids, Columbia, MD) and stored in the investigational pharmacy at Nationwide Children's Hospital. The supplement was then mailed to the donor mother. The dietitian instructed the mothers on supplementation. The mothers took either five 200-mg capsules of algal DHA/day or five 200-mg capsules of soy oil (placebo). The mothers were given the supplement for as long as they donated to the milk bank, which was 7–90 days. Mothers were instructed on clean pumping, collection, and delivery to the milk bank, and milk was pooled according to guidelines by The Human Milk Banking Association of North America. Milk was stored at −80°C, and then fatty acid concentrations were measured at baseline and Days 7, 14, 21, 28, and 84 by gas chromatography-flame ionization detection.15,16 Baseline dietary data were obtained using 3-day diet records prior to dietary supplementation and also at Weeks 1, 4, and 12, mailed to each participant and followed by instructions given over the phone by the clinical dietitian. The dietary intake data were collected and analyzed by a trained individual at The Ohio State University using Nutrition Data System for Research software developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN. Statistical analyses of nutrients were done using linear mixed models.17
Results
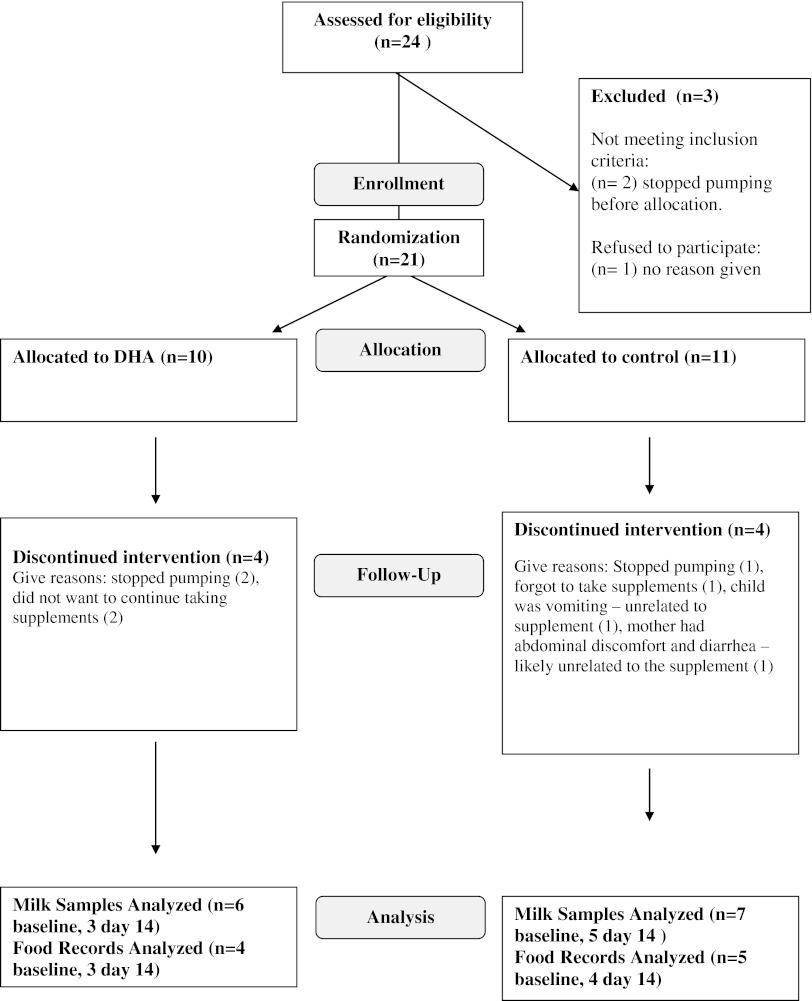
Twenty-one women at The Mothers' Milk Bank of Ohio were enrolled and randomly assigned to the DHA or placebo group (Fig. 1). No mother in the DHA group withdrew because of intolerance to the supplement. After allocation to the DHA supplement three mothers quit pumping prior to baseline collection (Day 0). Five mothers did not take the placebo intervention before milk analysis because of discontinued pumping (n=2), infant at home ill (n=1), low milk supply (n=1), or the mother became ill (n=1). By Day 14, four additional mothers had quit pumping in the DHA group and one in the placebo group.
FIG. 1.
The study design, enrollment, and enrollment using the CONSORT framework. DHA, docosahexaenoic acid.
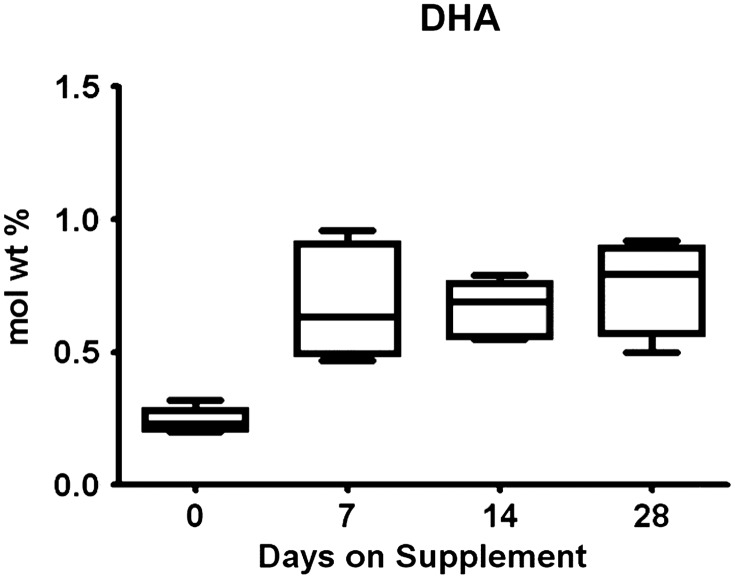
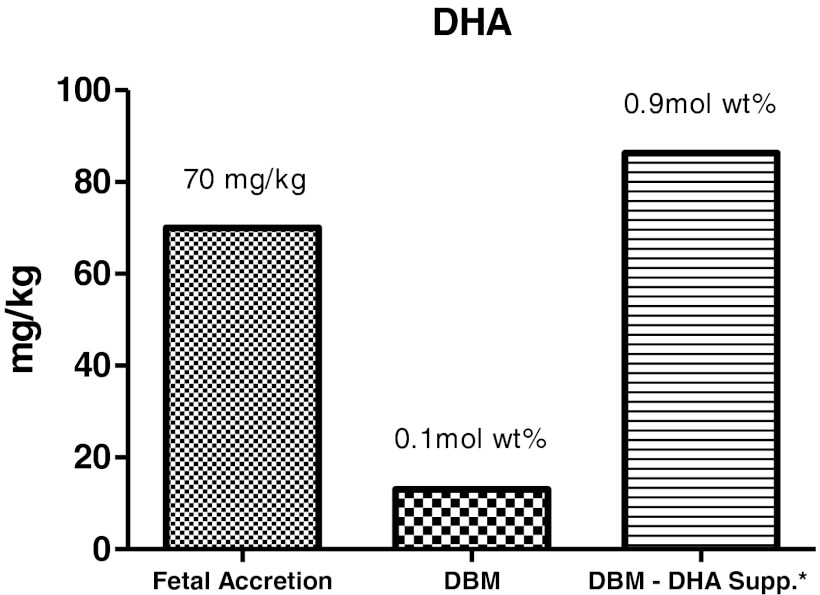
Baseline milk samples were obtained therefore in six women in the DHA group and seven in the placebo group (Fig. 1). No differences were found in maternal age or lactational stage between the groups (Table 1). Dietary intake of calories, protein, total fat, and DHA were similar across groups (Table 2). The dietary DHA (23 mg/day) was significantly lower than current recommendations for pregnancy and lactation (200 mg/day) in both groups prior to randomization (p<0.0001). The concentrations of the essential fatty acids and other long-chain fatty acids other than DHA were not different between groups. The absolute milk concentration of DHA was more than four times higher in the supplemented group by Day 14 (Table 3) and was significantly higher than baseline samples (p<0.05). The mole weight percent of the DHA concentration also improved significantly from baseline (p<0.01; Fig. 2). Figure 3 represents estimated intake of the fetus by intrauterine accretion in the first column, projected intake of the preterm infant by unsupplemented donor breastmilk if fed at 150 mL/kg/day in the second column, and maternal-supplemented DHA milk concentrations if fed to a preterm infant at 150 mL/kg/day in the third column. Based on the estimated volume of preterm infant milk intake of 150 mL/kg/day, the concentration of DHA in the supplemented donor milk met intrauterine accretion levels5 (Fig. 3).
Table 1.
Age and Lactational Stage of Mothers Contributing Milk in the Randomized Trial
| Placebo (n=7) | DHA (n=6) | p value | |
|---|---|---|---|
| Maternal age (years) | 30 (3.5) | 32 (4.2) | 0.527 |
| Lactational stage (weeks) | 19 (11) | 22 (17) | 0.687 |
Data are mean (SD) values.
Table 2.
Medians and 95% Confidence Intervals of Maternal Dietary Components
| |
Placebo supplement |
DHA supplement |
|
||||
|---|---|---|---|---|---|---|---|
| Baseline (n=4) | Day 14 (n=3) | Ratioa | Baseline (n=5) | Day 14 (n=4) | Ratioa | p valueb | |
| DHA (g)c | |||||||
| Median | 0.03 | 0.03 | 0.99 | 0.07 | 0.10 | 1.34 | 0.91 |
| 95% CI | (0.00, 0.08) | (0.00, 0.09) | — | (0.03, 0.13) | (0.04, 0.18) | — | |
| Linolenic acid (g) | |||||||
| Median | 2.67 | 2.38 | 0.89 | 1.83 | 1.90 | 1.04 | 0.75 |
| 95% CI | (1.42, 5.03) | (1.17, 4.86) | (0.40, 2.01) | (1.05, 3.20) | (1.03, 3.52) | (0.49, 2.19) | |
| Arachidonic acid (g) | |||||||
| Median | 0.08 | 0.06 | 0.81 | 0.09 | 0.12 | 1.23 | 0.77 |
| 95% CI | (0.02, 0.36) | (0.01, 0.39) | (0.06, 10.11) | (0.02, 0.39) | (0.02, 0.59) | (0.13, 11.31) | |
| EPA (g)d | |||||||
| Median | 0.01 | 0.01 | 0.90 | 0.02 | 0.05 | 2.82 | 0.28 |
| 95% CI | (0.00, 0.05) | (0.00, 0.05) | — | (0.00, 0.06) | (0.01, 0.17) | — | |
| Linoleic acid (g) | |||||||
| Median | 18.14 | 17.81 | 0.98 | 15.90 | 17.93 | 1.13 | 0.94 |
| 95% CI | (11.94, 27.56) | (10.71, 29.61) | (0.04, 24.95) | (10.81, 23.39) | (11.49, 27.69) | (0.10, 13.09) | |
| Protein (g) | |||||||
| Median | 98.87 | 100.53 | 1.02 | 94.72 | 93.91 | 0.99 | 0.96 |
| 95% CI | (61.97, 157.73) | (58.63, 172.40) | (0.46, 2.25) | (62.37, 143.83) | (58.86, 149.80) | (0.50, 1.96) | |
| Fat (g) | |||||||
| Median | 100.48 | 90.65 | 0.90 | 91.69 | 92.84 | 1.01 | 0.82 |
| 95% CI | (65.79, 153.48) | (55.58, 147.84) | (0.39, 2.10) | (62.77, 133.93) | (60.78, 141.81) | (0.50, 2.05) | |
| Carbohydrate (g) | |||||||
| Median | 325.15 | 321.76 | 0.99 | 311.97 | 305.79 | 0.98 | 0.99 |
| 95% CI | (230.26, 459.21) | (216.00, 479.29) | (0.39, 2.54) | (229.11, 424.79) | (216.55, 431.82) | (0.46, 2.10) | |
| Energy (kcal) | |||||||
| Median | 2,549.37 | 2,427.46 | 0.95 | 2,486.42 | 2403.54 | 0.97 | 0.96 |
| 95% CI | (1,875.07, 3,466.50) | (1,702.58, 3,461.30) | (0.58, 1.57) | (1,888.99, 3,272.80) | (1,767.82, 3,268.22) | (0.63, 1.49) | |
Medians and confidence intervals (CIs) were estimated using a linear mixed model applied to the natural log of the measures.
Day 14 median/baseline median.
(Ratio for DHA supplement)/(ratio for placebo supplement). All p values are unadjusted.
0.1 was added to each value prior to ln transformation. CIs for ratios could not be computed because of the required addition.
0.01 was added to each value prior to ln transformation. CIs for ratios could not be computed because of the required addition.
EPA, eicosapentaenoic acid.
Table 3.
Estimated Medians and 95% Confidence Intervals of Fatty Acid Levels in Donor Milk
| |
Placebo supplement |
DHA supplement |
|
||||
|---|---|---|---|---|---|---|---|
| Baseline (n=7) | Day 14 (n=3) | Ratioa | Baseline (n=6) | Day 14 (n=5) | Ratioa | p valueb | |
| DHA (mg) (absolute)a | |||||||
| Median | 16.3 | 10.86 | 0.67 | 13.97 | 48.95 | 3.50 | 0.07 |
| 95% CI | (9.56, 27.81) | (4.94, 23.85) | (0.13, 3.45) | (7.68, 25.44) | (24.81, 96.54) | (1.01, 12.19) | |
| DHA (mol wt %) | |||||||
| Median | 0.18 | 0.16 | 0.88 | 0.18 | 0.65 | 3.58 | 0.0045 |
| 95% CI | (0.11, 0.30) | (0.09, 0.28) | (0.55, 1.39) | (0.11, 0.30) | (0.38, 1.10) | (2.03, 6.32) | |
| Linolenic acid (mg) | |||||||
| Median | 109.76 | 85.09 | 0.78 | 99.79 | 107.24 | 1.07 | 0.89 |
| 95% CI | (60.76, 198.26) | (45.77, 158.17) | (0.54, 1.12) | (54.80, 181.74) | (58.06, 198.11) | (0.66, 1.75) | |
| Arachidonic acid (mg) | |||||||
| Median | 37.01 | 28.85 | 0.78 | 35.77 | 34.55 | 0.97 | 1.00 |
| 95% CI | (23.40, 58.56) | (16.17, 51.46) | (0.39, 1.57) | (21.93, 58.34) | (20.23, 58.98) | (0.46, 2.03) | |
| EPA (mg) | |||||||
| Median | 4.89 | 4.17 | 0.85 | 4.30 | 6.10 | 1.42 | 1.00 |
| 95% CI | (2.21, 10.78) | (1.55, 11.26) | (0.22, 3.33) | (1.85, 9.99) | (2.42, 15.37) | (0.35, 5.69) | |
| Linoleic acid (mg) | |||||||
| Median | 1,365.67 | 1,231.76 | 0.90 | 1,134.90 | 1,157.25 | 1.02 | 1.00 |
| 95% CI | (838.74, 2,223.42) | (734.14, 2,066.68) | (0.64, 1.27) | (691.18, 1,863.48) | (694.44, 1,928.70) | (0.65, 1.60) | |
Medians and their confidence intervals were estimated using a linear mixed model applied to natural log of the measures.
Day 14 median/baseline median.
(Ratio for DHA supplement)/(ratio for placebo supplement). The p values for DHA were unadjusted; other p values were adjusted using Holm's method.25
FIG. 2.
DHA contents in milk samples during the course of supplementation. DHA concentration was measured by gas chromatography, and concentrations expressed as mole weight percent of total fatty acid contents. Data were analyzed by mixed models. The concentration of DHA was significantly different from baseline at Days 7 and 14 in the supplemented group (p<0.01).
FIG. 3.
Estimated intake of the preterm infant fed the baseline donor breastmilk (DBM) compared with the supplemented milk (DBM-DHA Supp.) and compared with intrauterine fetal accretion of DHA. DHA-supplemented mothers had DHA concentrations in breastmilk similar to intrauterine accretion. The DHA level is calculated assuming an infant receives 150 mL/kg/day. *Maternal intake of 1 g of DHA/day supplemented for 7 days.
Discussion
Previous studies have established the influence of long-chain polyunsaturated fatty acid supplementation on the concentrations of breastmilk fatty acids.14,18 A full-term breastfed infant accretes approximately 1,900 mg of DHA in the first 6 months of life, whereas infants fed formulas with little or no DHA become DHA-depleted.19 In the Midwest, despite ingesting adequate amounts of calories and protein in reasonably healthy diets, the women in our population consumed very little DHA. In addition, it was apparent that despite the continued use of prenatal vitamins by the study participants, only a few actually received preformed DHA. Many of the vitamin supplements had sufficient quantities of α-linolenic (18:3n3), which did not translate to higher concentrations of DHA in the milk. Supplementation, for example, with flaxseed oil, high in α-linolenic acid, has been previously shown to not be effective in increasing DHA levels in breastmilk.20 Tracer studies have indicated that preformed DHA is a significantly more efficacious source for neural development than is α-linolenic acid.21 In other studies as well as the present study, increasing the intake of preformed DHA to 1 g/day yielded substantial increases in breastmilk contents. Furthermore, the neurological benefits of DHA supplementation have been described to be most significant for our most immature preterm infants weighing <1,250 g.22,23 In addition, preterm infants who have higher DHA plasma concentrations have been found to have less likelihood for chronic lung disease.24 In order to achieve intrauterine goals for dietary DHA and an opportunity to promote developmental indices and decrease chronic lung disease, an improved human milk concentration of DHA is imperative for feeding preterm infants.
Our findings support the previous reports that the level of human milk DHA can be raised by maternal supplementation.23 Furthermore, preterm infants fed human milk obtained from these supplemented women at 150 mL/kg/day would receive quantities sufficient to mimic fetal accretion. This emphasizes the importance of diet education for all mothers providing human milk to preterm infants and stresses the importance of further study to determine whether the currently recommended dose of 200 mg/day for pregnant and lactating women is actually sufficient. The primary limitation to our study was the nature of the donor milk banking and the small number of women in our final analysis because of cessation of breastfeeding their own infants. In addition, evaluations of digestion, absorption, and blood concentrations of DHA in the preterm infant ingesting this milk were not done and will be needed in future studies. A larger trial is necessary to examine diet education, milk concentrations, and the functional outcomes associated with various concentrations of dietary DHA in mothers donating milk.
Conclusions
The women from the Mothers' Milk Bank of Ohio studied in this report had low dietary intakes of DHA. Supplementation with a single-cell algal DHA product at 1 g/day improved dietary DHA and maternal milk concentrations to mimic intrauterine goals for the preterm infant. Other donor milk banks should evaluate maternal diets to determine if this is a regional experience or if supplementation of DHA at 1 g/day should be required for all mothers providing human milk.
Acknowledgments
We thank the mothers for donating human milk to infants in need; the staff at the Mothers' Milk Bank of Ohio; Diane Habash, Ph.D., R.D., for her support of the dietary analysis; Jill Bland, PharmD, for her help with storing and dispensing the supplements; Molly Augustine, B.S., for her assistance with fatty acid analysis; and The NIH Pediatric Research Loan Repayment Program. Intramural support was provided by the Research Institute, Nationwide Children's Hospital, and The Ohio State University, supported by Award number UL1RR025755 from the National Center for Research Resources. DHA and the placebo capsules were supplied by Martek Biosciences Corp. (now DSM Nutritional Lipids).
Disclosure Statement
The authors and the acknowledged individuals have no financial relationship with the products used in the study and have no declarations.
References
- 1.Hay WW., Jr. Nutritional requirements of extremely low birthweight infants. Acta Paediatr Suppl. 1994;402:94–99. doi: 10.1111/j.1651-2227.1994.tb13369.x. [DOI] [PubMed] [Google Scholar]
- 2.Neu J. Valentine C. Meetze W. Scientifically-based strategies for nutrition of the high-risk low birth weight infant. Eur J Pediatr. 1990;150:2–13. doi: 10.1007/BF01959470. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson SA. Special nutritional needs of infants for prevention of and recovery from bronchopulmonary dysplasia. J Nutr. 2001;131:942S–946S. doi: 10.1093/jn/131.3.942S. [DOI] [PubMed] [Google Scholar]
- 4.Poindexter BB. Langer JC. Dusick AM, et al. Early provision of parenteral amino acids in extremely low birth weight infants: Relation to growth and neurodevelopmental outcome. J Pediatr. 2006;148:300–305. doi: 10.1016/j.jpeds.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 5.Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005;26(Suppl A):S70–S75. doi: 10.1016/j.placenta.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Clandinin MT. Chappell JE. Leong S, et al. Intrauterine fatty acid accretion rates in human brain: Implications for fatty acid requirements. Early Hum Dev. 1980;4:121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- 7.Henriksen C. Haugholt K. Lindgren M, et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics. 2008;121:1137–1145. doi: 10.1542/peds.2007-1511. [DOI] [PubMed] [Google Scholar]
- 8.Clandinin MT. Chappell JE. Heim T, et al. Fatty acid utilization in perinatal de novo synthesis of tissues. Early Hum Dev. 1981;5:355–366. doi: 10.1016/0378-3782(81)90016-5. [DOI] [PubMed] [Google Scholar]
- 9.Mayes C. Burdge GC. Bingham A, et al. Variation in [U-13C] alpha linolenic acid absorption, beta-oxidation and conversion to docosahexaenoic acid in the pre-term infant fed a DHA-enriched formula. Pediatr Res. 2006;59:271–275. doi: 10.1203/01.pdr.0000196372.29648.7a. [DOI] [PubMed] [Google Scholar]
- 10.Lapillonne A. Jensen CL. Reevaluation of the DHA requirement for the premature infant. Prostaglandins Leukot Essent Fatty Acids. 2009;81:143–150. doi: 10.1016/j.plefa.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Brenna JT. Varamini B. Jensen RG, et al. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85:1457–1464. doi: 10.1093/ajcn/85.6.1457. [DOI] [PubMed] [Google Scholar]
- 12.Valentine CJ. Morrow G. Fernandez S, et al. Docosahexaenoic acid and amino acid contents in pasteurized donor milk are low for preterm infants. J Pediatr. 2010;157:906–910. doi: 10.1016/j.jpeds.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics. Pediatric Nutrition Handbook. 6th. American Academy of Pediatrics; Elk Grove Village, IL: 2009. [Google Scholar]
- 14.Makrides M. Neumann MA. Gibson RA. Effect of maternal docosahexaenoic acid (DHA) supplementation on breast milk composition. Eur J Clin Nutr. 1996;50:352–357. [PubMed] [Google Scholar]
- 15.Bligh EG. Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 16.Folch J. Lees M. Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 17.Box G. Hunter JS. Hunter W. Statistics for Experiments: An Introduction to Design, Data Analysis and Model Building. 2nd. John Wiley & Sons; New York: 2005. [Google Scholar]
- 18.Jensen CL. Maude M. Anderson RE, et al. Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids. Am J Clin Nutr. 2000;71(1 Suppl):292S–299S. doi: 10.1093/ajcn/71.1.292s. [DOI] [PubMed] [Google Scholar]
- 19.Cunnane SC. Francescutti V. Brenna JT, et al. Breast-fed infants achieve a higher rate of brain and whole body docosahexaenoate accumulation than formula-fed infants not consuming dietary docosahexaenoate. Lipids. 2000;35:105–111. doi: 10.1007/s11745-000-0501-6. [DOI] [PubMed] [Google Scholar]
- 20.Francois CA. Connor SL. Bolewicz LC, et al. Supplementing lactating women with flaxseed oil does not increase docosahexaenoic acid in their milk. Am J Clin Nutr. 2003;77:226–233. doi: 10.1093/ajcn/77.1.226. [DOI] [PubMed] [Google Scholar]
- 21.Su HM. Bernardo L. Mirmiran M, et al. Dietary 18:3n-3 and 22:6n-3 as sources of 22:6n-3 accretion in neonatal baboon brain and associated organs. Lipids. 1999;34(Suppl):S347–S350. doi: 10.1007/BF02562339. [DOI] [PubMed] [Google Scholar]
- 22.Helland IB. Smith L. Saarem K, et al. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111:e39–e44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 23.Makrides M. Gibson RA. McPhee AJ, et al. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: A randomized controlled trial. JAMA. 2010;304:1675–1683. doi: 10.1001/jama.2010.1507. [DOI] [PubMed] [Google Scholar]
- 24.Martin CR. Dasilva DA. Cluette-Brown JE, et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J Pediatr. 2011;159:743–749. doi: 10.1016/j.jpeds.2011.04.039. e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]