Abstract
Objectives
Recurrent urinary tract infections (UTIs) are a problem affecting both women and men. Animal experiments and in vitro studies indicate that statins might prevent recurrent UTIs. We assessed the effects of pravastatin on UTI antibiotic prescribing among adults.
Methods
A post hoc analysis was conducted with data from PREVEND IT, a trial among participants randomized to receive pravastatin, fosinopril or placebo in a 2 × 2 factorial design over 4 years. Trial data were linked to the pharmacy prescription database IADB.nl. The primary outcome was the number of prescriptions with a nitrofuran derivate, a sulphonamide or trimethoprim as a proxy for UTI antibiotic prescribing. Generalized estimating equations were used to estimate the effect on the number of UTI antibiotic prescriptions. Cox regression was used to determine the effect on first and second (recurrent) UTI antibiotic prescriptions.
Results
Of the 864 trial participants, 655 were eligible for analysis. During an average follow-up of 3.8 years, 112 (17%) participants received at least one UTI antibiotic prescription. Intention-to-treat analyses showed that pravastatin was associated with a reduced total number of UTI antibiotic prescriptions (relative risk, 0.43; 95% CI, 0.21–0.88) and occurrence of second UTI antibiotic prescriptions [hazard ratio (HR), 0.25; 95% CI, 0.08–0.77]. No significant effect on occurrence of first UTI antibiotic prescriptions was found (HR, 0.83; 95% CI, 0.57–1.20). Fosinopril was associated with an increased occurrence of first UTI antibiotic prescriptions (HR, 1.82; 95% CI, 1.16–2.88). Combination therapy with fosinopril and pravastatin did not significantly influence the number of UTI antibiotic prescriptions.
Conclusions
This study suggests that pravastatin can reduce the occurrence of recurrent UTIs. Larger studies among patients with recurrent UTIs are warranted.
Keywords: cystitis, statin, bacterial invasion
Introduction
Urinary tract infections (UTIs) are one of the most common bacterial infections in humans.1 Cystitis in particular is very common, with an annual incidence of 70 per 1000 women and 10 per 1000 men.1 Recurrent UTIs are a considerable problem, affecting ∼25% of women within 6 months of an acute UTI episode.2 Recurrent UTIs are also a problem in men.3
Recently, it has been shown that uropathogenic Escherichia coli are invasive to bladder and kidney epithelial cells.2 Bacterial invasion facilitates the establishment of a quiescent intracellular reservoir (QIR).2 The bacteria that form the QIR can persist for months following initial infection, resist antibiotic treatment4 and can serve as the source for recurrent UTIs.5
Bacterial invasion into the bladder epithelium involves Rac1, a Rho GTPase.6–9 Because statins can reduce the amount of Rac1 associated with the membrane,10–14 they might inhibit bacterial invasion. Pre-clinical studies indicate that statins indeed can reduce bacterial invasion.9,15–17
This might prevent the formation of a QIR. Therefore, we hypothesized that statins may reduce the occurrence of recurrent UTIs, as their source could be removed. Statin treatment may result in a decreased duration or severity of first UTIs, but their occurrence is most likely less affected, since removing the source of recurrent UTIs does not substantially influence the occurrence of first (non-recurrent) UTIs.
Two observational studies assessed the effect of statins on the risk of contracting UTIs. One found that statin therapy was associated with a 9% decreased UTI risk,18 whereas the other observed a 5% increased risk.19 These studies were vulnerable to unmeasured confounding bias, because both studies were non-randomized and important risk factors for UTIs, such as kidney disorders and/or urinary tract abnormalities, were not measured and patients having these conditions were not excluded.
Hence, we investigated the effect of statins on the occurrence of (recurrent) UTIs using a randomized design. Data from the Prevention of REnal and Vascular ENdstage Disease Intervention Trial (PREVEND IT)20 were linked to a large prescription database to estimate post hoc the effect of pravastatin on the occurrence of UTIs compared with placebo. We further assessed whether the effect was larger for subsequent UTIs than for first UTIs.
Materials and methods
PREVEND IT is a randomized, double-blind, placebo-controlled trial with a 2 × 2 factorial design, which aimed to determine whether treatment with pravastatin and/or fosinopril can prevent cardiovascular and renal disease in non-hypertensive, non-hypercholesterolaemic adults with persistent microalbuminuria. Participants were randomized to 40 mg of pravastatin or matching placebo and to 20 mg of fosinopril or matching placebo. Details of the PREVEND IT objectives, design and methods have been described previously20,21 and are summarized below.
The PREVEND IT study protocol was approved by the institutional review board of the University Medical Center Groningen and was conducted in accordance with the guidelines of the Declaration of Helsinki. Informed consent was obtained from all participants before randomization. The key entry criteria for participation in PREVEND IT were persistent microalbuminuria (one urinary albumin concentration >10 mg/L in an early morning spot urine test and at least one of 15–300 mg/24 h in two 24 h urine samples), absence of antihypertensive and lipid-lowering medication, blood pressure <160/100 mmHg and total cholesterol <8.0 or <5.0 mmol/L in the case of previous myocardial infarction. From April 1998 to June 1999, 864 subjects were included in PREVEND IT and were randomized to the study medication for 4 years.
Most participants in PREVEND IT were inhabitants of the city of Groningen. The IADB.nl database (IADB), a community-based pharmacy database, contains detailed patient-specific drug prescription information on almost all inhabitants of the city of Groningen22 and was linked to PREVEND IT data. The IADB contains, among other data, information on the date of prescription, number of days the drug was prescribed for and the number of defined daily doses based on the WHO definition.22 Prescription drugs are classified according to the Anatomical Therapeutic Chemical (ATC) system and the IADB population is considered representative of the Dutch population in terms of drug use.23 All PREVEND IT participants included in this study gave informed consent to link their data with pharmacy-dispensing data.
For the present post hoc analyses, individuals were excluded if no pharmacy data during the 6 months prior to the start of the trial could be linked. Further, individuals who received a prescription of nitrofurantoin, a sulphonamide or trimethoprim (used as a proxy for UTI antibiotic prescribing, see outcome definitions) during this pre-trial period were excluded.
Outcome definitions
The primary outcome was defined as the number of prescriptions with nitrofurantoin (ATC code J01XE) or sulphonamides or trimethoprim (ATC code J01E) during follow-up. To exclude relapses due to insufficient or incorrect treatment, a new UTI antibiotic prescription was defined as a UTI antibiotic prescription occurring ≥30 days after a previous UTI antibiotic prescription. During the research period (1998–2003), in 96% of the cases that nitrofurantoin was prescribed in the Netherlands, it was prescribed for a UTI.24 For sulphonamides or trimethoprim the corresponding specificity was 82%.24 The sensitivity of our proxy was estimated at 75%.24,25
Statistical analyses
Statistical analyses were performed according to the intention-to-treat (ITT) and per-protocol principles, with the use of two-sided tests and STATA 12 and SPSS 18 software.
For ITT analyses, follow-up time was defined as the period from the date of the start of study medication use to the end of the trial (4 years) or right censoring (loss to follow-up) in the prescription database. The association between the treatment arm and number of UTI antibiotic prescriptions was determined using a multivariate negative binomial generalized estimating equation (GEE) with an autoregressive correlation structure and robust standard errors, and the results are presented as the relative risk (RR) with the corresponding 95% confidence interval (95% CI). We clustered on the patient level, because the risk of contracting a UTI increases after a first UTI2,26,27 and UTI antibiotic prescriptions within one person are consequently correlated, especially when following shortly one after another. ITT time-to-event analyses were performed with Cox regression to estimate the effect on the first (time to first UTI antibiotic prescription) or subsequent UTI antibiotic prescriptions (time between first and second UTI antibiotic prescription), with the results presented as hazard ratios (HR) and 95% CIs.
Per-protocol analyses were performed using the same regression techniques as for the ITT analyses. Follow-up time was defined as the period from the start of study medication use to the moment the participant did not adhere to the study protocol. Possible reasons were non-adherence to the study medication, crossover between treatment groups, use of study medication outside the study protocol or right censoring in the prescription database.
We further explored in separate analyses the effect of fosinopril. Treatment with angiotensin-converting enzyme inhibitors (ACEIs) can result in a decrease of the urine output in healthy elderly persons.28 We therefore secondarily hypothesized that fosinopril may increase the risk of UTIs and that effect modification may be present for recurrent infections, but not for first infections. Effect modification on an additive scale was assessed by incorporating an interaction term between fosinopril and pravastatin into the models. Because power calculations showed that our study likely lacked statistical power for identifying effect modification, especially for the analysis of time between first and second events, a P value of <0.2 was considered significant for analyses of interactions.29 For Cox regression analyses, we used the delta method to calculate the CIs for the relative excess risk due to interaction (RERI) on an additive scale.30 Given the biological mechanism that could explain an interaction between fosinopril and pravastatin for recurrent infections, pravastatin and combination therapy were analysed separately. We also calculated the effect of pravastatin, regardless of possible effect modification by fosinopril.
Results
ITT analyses
Of the 864 trial participants, 655 could be included in the analyses (Table 1). Reasons for exclusion were receiving a UTI antibiotic prescription in the 6 months prior to the study (placebo, n = 6; pravastatin, n = 3; fosinopril, n = 4; and combination therapy, n = 3) or having no pharmacy data available during the full 6 months prior to the study (placebo, n = 41; pravastatin, n = 56; fosinopril, n = 51; and combination therapy, n = 45). Excluded patients were more frequently male, were slightly younger and had a higher glomerular filtration rate than patients that met the inclusion criteria.
Table 1.
Baseline characteristics (n = 655)
| Characteristics | Placebo (n = 169) | Pravastatin (n = 158) | Fosinopril (n = 160) | Pravastatin + fosinopril (n = 168) | Excluded patients (n = 209) |
|---|---|---|---|---|---|
| Age (years) | 50.6 ± 11.6 | 51.5 ± 11.7 | 50.3 ± 11.5 | 51.5 ± 12.1 | 47.6 ± 11.7 |
| Male gender (%) | 59.2 | 65.8 | 59.4 | 64.9 | 73.2 |
| Systolic blood pressure (mm Hg) | 131.8 ± 16.0 | 132.5 ± 17.4 | 132.0 ± 16.0 | 130.9 ± 16.6 | 130.7 ± 15.0 |
| Diastolic blood pressure (mm Hg) | 76.6 ± 9.7 | 76.9 ± 8.3 | 76.5 ± 9.0 | 76.3 ± 9.0 | 75.7 ± 9.1 |
| Total cholesterol (mmol/L) | 5.6 ± 1.0 | 5.6 ± 0.9 | 5.8 ± 1.0 | 5.8 ± 1.0 | 5.6 ± 1.0 |
| Serum creatinine (mmol/L) | 82.6 ± 13.5 | 83.4 ± 12.9 | 84.4 ± 14.1 | 86.6 ± 14.7 | 85.0 ± 13.3 |
| Urinary albumin excretion (mg/24 h)a | 24.6 (26.3) | 22.1 (22.4) | 25.5 (21.8) | 23.9 (26.8) | 22.6 (23.5) |
| eGFR, (mL/min/1.73 m2) | 73.6 ± 28.9 | 70.3 ± 32.6 | 73.4 ± 28.1 | 70.8 ± 30.2 | 84.2 ± 13.2 |
| Body mass index (kg/m2) | 27.5 ± 4.9 | 26.9 ± 4.7 | 27.0 ± 4.3 | 27.0 ± 4.1 | 26.1 ± 4.3 |
| Smoking (%) | 52.1 | 49.4 | 40.6 | 49.4 | 52.2 |
| Blood pressure-lowering medication (%) | 2.4 | 1.3 | 0 | 3.0 | 1.9 |
| Glucose-lowering medication (%) | 1.8 | 1.3 | 0 | 3.0 | 0 |
| Lipid-lowering medication (%) | 1.2 | 0.6 | 1.3 | 1.2 | 1.0 |
Abbreviations: eGFR, estimated glomerular filtration rate.
aValues are medians (IQR).
During an average follow-up of 3.8 years, 17 subjects (11%) allocated to pravastatin received at least one UTI antibiotic prescription. For placebo, fosinopril and combination therapy, these numbers were 34 (20%), 30 (19%) and 31 (18%), respectively. Of those subjects allocated to pravastatin that received a first UTI antibiotic prescription, four (24%) subjects experienced also a second UTI during follow-up. For subjects allocated to placebo, fosinopril and combination therapy, these figures were 16 (47%), 15 (50%) and 11 (35%), respectively. Of all men, 38 (19%) received a UTI antibiotic prescription, while 74 (60%) women received a UTI antibiotic prescription during follow-up. The use of other antibiotics used to treat UTIs and commonly prescribed drugs during follow-up was similar in all groups (Table 2).
Table 2.
Drug use during follow-upa
| Drug | Placebo | Pravastatin | Fosinopril | Pravastatin + fosinopril |
|---|---|---|---|---|
| Total other antibiotics | 84 (50%) | 88 (56%) | 79 (49%) | 94 (56%) |
| Other antibiotics used for UTI treatment (amoxicillin, amoxicillin/clavulanate, quinolones) | 47 (28%) | 44 (28%) | 44 (28%) | 54 (32%) |
| NSAIDs | 86 (51%) | 88 (56%) | 85 (53%) | 98 (58%) |
| Drugs for acid-related disorders | 33 (20%) | 33 (21%) | 39 (24%) | 38 (23%) |
| Antifungals for dermatological use | 32 (19%) | 33 (21%) | 36 (23%) | 40 (24%) |
Abbreviations: NSAIDs, non-steroidal anti-inflammatory drugs; UTI, urinary tract infection.
aData presented as number of persons receiving at least one prescription during follow-up.
GEE analysis showed that pravastatin reduced the number of UTI antibiotic prescriptions (RR, 0.43; 95% CI, 0.21–0.88) compared with placebo. Pravastatin further reduced the hazard of a first UTI antibiotic prescription (HR, 0.58; 95% CI, 0.32–1.03; Figure 1) and second UTI antibiotic prescription (HR, 0.25; 95% CI, 0.08–0.77; Table 3). Allocation to combination therapy was not associated with the number of UTI antibiotic prescriptions (RR, 1.04; 95% CI, 0.57–1.89) or the hazard of a first UTI antibiotic prescription (HR, 1.07; 95% CI, 0.66–1.75). A non-significant reduction in the occurrence of second UTI antibiotic prescriptions was observed in those patients (HR, 0.48; 95% CI, 0.22–1.05).
Figure 1.
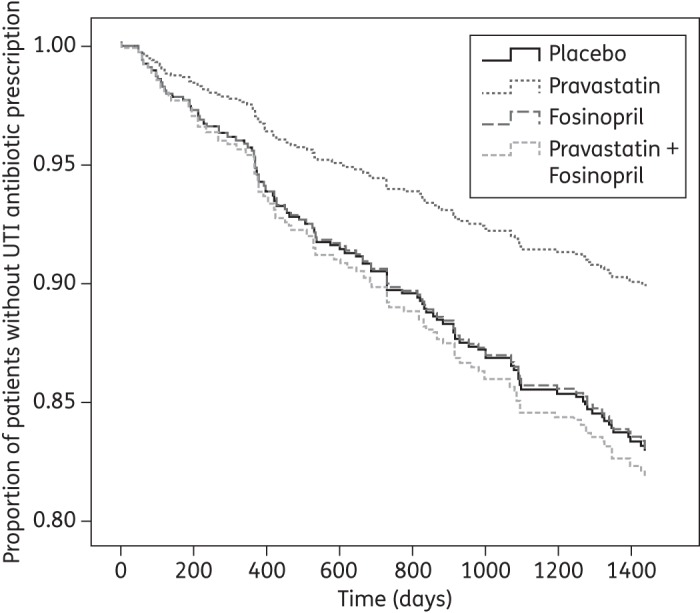
ITT time to first UTI antibiotic prescription after starting placebo, pravastatin, fosinopril or combination therapy with pravastatin and fosinopril. Survival curves are adjusted for gender.
Table 3.
ITT analyses
| Characteristics | Total number of UTIs | Risk of UTI (RR, 95% CI)a | Hazard of first UTI (HR, 95% CI)a | Hazard of second UTI (HR, 95% CI)b |
|---|---|---|---|---|
| Placebo | 66 | Ref. | Ref. | Ref. |
| Pravastatin | 25 | 0.43 (0.21–0.88) | 0.58 (0.32–1.03) | 0.25 (0.08–0.77) |
| Pravastatin + fosinopril | 62 | 1.04 (0.57–1.89) | 1.07 (0.66–1.75) | 0.48 (0.22–1.05) |
| Pravastatin versus no pravastatin | ||||
| no pravastatin | 130 | Ref. | Ref. | Ref. |
| pravastatin | 87 | 0.71 (0.44–1.15) | 0.83 (0.57–1.20) | 0.48 (0.25–0.89) |
Abbreviations: UTI, urinary tract infection; RR, relative risk; CI, confidence interval; HR, hazard ratio; Ref., reference category.
aAdjusted for gender.
bAdjusted for urinary albumin excretion.
At an α level of 0.20, there was a significant interaction on an additive scale between fosinopril and pravastatin, when evaluating the time between first and second UTI antibiotic prescription (RERI, −4.13; 80% CI, −8.07 to −0.19) and the total number of UTI antibiotic prescriptions (P = 0.10). In contrast, there was no significant interaction between fosinopril and pravastatin for the time to first UTI antibiotic prescription (RERI, −0.59; 80% CI, −1.78–0.60).
Pravastatin versus no pravastatin and fosinopril versus no fosinopril
When pravastatin was compared with no pravastatin, a non-significant reduction in the risk of receiving UTI antibiotic prescriptions (RR, 0.71; 95% CI, 0.44–1.15) was found. Pravastatin treatment further resulted in a non-significant reduction in the hazard of receiving a first UTI antibiotic prescription (HR, 0.83; 95% CI, 0.57–1.20), but a significant reduction in the hazard of a second UTI antibiotic prescription (HR, 0.48; 95% CI, 0.25–0.89; Table 3). When compared with no fosinopril, allocation to fosinopril resulted in a non-significant increase in the hazard of receiving a first UTI antibiotic prescription (HR, 1.28; 95% CI, 0.89–1.86).
Per-protocol analyses
During an average time at risk of 2.7 years, 12 subjects (8%) allocated to pravastatin received at least one UTI antibiotic prescription. These figures were 18 (11%), 24 (15%) and 24 (14%) for placebo, fosinopril and combination therapy, respectively.
Of the pravastatin-treated subjects that received a first UTI antibiotic prescription, two (17%) also received a second one. For placebo, fosinopril and combination therapy, these numbers were 7 (39%), 10 (42%) and 8 (33%), respectively. In total, 27 (13%) males and 51 (43%) females received a UTI antibiotic prescription.
Per-protocol analysis showed that pravastatin resulted in a non-significant reduction in the frequency of UTI antibiotic prescriptions (RR, 0.54; 95% CI, 0.23–1.28) and the occurrence of first UTI antibiotic prescriptions (HR, 0.83; 95% CI, 0.40–1.73; Figure 2) when compared with placebo (Table 4). The occurrence of second UTI antibiotic prescriptions was significantly reduced (HR, 0.19; 95% CI, 0.38–0.95) with pravastatin treatment.
Figure 2.
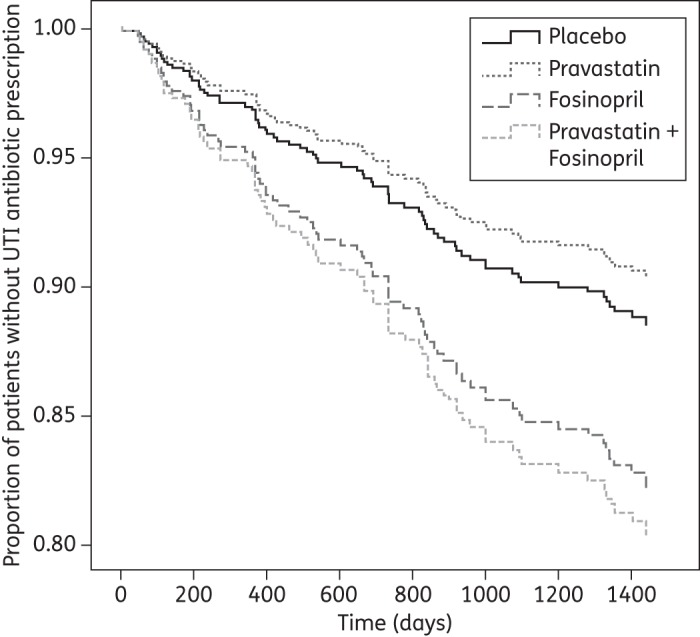
Per-protocol time to first UTI antibiotic prescription after starting placebo, pravastatin, fosinopril or combination therapy with pravastatin and fosinopril. Survival curves are adjusted for gender.
Table 4.
Per-protocol analyses
| Characteristics | Total number of UTIs | Risk of UTI (RR, 95% CI)a | Hazard of first UTI (HR, 95% CI)a | Hazard of second UTI (HR, 95% CI)b |
|---|---|---|---|---|
| Placebo | 36 | Ref. | Ref. | Ref. |
| Pravastatin | 15 | 0.54 (0.23–1.28) | 0.83 (0.40–1.73) | 0.19 (0.38–0.95) |
| Pravastatin + fosinopril | 41 | 1.39 (0.64–3.02) | 1.79 (0.97–3.31) | 0.43 (0.15–1.23) |
| Pravastatin versus no pravastatin | ||||
| no pravastatin | 80 | Ref. | Ref. | Ref. |
| pravastatin | 56 | 0.79 (0.46–1.37) | 1.02 (0.65–1.59) | 0.53 (0.46–1.37) |
Abbreviations: UTI, urinary tract infection; RR, relative risk; CI, confidence interval; HR, hazard ratio; Ref., reference category
aAdjusted for gender.
bAdjusted for urinary albumin excretion.
Combination therapy resulted in a non-significant increase in the risk of receiving UTI antibiotic prescriptions (RR, 1.39; 95% CI, 0.64–3.02) and the hazard of a first UTI antibiotic prescription (HR, 1.79; 95% CI, 0.97–3.31). This combination therapy resulted in a non-significant reduction in the occurrence of second UTI antibiotic prescriptions (HR, 0.43; 95% CI, 0.15–1.23).
Pravastatin versus no pravastatin and fosinopril versus no fosinopril
When comparing pravastatin with no pravastatin, a non-significant reduction in the frequency of UTI antibiotic prescriptions in the pravastatin treatment group was found (RR, 0.79; 95% CI, 0.46–1.37) (Table 4). Pravastatin treatment had no effect on the time to first UTI antibiotic prescription (HR, 1.02; 95% CI, 0.65–1.59) and resulted in a non-significant reduction in the hazard of receiving a second UTI antibiotic prescription (HR, 0.53; 95% CI, 0.46–1.37). If fosinopril was compared with no fosinopril therapy, a significant increase in the hazard of receiving a first UTI antibiotic prescription was found (HR, 1.82; 95% CI, 1.16–2.88).
Discussion
We found that allocation to pravastatin without coadministration of fosinopril was associated with a reduction in the number of UTI antibiotic prescriptions and the hazard of a second (recurrent) UTI antibiotic prescription, but had no influence on first UTI antibiotic prescriptions. The results of the per-protocol and ITT analyses were not substantially different from each other. The observed difference between second and first UTI antibiotic prescriptions is compatible with our hypothesis that statins exert a higher effect on recurrent UTIs than on first UTIs.
Per-protocol analysis further showed an increased hazard of receiving a first UTI antibiotic prescription in patients using fosinopril, indicating that fosinopril increases the risk of contracting UTIs.
Together with the finding that exfoliated intracellular bacterial communities and filamentous bacteria are present in the urine samples of women with acute cystitis,31 our results indicate that QIR formation also occurs in humans. The finding that pravastatin can reduce the occurrence of (recurrent) UTI antibiotic prescriptions is further supported by the correlation found between elevated cholesterol levels and an increased incidence of UTIs in children.32
Previously, a population-based non-randomized study observed a small increased risk of contracting UTIs associated with statin use (HR, 1.05; 99% CI, 1.00–1.11).19 By exploring the effect on time to first events, most of the UTIs in that study were likely first UTIs, on which the effect of statin therapy in our study was limited. This limited effect on first UTIs might also explain why no significant effect is found on the total number of UTI antibiotic prescriptions in our per-protocol analysis. After all, more than half of the total number of UTI antibiotic prescriptions was a first UTI antibiotic prescription.
Another non-randomized study observed a smaller decrease in the number of UTIs associated with statin use (OR, 0.91; 95% CI, 0.85–0.98)18 than we estimated using either the per-protocol (RR, 0.54; 95% CI, 0.23–1.28) or ITT analyses (RR, 0.43; 95% CI, 0.21–0.88). This difference in the magnitude of the effect estimate might be caused by unmeasured confounding due to the non-randomized character of that study and the lack of information on potentially important risk factors for UTIs.18
An alternative explanation of our findings could be that UTIs might have been treated with other antibiotics more frequently in the pravastatin group. Therefore, we evaluated the use of amoxicillin, amoxicillin/clavulanate and quinolones as, together with our proxy, these antibiotics cover >98% of all UTIs that are treated with antibiotics. The similar use of these other antibiotics is not in accordance with this alternative explanation.
Another alternative hypothesis could be that by reducing membrane-associated Rac1, patients on pravastatin have a much higher bacterial load prior to active disease and are thus be more likely to have systemic disease instead of UTIs. However, the number of persons that received other antibiotics was similar between the placebo and pravastatin groups, indicating that patients allocated to pravastatin did not receive more alternative antibiotics. We did not assess whether patients on pravastatin were more frequently hospitalized for bacteraemia or sepsis, as such hospitalizations are likely very uncommon in our small and relatively healthy study population.33,34
The similar use of other commonly used drugs among the different treatment groups indicates that the pravastatin-treated patients were also not less likely to receive drug prescriptions in general.
An important strength of our study is the analysis of data from a randomized placebo-controlled trial, thereby limiting potential unmeasured confounding. Although we excluded some patients, primarily because of unsuccessful linkage of pharmacy data to study participants, measured potential confounders were equally distributed between treatment groups for the primary analysis, except for a small difference in gender. This indicates that unmeasured potential confounders were also likely equally distributed between the treatment groups.
Our study has potential limitations. First, we used specific antibiotic prescriptions obtained from a pharmacy description database as a proxy for UTIs. The sensitivity and specificity are based on studies using data from the Second Dutch National Survey of General Practice (DNSGP-2).24,25 The patient population of DNSGP-2 partly overlaps with that of IADB.35,36 Moreover, because Akkerman et al.24 and Ong et al.25 both estimated the sensitivity and specificity of our proxy using data from the same time period as PREVEND IT took place, both high test characteristics are likely also applicable to our study.
In the placebo group, 34 subjects received a first UTI antibiotic prescription, while based on age- and gender-specific figures from the general Dutch population (Statistics Netherlands), 22 first UTIs would be expected. However, persons with microalbuminuria are at increased risk of developing de novo renal function impairment.37 Because an impaired renal function might increase the risk for UTIs,38–40 the study population might have had an increased risk of contracting a UTI compared with the general population.
Although misclassification of the outcome may have occurred, we expect this to be random, because we used the same objective outcome (i.e. antibiotic prescriptions) for the different treatment groups. Random misclassification of the outcome across treatment groups biases the effect towards a null finding (no effect). Therefore, using a proxy has likely not resulted in an overestimation of the effect of pravastatin.
Second, although we excluded patients (n = 16) who had a UTI in the previous 6 months based on antibiotic prescriptions, it is still possible that patients with asymptomatic UTIs during these months were included. If these patients were unevenly distributed between treatment groups, this could have resulted in different baseline risks for the different treatment groups.
Third, although a decrease in the urine output has been shown in previous studies,28 no studies are available that showed that ACEIs also increase the occurrence of UTIs. Because bacterial clearance from the urinary tract is partially dependent on urine output,41 ACEIs might increase the frequency of UTIs.
Because patients might stop taking fosinopril after experiencing an adverse effect and the increased risk for UTIs is likely quickly reversible by non-adherence,42,43 the estimated increased hazard of receiving a first UTI antibiotic prescription in patients on fosinopril treatment was more clearly present in per-protocol analysis than in ITT analysis.
This increased risk might be partly due to the concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs).28 Of patients treated with fosinopril, 21% received three or more NSAID prescriptions during the time at risk. Moreover, many people buy NSAIDs over the counter. The interaction between fosinopril and pravastatin, when evaluating the time between first and second events, further supports the hypothesis that fosinopril can increase the risk of contracting UTIs, due to reduced urine output. Therefore, we do feel that the separated analyses for pravastatin and combination therapy best present the true effect of pravastatin for outcomes including recurrent events.
Fourth, this study was not designed and powered for the current post hoc analysis. Therefore, statistical significance could not be reached for some of the secondary analyses. Larger studies with preferably hard endpoints instead of proxies are needed.
Finally, our study sample was predominantly healthy and male, and we excluded patients with antibiotic prescriptions for UTIs in the previous 6 months, which could limit the generalizability of our results. It is not likely that such individuals who have no recurrent UTIs and no other indication for treatment with statins will be treated with a statin to prevent UTIs. Therefore, future research should preferably focus on individuals with recurrent UTIs and/or a high risk for recurrent UTIs.
In summary, this post hoc analysis suggests that pravastatin can reduce the risk of recurrent UTIs, possibly by inhibiting bacterial invasion. Larger studies with persons that have a high risk for recurrent UTIs are needed.
Funding
This study was carried out as part of our routine work.
Transparency declarations
None to declare.
References
- 1.van Pinxteren B, van Vliet SM, Wiersma TJ, et al. Summary of the practice guideline 'urinary-tract infections’ (second revision) from the Dutch college of general practitioners. Ned Tijdschr Geneeskd. 2006;150:718–22. [PubMed] [Google Scholar]
- 2.Hunstad DA, Justice SS. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu Rev Microbiol. 2010;64:203–21. doi: 10.1146/annurev.micro.112408.134258. [DOI] [PubMed] [Google Scholar]
- 3.Lipsky BA. Urinary tract infections in men: epidemiology, pathophysiology, diagnosis, and treatment. Ann Intern Med. 1989;110:138–50. doi: 10.7326/0003-4819-110-2-138. [DOI] [PubMed] [Google Scholar]
- 4.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–9. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schilling JD, Mulvey MA, Vincent CD, et al. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol. 2001;166:1148–55. doi: 10.4049/jimmunol.166.2.1148. [DOI] [PubMed] [Google Scholar]
- 6.Duncan MJ, Li G, Shin JS, et al. Bacterial penetration of bladder epithelium through lipid rafts. J Biol Chem. 2004;279:18944–51. doi: 10.1074/jbc.M400769200. [DOI] [PubMed] [Google Scholar]
- 7.Martinez JJ, Hultgren SJ. Requirement of rho-family GTPases in the invasion of type 1-piliated uropathogenic Escherichia coli. Cell Microbiol. 2002;4:19–28. doi: 10.1046/j.1462-5822.2002.00166.x. [DOI] [PubMed] [Google Scholar]
- 8.Song J, Bishop BL, Li G, et al. TLR4-initiated and cAMP-mediated abrogation of bacterial invasion of the bladder. Cell Host Microbe. 2007;1:287–98. doi: 10.1016/j.chom.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnham CA, Shokoples SE, Tyrrell GJ. Rac1, RhoA, and Cdc42 participate in HeLa cell invasion by group B Streptococcus. FEMS Microbiol Lett. 2007;272:8–14. doi: 10.1111/j.1574-6968.2007.00768.x. [DOI] [PubMed] [Google Scholar]
- 10.Cordle A, Koenigsknecht-Talboo J, Wilkinson B, et al. Mechanisms of statin-mediated inhibition of small G-protein function. J Biol Chem. 2005;280:34202–9. doi: 10.1074/jbc.M505268200. [DOI] [PubMed] [Google Scholar]
- 11.Rashid M, Tawara S, Fukumoto Y, et al. Importance of Rac1 signaling pathway inhibition in the pleiotropic effects of HMG-CoA reductase inhibitors. Circ J. 2009;73:361–70. doi: 10.1253/circj.cj-08-0817. [DOI] [PubMed] [Google Scholar]
- 12.Dechend R, Gieffers J, Dietz R, et al. Hydroxymethylglutaryl coenzyme A reductase inhibition reduces Chlamydia pneumoniae-induced cell interaction and activation. Circulation. 2003;108:261–5. doi: 10.1161/01.CIR.0000083367.93022.78. [DOI] [PubMed] [Google Scholar]
- 13.Antoniades C, Bakogiannis C, Tousoulis D, et al. Preoperative atorvastatin treatment in CABG patients rapidly improves vein graft redox state by inhibition of Rac1 and NADPH-oxidase activity. Circulation. 2010;122:S66–73. doi: 10.1161/CIRCULATIONAHA.109.927376. [DOI] [PubMed] [Google Scholar]
- 14.Maack C, Kartes T, Kilter H, et al. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108:1567–74. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 15.Zaas DW, Duncan M, Rae Wright J, et al. The role of lipid rafts in the pathogenesis of bacterial infections. Biochim Biophys Acta. 2005;1746:305–13. doi: 10.1016/j.bbamcr.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Horn MP, Knecht SM, Rushing FL, et al. Simvastatin inhibits Staphylococcus aureus host cell invasion through modulation of isoprenoid intermediates. J Pharmacol Exp Ther. 2008;326:135–43. doi: 10.1124/jpet.108.137927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosch JW, Boyd AR, Hinojosa E, et al. Statins protect against fulminant pneumococcal infection and cytolysin toxicity in a mouse model of sickle cell disease. J Clin Invest. 2010;120:627–35. doi: 10.1172/JCI39843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming DM, Verlander NQ, Elliot AJ, et al. An assessment of the effect of statin use on the incidence of acute respiratory infections in England during winters 1998–1999 to 2005–2006. Epidemiol Infect. 2010;138:1281–8. doi: 10.1017/S0950268810000105. [DOI] [PubMed] [Google Scholar]
- 19.Smeeth L, Douglas I, Hall AJ, et al. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials. Br J Clin Pharmacol. 2009;67:99–109. doi: 10.1111/j.1365-2125.2008.03308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diercks GF, Janssen WM, van Boven AJ, et al. Rationale, design, and baseline characteristics of a trial of prevention of cardiovascular and renal disease with fosinopril and pravastatin in nonhypertensive, nonhypercholesterolemic subjects with microalbuminuria (the Prevention of REnal and Vascular ENdstage Disease Intervention Trial [PREVEND IT]) Am J Cardiol. 2000;86:635–8. doi: 10.1016/s0002-9149(00)01042-0. [DOI] [PubMed] [Google Scholar]
- 21.Asselbergs FW, Diercks GF, Hillege HL, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809–16. doi: 10.1161/01.CIR.0000146378.65439.7A. [DOI] [PubMed] [Google Scholar]
- 22.Pharmacy-dispensing Database of IADB.nl. http://www.iadb.nl. (2 October 2012, date last accessed)
- 23.Tobi H, van den Berg P, de Jong-van den Berg L. The InterAction database: synergy of science and practice in pharmacy. Lect Notes Comput Sci. 2000;1933:93–108. [Google Scholar]
- 24.Akkerman AE, Kuyvenhoven MM, Verheij TJ, et al. Antibiotics in Dutch general practice: nationwide electronic GP database and national reimbursement rates. Pharmacoepidemiol Drug Saf. 2008;17:378–83. doi: 10.1002/pds.1501. [DOI] [PubMed] [Google Scholar]
- 25.Ong DS, Kuyvenhoven MM, van Dijk L, et al. Antibiotics for respiratory, ear and urinary tract disorders and consistency among GPs. J Antimicrob Chemother. 2008;62:587–92. doi: 10.1093/jac/dkn230. [DOI] [PubMed] [Google Scholar]
- 26.Russo TA, Stapleton A, Wenderoth S, et al. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J Infect Dis. 1995;172:440–5. doi: 10.1093/infdis/172.2.440. [DOI] [PubMed] [Google Scholar]
- 27.Foxman B. Recurring urinary tract infection: incidence and risk factors. Am J Public Health. 1990;80:331–3. doi: 10.2105/ajph.80.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juhlin T, Bjorkman S, Hoglund P. Cyclooxygenase inhibition causes marked impairment of renal function in elderly subjects treated with diuretics and ACE-inhibitors. Eur J Heart Fail. 2005;7:1049–56. doi: 10.1016/j.ejheart.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Marshall SW. Power for tests of interaction: effect of raising the type I error rate. Epidemiol Perspect Innov. 2007;4:4. doi: 10.1186/1742-5573-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–6. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Rosen DA, Hooton TM, Stamm WE, et al. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulati S, Kher V, Arora P, et al. Urinary tract infection in nephrotic syndrome. Pediatr Infect Dis J. 1996;15:237–40. doi: 10.1097/00006454-199603000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen G, Schonheyder HC, Sorensen HT. Source of infection and other factors associated with case fatality in community-acquired bacteremia—a Danish population-based cohort study from 1992 to 1997. Clin Microbiol Infect. 2003;9:793–802. doi: 10.1046/j.1469-0691.2003.00599.x. [DOI] [PubMed] [Google Scholar]
- 34.Elhanan G, Sarhat M, Raz R. Empiric antibiotic treatment and the misuse of culture results and antibiotic sensitivities in patients with community-acquired bacteraemia due to urinary tract infection. J Infect. 1997;35:283–8. doi: 10.1016/s0163-4453(97)93194-7. [DOI] [PubMed] [Google Scholar]
- 35.Second Dutch National Survey of General Practice. www.nivel.nl/pdf/ns2_r0_h14.pdf. (2 October 2012, date last accessed)
- 36.Pechlivanoglou P, van der Veen WJ, Bos JH, et al. Analyzing generic and branded substitution patterns in the Netherlands using prescription data. BMC Health Serv Res. 2011;11:89. doi: 10.1186/1472-6963-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verhave JC, Gansevoort RT, Hillege HL, et al. An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int Suppl. 2004;92:S18–21. doi: 10.1111/j.1523-1755.2004.09205.x. [DOI] [PubMed] [Google Scholar]
- 38.Funfstuck R, Ott U, Naber KG. The interaction of urinary tract infection and renal insufficiency. Int J Antimicrob Agents. 2006;28(Suppl 1):S72–7. doi: 10.1016/j.ijantimicag.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert DN. Urinary tract infections in patients with chronic renal insufficiency. Clin J Am Soc Nephrol. 2006;1:327–31. doi: 10.2215/CJN.01931105. [DOI] [PubMed] [Google Scholar]
- 40.Yamasaki T, Naganuma T, Iguchi T, et al. Association between chronic kidney disease and small residual urine volumes in patients with benign prostatic hyperplasia. Nephrology (Carlton) 2011;16:335–9. doi: 10.1111/j.1440-1797.2010.01430.x. [DOI] [PubMed] [Google Scholar]
- 41.Beetz R. Mild dehydration: a risk factor of urinary tract infection? Eur J Clin Nutr. 2003;57(Suppl 2):S52–8. doi: 10.1038/sj.ejcn.1601902. [DOI] [PubMed] [Google Scholar]
- 42.Navis G, Faber HJ, de Zeeuw D, et al. ACE inhibitors and the kidney. A risk–benefit assessment. Drug Saf. 1996;15:200–11. doi: 10.2165/00002018-199615030-00005. [DOI] [PubMed] [Google Scholar]
- 43.Whelton A, Schulman G, Wallemark C, et al. Effects of celecoxib and naproxen on renal function in the elderly. Arch Intern Med. 2000;160:1465–70. doi: 10.1001/archinte.160.10.1465. [DOI] [PubMed] [Google Scholar]


