Abstract
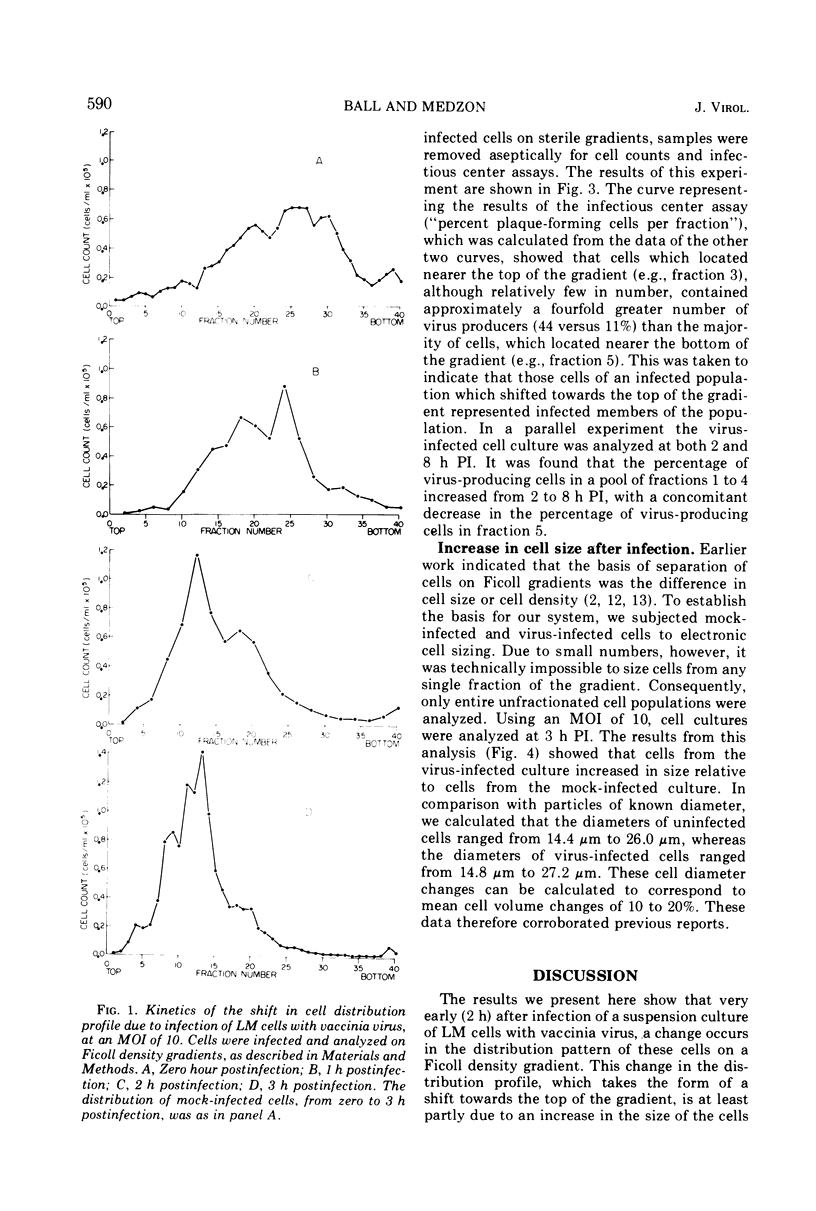
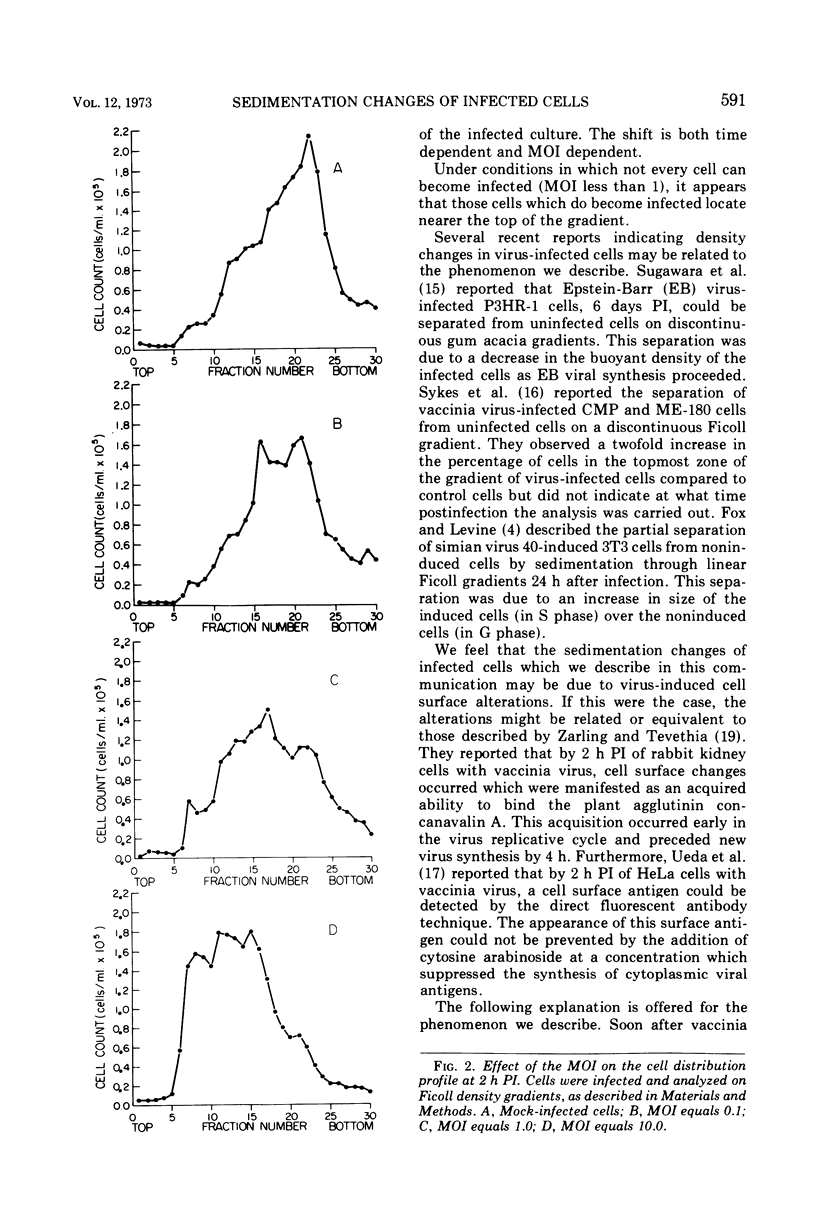
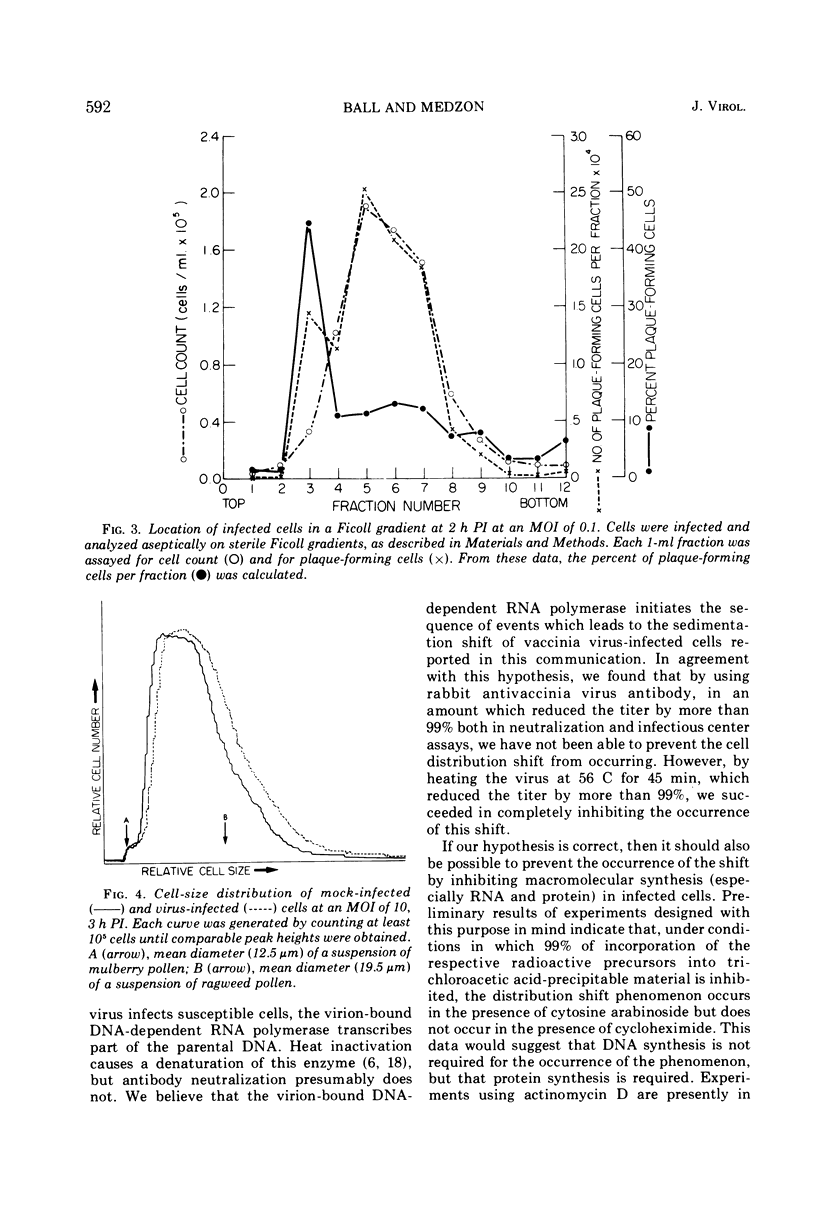
By 2 h postinfection, LM cells infected with vaccinia virus show a shift in their distribution when separated on a Ficoll density gradient. This shift is dependent on both time and the multiplicity of infection and is due, at least in part, to an increase in cell size. Those cells which do shift in position in the gradient represent infected members of the population. Physical changes induced in virus-infected cells can be utilized for studying early events in virus replication.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayad S. R., Fox M., Winstanley D. The use of ficoll gradient centrifugation to produce synchronous mouse lymphoma cells. Biochem Biophys Res Commun. 1969 Nov 6;37(4):551–558. doi: 10.1016/0006-291x(69)90844-4. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Summers D. F., Levintow L. Characterization of ribonuclease-resistant RNA from poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1273–1281. doi: 10.1073/pnas.54.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone C. W., Harell G. S., Bond H. E. The resolution of mixtures of viable mammalian cells into homogeneous fractions by zonal centrifugation. J Cell Biol. 1968 Feb;36(2):369–378. doi: 10.1083/jcb.36.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIRNS J. The initiation of vaccinia infection. Virology. 1960 Jul;11:603–623. doi: 10.1016/0042-6822(60)90103-3. [DOI] [PubMed] [Google Scholar]
- Esteban M., Metz D. H. Early virus protein synthesis in vaccinia virus-infected cells. J Gen Virol. 1973 May;19(2):201–206. doi: 10.1099/0022-1317-19-2-201. [DOI] [PubMed] [Google Scholar]
- Fox T. O., Levine A. J. Relationship between virus-induced cellular deoxyribonucleic acid synthesis and transformation by simian virus 40. J Virol. 1971 Apr;7(4):473–477. doi: 10.1128/jvi.7.4.473-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groyon R. M., Kniazeff A. J. Vaccinia virus infection of synchronized pig kidney cells. J Virol. 1967 Dec;1(6):1255–1264. doi: 10.1128/jvi.1.6.1255-1264.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K. The poxviruses. Annu Rev Microbiol. 1968;22:359–390. doi: 10.1146/annurev.mi.22.100168.002043. [DOI] [PubMed] [Google Scholar]
- MARCUS P. I., ROBBINS E. VIRAL INHIBITION IN THE METAPHASE-ARREST CELL. Proc Natl Acad Sci U S A. 1963 Dec;50:1156–1164. doi: 10.1073/pnas.50.6.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald H. R., Miller R. G. Synchronization of mouse L-cells by a velocity sedimentation technique. Biophys J. 1970 Sep;10(9):834–842. doi: 10.1016/S0006-3495(70)86338-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantani M., Kato S. Lack of virus-induced cell surface antigen in metaphase-arrest cells infected with poxvirus. Biken J. 1972 Jun;15(2):105–109. [PubMed] [Google Scholar]
- Medzon E. L., Bauer H. Structural features of vaccinia virus revealed by negative staining, sectioning, and freeze-etching. Virology. 1970 Apr;40(4):860–867. doi: 10.1016/0042-6822(70)90132-7. [DOI] [PubMed] [Google Scholar]
- Medzon E. L., Merchant D. J. Interaction of the LM cell surface with methylcellulose and vaccinia virus. Mode of action and implications for large scale vaccine production. In Vitro. 1971 Jul-Aug;7(1):46–58. doi: 10.1007/BF02619004. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Pretlow T. G., 2nd, Boone C. W. Separation of mammalian cells using programmed gradient sedimentation. Exp Mol Pathol. 1969 Oct;11(2):139–152. doi: 10.1016/0014-4800(69)90003-3. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Mizuno F., Osato T. Density changes of cultured Burkitt lymphoma cells following EB viral synthesis. Nat New Biol. 1971 Sep 22;233(38):106–107. doi: 10.1038/newbio233106a0. [DOI] [PubMed] [Google Scholar]
- Sykes J. A., Whitescarver J., Briggs L., Anson J. H. Separation of tumor cells from fibroblasts with use of discontinuous density gradients. J Natl Cancer Inst. 1970 Apr;44(4):855–864. [PubMed] [Google Scholar]
- Ueda Y., Ito M., Tagaya I. A specific surface antigen induced by poxvirus. Virology. 1969 May;38(1):180–182. doi: 10.1016/0042-6822(69)90141-x. [DOI] [PubMed] [Google Scholar]
- Woodson B. Recent progress in poxvirus research. Bacteriol Rev. 1968 Jun;32(2):127–137. doi: 10.1128/br.32.2.127-137.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Tevethia S. S. Expression of concanavalin A binding sites in rabbit kidney cells infected with vaccinia virus. Virology. 1971 Jul;45(1):313–316. doi: 10.1016/0042-6822(71)90140-1. [DOI] [PubMed] [Google Scholar]


