Abstract
Background
Mycobacterium xenopi is a common agent of non-tuberculous mycobacterial lung diseases in Europe. However, an optimal treatment regimen for M. xenopi infection has not yet been established. Appropriate in vitro and in vivo model systems are needed for characterization of the activity of potential drugs and drug combinations against M. xenopi.
Methods
We utilized three experimental platforms to analyse the anti-M. xenopi activity of single and combination drug regimens. First, we determined the bacteriostatic and bactericidal activities of drugs alone and in combination in vitro. Second, we used serum from treated mice to evaluate drug activities ex vivo. Third, we analysed M. xenopi growth in four strains of mice (BALB/c, C57BL/6, beige and athymic nude) and developed a mouse model of chemotherapy for this infection.
Results
Two-drug combinations of ethambutol with rifampicin, rifapentine or moxifloxacin, and of clarithromycin with moxifloxacin were bactericidal in vitro, and the combination of ethambutol and rifampicin with either clarithromycin or moxifloxacin showed significant bactericidal activity ex vivo. Nude mice were the most susceptible strain to M. xenopi infection, and in this model amikacin-containing regimens were the most effective against M. xenopi. No difference in activity was found between regimens containing clarithromycin and moxifloxacin in vivo.
Conclusion
The ethambutol/rifampicin combination with clarithromycin or moxifloxacin had significant bactericidal activity against M. xenopi. The nude mouse, being highly susceptible to M. xenopi, can be utilized for in vivo chemotherapy studies for this infection.
Keywords: drug susceptibility testing, clarithromycin, ethambutol, nude mouse
Introduction
Mycobacterium xenopi is a leading cause of non-tuberculous mycobacterial (NTM) lung disease in Europe, second only to the Mycobacterium avium complex (MAC), and is largely associated with underlying obstructive lung disease.1–4 Prognosis of patients infected with M. xenopi is poor, with mortality ranging from 51% to 69% within 5 years of diagnosis.1,2,5 In addition to the comorbid pulmonary conditions of most M. xenopi-infected individuals, the lack of defined, effective treatment regimens for these patients contributes to this high mortality rate.
The treatment of NTM diseases is notoriously difficult, as most of the standard drugs used to treat tuberculous mycobacterial infections are ineffective against NTM species. In the early 1990s, it was reported that the macrolide clarithromycin was effective against MAC infections in AIDS patients.6 However, this study and others have indicated that clarithromycin, like most other mycobacterial drugs, should be administered in a combination regimen to prevent the emergence of drug-resistant bacilli.7,8 Since this breakthrough finding, clarithromycin has been demonstrated to be effective against a number of NTM species, and has become one of the cornerstone drugs in the treatment of NTM diseases.4,9 In addition, clarithromycin has been demonstrated to work synergistically with drugs, such as those used for tuberculosis (TB) treatment, that alone are not effective against NTM diseases.10–12 However, the optimal combination regimen for many NTM infections, including M. xenopi, has yet to be established.
The current treatment guidelines for M. xenopi pulmonary infection only suggest that a combination of clarithromycin, rifampicin and ethambutol should be administered for at least 12 months, and that a quinolone, preferably moxifloxacin, could be substituted for one of the recommended drugs.4 These guidelines are based on a small number of reports, none of which clearly identified an optimal treatment strategy. The lack of validated chemotherapy model systems for M. xenopi infection has hindered the development and optimization of drug regimens effective against this organism. Only two studies have examined the effect of therapy in M. xenopi-infected mice.13,14 In both of these studies, the mice were infected by intravenous injection, thus not mimicking the natural aerosol route of infection, and the drug regimens tested did not reflect what is commonly utilized in human patients.
Our objective was to utilize and develop in vitro and in vivo model systems, respectively, for the analysis of drugs and drug combinations for the treatment of M. xenopi infection, and in this communication we describe a series of experiments designed to achieve this goal. First, we examined the anti-M. xenopi activity of key two-drug combinations in vitro to systematically characterize any additive or synergistic effects between the drugs, as has been reported for other NTM diseases.10–12 Second, we evaluated the bacteriostatic and bactericidal activity of clinically relevant drug combinations ex vivo in the serum of treated mice. Third, we analysed the growth of M. xenopi following aerosol infection in four different strains of mice: the immune-competent BALB/c and C57BL/6 mice and the immune-compromised beige (C57Bl/6J bgj/bgj) and nude (nu/nu athymic) mice. Beige mice are defective in natural killer cell production and have been demonstrated to be more susceptible to MAC infection than immune-competent mouse strains,15,16 and the nude mouse, lacking a thymus, cannot generate mature T lymphocytes and thus represents an AIDS-like immune-deficient host. Finally, having found nude mice to be the most susceptible to M. xenopi aerosol infection, we then utilized this model to study the effectiveness of clinically relevant drug combination regimens in vivo.
Materials and methods
Bacterial culture
M. xenopi strain ATCC 19971 was passaged in mice, frozen in 1 mL aliquots and stored at −80°C before use, according to our standard procedures. This method is known to maintain the virulence of M. avium.17 For each experiment, an aliquot was thawed and subcultured at 42°C in Middlebrook 7H9 broth (Difco, Detroit, MI, USA) supplemented with 10% oleic acid–albumin–dextrose–catalase [OADC (Difco)] and 0.05% Tween 80 (Sigma, St Louis, MO, USA). M. xenopi cultures were allowed to grow for ∼4 weeks before being utilized for an experiment or infection.
Animals
All animal experiments were performed with 6-week-old female mice. Swiss, BALB/c and nude mice were purchased from Charles River (Wilmington, MA, USA) and beige mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All reported research was approved by the Johns Hopkins University Animal Care and Use Committee (ACUC), protocol number MO09M404. Animals were cared for in accordance with the ACUC guidelines of Johns Hopkins University.
Antimicrobials
Moxifloxacin, rifampicin, rifapentine, amikacin, ethambutol and isoniazid were purchased from Sigma. Clarithromycin was donated by Abbott Laboratories (Abbott Park, IL, USA) and linezolid was donated by Pfizer (Groton, CT, USA). Stock solutions of moxifloxacin, rifampicin, rifapentine, amikacin, ethambutol and isoniazid were prepared by dissolving the drugs in sterile water. Stock solutions of clarithromycin and linezolid were prepared by first dissolving the drugs in DMSO and subsequently diluting in sterile water. All stock solutions were stored at 4°C for up to 1 week.
MIC assays
For MIC determination on solid media, drugs were incorporated into 7H11 agar (Difco) supplemented with 10% OADC to obtain 2-fold concentration dilutions (in mg/L) of 0.06–4 for moxifloxacin, 0.25–16 for rifampicin, 0.06–4 for rifapentine, 2–32 for amikacin, 1–32 for ethambutol, 0.25–2 for isoniazid, 0.03–4 for clarithromycin and 0.5–8 for linezolid. As acidic pH has a negative impact on the in vitro potency of macrolides and aminoglycosides,18,19 clarithromycin and amikacin were also incorporated into Mueller–Hinton agar (pH 7.3; Difco) supplemented with 10% OADC, in addition to 7H11 agar (pH 6.6). For inoculation, the turbidity of the M. xenopi culture was adjusted with sterile PBS to an optical density at 600 nm (OD600) of 1.0. Then, 0.5 mL of 10−3 and 10−4 culture dilutions was inoculated onto control and drug-containing plates. The plates were incubated for 28 days at 42°C. The MIC was defined as the lowest drug concentration that inhibited ≥99% of the cfu count compared with the cfu count on drug-free control medium. Each MIC test was performed in duplicate.
For MIC determination in liquid media, serial 2-fold dilutions of each drug at the same range of concentrations used for the MIC assays on solid media were prepared in 2.5 mL of 7H9 broth supplemented with 10% OADC but without Tween 80. Drug-free and drug-containing broth aliquots were then inoculated with 0.1 mL of the same broth culture that was used to determine MICs on 7H11 agar. After 14 days of incubation at 42°C, when growth was visible in drug-free control cultures, the MIC was defined as the lowest drug concentration that completely inhibited bacterial growth as determined by the naked eye. Each MIC test was performed in duplicate.
MBC assay
For each drug, the liquid media in the MIC culture tubes that did not exhibit any visible bacterial growth (i.e. the broth vials containing drug at the MIC and higher concentrations) were plated on 7H11 agar (supplemented with 10% OADC) plates for cfu counts. The MBC was defined as the lowest concentration of drug that killed ≥99% of the cfu compared with the cfu count of the initial inoculum used for the MIC assays.
Fractional inhibitory coefficient index (FICI)
The combination activities of moxifloxacin/ethambutol, rifampicin/ethambutol, rifapentine/ethambutol and moxifloxacin/clarithromycin against M. xenopi were studied by the chequerboard titration technique20 in 7H9 broth supplemented with 10% OADC but without Tween 80. Serial 2-fold dilutions of each drug, ranging from 0.25 to 4.0 × MIC, were prepared. Each drug-free and drug-containing sample of 2.5 mL 7H9 broth was inoculated with 0.1 mL of M. xenopi culture suspension at an OD600 of 1.0. The inoculated samples were incubated for 14 days, and the MIC for each drug combination was defined as the lowest concentration that completely inhibited bacterial growth as determined by the naked eye. These results were used to calculate the FICI as follows: FICI = (MICdrug A in combination/MICdrug A alone) + (MICdrug B in combination/MICdrug B alone). According to the official recommendations, combinations were considered synergistic if the FICI was ≤0.5, additive when the FICI was >0.5 but <4.0, and antagonistic when the FICI was ≥4.0.21
To determine the fractional bactericidal concentration index (FBCI), the MBC of drug combinations was calculated by determining cfu counts (on 7H11 agar with 10% OADC) from the broth vials with no visible growth. MBC values were used to calculate the FBCI in the same way as the FICI was calculated, and the results were interpreted according to the definitions of synergism, indifference and antagonism as for the FICI.
Ex vivo study: serum inhibitory titre (SIT) and serum bactericidal titre (SBT)
Sixty 6-week-old female outbred Swiss mice were randomized in four subgroups of 15 mice, and each group received a single dose of the following drug combinations: clarithromycin/ethambutol/rifampicin, clarithromycin/ethambutol/rifampicin/amikacin, moxifloxacin/ethambutol/rifampicin or moxifloxacin/ethambutol/rifampicin/amikacin. Drug doses were as follows: 10 mg/kg for rifampicin and 100 mg/kg for clarithromycin, moxifloxacin, amikacin and ethambutol. Solutions of rifampicin, clarithromycin and moxifloxacin were prepared in sterile 0.05% agarose solution, and amikacin and ethambutol were prepared in sterile normal saline. All drugs were administered in a volume of 0.2 mL by oesophageal cannula, except amikacin, which was administered subcutaneously. Rifampicin was given 1 h before the other drugs to avoid adverse pharmacokinetic interactions. Two hours after rifampicin dosing (which was 1 h after administration of the other drugs), mice were anaesthetized with isoflurane and exsanguinated by cardiac puncture. Blood was collected in sterile microcentrifuge tubes, rested for 30 min at room temperature and centrifuged for serum separation. Serum samples within each mouse treatment group were pooled, and 2.5 mL was combined with 2.5 mL of 7H9 broth (supplemented with 10% OADC but without Tween 80) to obtain the 1/2 dilution. The 1/2 dilution was then serially diluted to obtain 1/4, 1/8, 1/16 and 1/32 serum dilutions. All diluted serum samples and controls (prepared with plain mouse serum at 1/2 dilution) were inoculated with 0.1 mL of the same mycobacterial suspension at an OD600 of 1.0 and were used to determine MIC and MBC as described above. The SIT was defined as the highest dilution of the serum that prevented visible growth, and the SBT was defined as the highest dilution of the serum that induced a 99% reduction of the inoculum cfu count.
Mouse model development
From each mouse strain (BALB/c, C57BL/6, nude and beige), 30 females aged 6 weeks (120 mice in total) were simultaneously aerosol-infected with 7H9 broth containing 9.48 log10 cfu/mL of M. xenopi using the Inhalation Exposure System (Glas-Col Inc., Terre Haute, IN, USA). Each mouse was weighed weekly from the day after infection until the day of sacrifice. Three animals from each group were sacrificed on day 1 to establish the baseline values of spleen weight and organ cfu counts. To assess if any of the four mouse strains could be used as a chemotherapy model for M. xenopi, we initiated clarithromycin treatment for 10 mice from each strain group, starting 28 days post-infection. Oral therapy with clarithromycin (100 mg/kg in 0.2 mL) was administered 5 days a week by oesophageal cannula (gavage).
Three of the untreated mice in each group were sacrificed at weeks 1, 2, 3, 4, 8, 12 and 23 post-infection, and three mice in each of the clarithromycin-treated groups were sacrificed at 1 and 2 months post-treatment initiation. Lungs and spleen were examined for gross lesions. For each mouse (treated and untreated), one lung was fixed in formalin for histopathology analyses. The remaining lung and the spleen were homogenized in 1 mL sterile PBS using glass homogenizers. Undiluted specimen homogenates were plated on selective 7H11 agar [containing 50 mg/L cycloheximide, 50 mg/L carbenicillin, 200 U/mL polymyxin B and 20 mg/L trimethoprim (Difco)]. Serial dilutions of homogenates were plated in duplicate on selective 7H11 for cfu enumeration. For homogenates from clarithromycin-treated mice, the selection of clarithromycin-resistant mutants during treatment was directly assessed by plating lung suspensions on selective 7H11 agar also containing 8 mg/L clarithromycin, which is a concentration equal to 4 × MIC for M. xenopi.
Combination therapy in nude mice
One-hundred-and-twenty 6-week-old female nude mice (n = 120) were simultaneously aerosol-infected using 7H9 broth containing 9.44 log10 cfu/mL M. xenopi using the Inhalation Exposure System. The mice were randomized into six subgroups, and 28 days post-infection treatment was initiated with one of the following drug combinations: clarithromycin/ethambutol/rifampicin (20 mice), clarithromycin/ethambutol/rifampicin/amikacin (20 mice), moxifloxacin/ethambutol/rifampicin (20 mice), moxifloxacin/ethambutol/rifampicin/amikacin (20 mice) and clarithromycin/moxifloxacin (10 mice); 30 mice received no treatment. Drug doses were 10 mg/kg for rifampicin and 100 mg/kg for clarithromycin, moxifloxacin, amikacin and ethambutol. All drug solutions were prepared as described above for the ex vivo SIT/SBT experiments. All drugs were administered in a total volume of 0.2 mL by oesophageal cannula, except amikacin, which was administered subcutaneously. Rifampicin was given 1 h before other drugs to avoid adverse pharmacokinetic interactions.22 Five animals from the untreated group were sacrificed on day −27 (day 1 post-infection) to establish the baseline values of lung cfu, and on day 0 (just prior to starting treatment). At weeks 2, 4, 8 and 12 post-treatment, five animals from each group (untreated mice, rifampicin/ethambutol/clarithromycin, rifampicin/ethambutol/moxifloxacin, rifampicin/ethambutol/clarithromycin/amikacin and rifampicin/ethambutol/moxifloxacin/amikacin) were sacrificed, and five mice from the moxifloxacin/clarithromycin group were sacrificed at weeks 4 and 12. Lungs were homogenized and plated for cfu enumeration as described above.
Results
Single-drug MIC and MBC values
The MICs of rifampicin, rifapentine, clarithromycin, moxifloxacin, ethambutol, amikacin, linezolid and isoniazid for M. xenopi and the associated MBC values are presented in Table 1. Except for amikacin, the MIC values measured in broth were at least one dilution above the values measured on solid media. The MIC values for the most commonly recommended drugs for M. xenopi treatment, namely clarithromycin, rifampicin, ethambutol and amikacin, were close to or higher than the peak serum concentrations obtained at the standard doses in humans.23–29 The MIC values for both clarithromycin and amikacin analysed on Mueller–Hinton agar (pH 7.3) were one dilution lower than on 7H11 agar (pH 6.6): 0.5 mg/L for clarithromycin and 4 mg/L for amikacin. For those drugs for which the MBC was determined, rifampicin and ethambutol had MBCs that were above the human peak serum concentrations, while the MBC of moxifloxacin was near the peak serum concentration, thus indicating that none of these drugs alone was bactericidal for M. xenopi.
Table 1.
Single-drug MIC and MBC values (mg/L) for M. xenopi in relation to the reported peak serum concentrationa (Cmax) in humans
| Drug |
||||||||
|---|---|---|---|---|---|---|---|---|
| RIF | RFP | CLR | MXF | EMB | AMK | LZD | INH | |
| MIC: 7H11 agar | 8 | 1 | 1 | 1 | 8 | 16 | 8 | >2 |
| MIC: 7H9 broth | >16 | >4 | >4 | 2 | 16 | 16 | ND | ND |
| MBC | >16 | 4 | >4 | 2 | 16 | ND | ND | ND |
| Cmax | 10 | 10 | 2.5–3 | 3–4 | 3–4 | 20–40 | 13–18 | 4 |
FICI and FBCI for two-drug combinations
The chequerboard analysis of two-drug combinations demonstrated that all tested combinations (rifampicin/ethambutol, rifapentine/ethambutol, moxifloxacin/ethambutol and moxifloxacin/clarithromycin) were synergistic for bacteriostatic and bactericidal activities against M. xenopi (Table 2). The MIC of rifampicin alone was >16 mg/L, whereas the MIC of rifampicin combined with ethambutol was 2 mg/L; the MIC of clarithromycin alone was >4 mg/L, whereas the MIC of clarithromycin combined with moxifloxacin was 0.5 mg/L; and the MIC of moxifloxacin alone was 2 mg/L, whereas the MIC of moxifloxacin combined with ethambutol was 0.5 mg/L. Notably, no antagonism was observed between clarithromycin and moxifloxacin.
Table 2.
MIC and MBC values (mg/L) and the associated FICI and FBCI of two-drug combinations for M. xenopi
| FICI |
FBCI |
|||||
|---|---|---|---|---|---|---|
| Drugs | MIC | value | interpretation | MBC | value | interpretation |
| RIF/EMB | 2/4 | <0.325 | synergism | 2/4 | 0.325 | synergism |
| RFP/EMB | 0.25/4 | <0.325 | synergism | 0.5/4 | 0.325 | synergism |
| MXF/EMB | 0.5/2 | <0.325 | synergism | 0.5/2 | 0.325 | synergism |
| MXF/CLR | 0.5/0.5 | <0.325 | synergism | 0.5/2 | <0.5 | synergism |
RIF, rifampicin; RFP, rifapentine; CLR, clarithromycin; MXF, moxifloxacin; EMB, ethambutol.
Ex vivo SIT and SBT for multidrug combinations
The serum bacteriostatic and bactericidal activities of clinically relevant drug combinations (i.e. those combinations often administered to humans infected with M. xenopi) were determined after drug administration in outbred Swiss mice. This mouse strain was used to recapitulate drug metabolism differences in outbred (human) hosts. The most active drug combination in mouse sera was rifampicin/ethambutol/clarithromycin, which inhibited the growth of M. xenopi up to a serum dilution of 1/32 (Table 3).
Table 3.
SIT and SBT values of multidrug combinations for M. xenopi
| Serum dilution |
||
|---|---|---|
| Drug combination | SIT | SBT |
| Clarithromycin/ethambutol/rifampicin | >1/32 | 1/32 |
| Clarithromycin/ethambutol/rifampicin/amikacin | 1/8 | 1/8 |
| Moxifloxacin/ethambutol/rifampicin | 1/16 | 1/16 |
| Moxifloxacin/ethambutol/rifampicin/amikacin | 1/16 | 1/8 |
Sera were obtained from Swiss mice after one dose of each of the drug combinations.
Development of a disease and chemotherapy mouse model for M. xenopi infection
Characterization of M. xenopi infection in different mouse strains
Following aerosol infection of the immune-competent mouse strains, BALB/c and C57BL/6, the lung cfu of M. xenopi increased steadily for 4 weeks, at which point the bacterial load plateaued at around 6.5 log10 cfu/lung (Table 4). Aerosol infection of the immune-deficient nude and beige mice resulted in a continual increase in lung cfu until the end of the experiment at week 23, with the nude mice being the most susceptible to M. xenopi (>8 log10 cfu/lung at week 23). In all strains of mice, M. xenopi disseminated to the spleen, and by 12 weeks post-infection the bacterial burdens in the spleens were 4.14, 5.43, 6.07 and 6.83 log10 cfu in BALB/C, C57BL/6, beige and nude mice, respectively (Table 5).
Table 4.
Mean log10 cfu/lung (standard deviation) of M. xenopi in four strains of mice with and without clarithromycin treatment from week 4 to week 12 post-infection
| Strain and treatment | Timepoint post-infection |
||||||
|---|---|---|---|---|---|---|---|
| day 1 | week 1 | week 2 | week 3 | week 4 | week 8 | week 12 | |
| C57Bl/6 | |||||||
| UT | 4.78 (0.05) | 5.07 (0.17) | 5.56 (0.11) | 5.92 (0.09) | 6.97 (0.39) | 6.72 (0.62) | 6.51 (0.28) |
| CLR | 5.51 (0.31) | 5.49 (0.19) | |||||
| BALB/c | |||||||
| UT | 4.79 (0.07) | 4.92 (0.27) | 5.33 (0.19) | 5.57 (0.07) | 6.50 (0.20) | 6.35 (0.20) | 6.43 (0.15) |
| CLR | 4.27 (0.17) | 4.59 (0.25) | |||||
| Nude | |||||||
| UT | 4.70 (0.12) | 5.08 (0.52) | 5.76 (0.08) | 5.75 (0.14) | 5.56 (0.11) | 6.93 (0.83) | 7.69 (0.01) |
| CLR | 5.13 (0.66) | 5.33 (0.85) | |||||
| Beige | |||||||
| UT | 4.70 (0.12) | 4.78 (0.20) | 5.74 (0.16) | 5.99 (0.01) | 6.86 (0.08) | 6.60 (0.17) | 6.63 (0.13) |
| CLR | 4.88 (0.36) | 5.13 (0.72) | |||||
UT, untreated; CLR, clarithromycin at 100 mg/kg/day, 5 days per week.
Table 5.
Mean log10 cfu/spleen (standard deviation) of M. xenopi in four strains of mice with and without clarithromycin treatment from week 4 to week 12 post-infection
| Week 2 | Week 4 | Week 8 | Week 12 | |
|---|---|---|---|---|
| C57Bl/6 | ||||
| UT | 4.85 (0.06) | 4.68 (0.03) | 5.51 (0.32) | 5.43 (0.26) |
| CLR | 4.68 (0.02) | 2.98 (0.36) | ||
| BALB/c | ||||
| UT | 4.94 (0.15) | 4.68 (0.04) | 4.28 (0.17) | 4.14 (0.87) |
| CLR | 4.60 (0.03) | 2.11 (0.38) | ||
| Nude | ||||
| UT | 4.89 (0.11) | 4.66 (0.02) | 5.13 (0.66) | 6.83 (0.10) |
| CLR | 4.59 (0.04) | 3.39 (0.01) | ||
| Beige | ||||
| UT | 4.91 (0.09) | 4.70 (0.04) | 4.88 (0.36) | 6.07 (0.63) |
| CLR | 4.58 (0.04) | 3.69 (0.02) | ||
UT, untreated; CLR, clarithromycin 100 mg/kg/day, 5 days a week from week 4 to week 12.
During the entire 23-week course of M. xenopi infection, none of the mice in any group appeared sick, and none died. The body and spleen weights in all groups steadily increased, even those of nude mice. The ratio of spleen weight-to-body weight remained rather constant, in the range of 0.50%–0.75% in all untreated mice. All of the untreated mice exhibited gross lung lesions, and these lesions were more diffuse in the nude mice. Acid-fast bacilli (AFB) were visible in lung sections of all four mouse strains (Figure 1). However, the lung sections from the infected nude mice exhibited clumps of AFB that were not visible in the other mouse strains.
Figure 1.
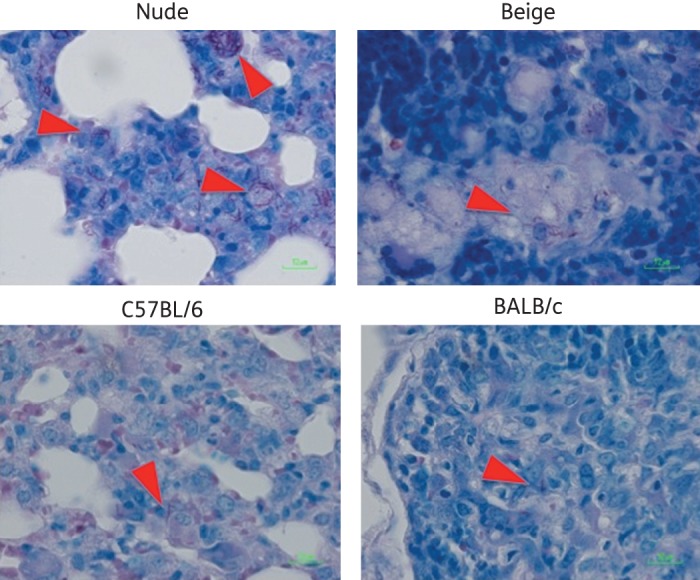
Lung histopathology in four strains of mice 12 weeks post-aerosol infection with M. xenopi. Lung sections were visualized with the Ziehl–Neelsen stain, and red arrowheads indicate AFB. Images were captured at ×50 magnification. This figure appears in colour in the online version of JAC and in black and white in the print version.
Impact of clarithromycin treatment
Administration of clarithromycin was initiated at 4 weeks post-infection, and within 1month the bacterial burden was significantly reduced by 1.5–2 log10 cfu/lung in all four strains of mice (Table 4). However, no additional fall in cfu was observed after an additional month of clarithromycin treatment. No clarithromycin-resistant M. xenopi were selected from any of the mice during this experiment. All of the treated mice exhibited a reduction in gross lesions compared with their untreated counterparts. This reduction was especially marked in the nude mice, whose treated lungs appeared almost free of lesions. In all treated mice, AFB were not visible in lung sections (data not shown).
Multidrug combination therapy in M. xenopi-infected nude mice
Our data indicated that the nude mouse was highly susceptible to M. xenopi aerosol infection, and the impact of treatment on the lung infection could be clearly monitored in this strain. Thus, we utilized the nude mouse aerosol infection model to analyse clinically relevant multidrug combination therapy regimens for treatment of M. xenopi infection. One-hundred-and-twenty female nude mice were simultaneously aerosol-infected with M. xenopi at a dose of 5.17 log10 cfu/lung (determined the day after infection). The mice were randomized into six treatment groups (no treatment, clarithromycin/ethambutol/rifampicin, clarithromycin/ethambutol/rifampicin/amikacin, moxifloxacin/ethambutol/rifampicin, moxifloxacin/ethambutol/rifampicin/amikacin and clarithromycin/moxifloxacin) and treatment was initiated 4 weeks post-infection, when the lung bacterial load had reached 6.63 log10 cfu. The impact of the different treatment regimens on M. xenopi in the mouse lungs is presented in Table 6. The most efficacious regimens were the two containing amikacin, while the moxifloxacin/clarithromycin two-drug regimen was less effective at killing M. xenopi.
Table 6.
Impact of different multidrug treatment regimens on the mean log10 cfu/lung (standard deviation) of M. xenopi in nude mice infected 28 days prior to treatment
| Timepoint relative to the start of treatment |
||||||
|---|---|---|---|---|---|---|
| Group | day −27 | day 0 | week 2 | week 4 | week 8 | week 12 |
| Untreated | 5.17 (0.07) | 6.63 (0.42) | 6.95 (0.43) | 6.93 (0.43) | 7.76 (0.57) | 7.79 (0.49) |
| CLR/EMB/RIF | 5.75 (0.33) | 6.57 (0.77) | 5.68 (0.31) | 4.69 (0.24) | ||
| CLR/EMB/RIF/AMK | 5.86 (0.34) | 5.22 (0.40) | 4.83 (0.25) | 4.58 (0.12) | ||
| MXF/EMB/RIF | 6.42 (0.29) | 6.19 (0.56) | 5.97 (0.94) | 5.57 (0.17) | ||
| MXF/EMB/RIF/AMK | 5.67 (0.27) | 5.25 (0.37) | 4.49 (0.34) | 4.23 (0.36) | ||
| MXF/CLR | 6.07 (0.23) | 5.23 (0.14) | ||||
CLR, clarithromycin (100 mg/kg/day); EMB, ethambutol (100 mg/kg/day); RIF, rifampicin (10 mg/kg/day); AMK, amikacin (100 mg/kg/day); MXF, moxifloxacin (100 mg/kg/day). Drugs were administered 5 days per week.
Discussion
Although M. xenopi infection is a common cause of NTM lung disease in Europe, an optimal treatment strategy has not been defined. Our objective was to characterize the bacteriostatic and bactericidal activities of commonly used anti-mycobacterial drugs alone and in combination against M. xenopi and to develop a mouse model of M. xenopi infection and chemotherapy. Our data confirm the already well-established natural resistance of M. xenopi to most of the indicated antibiotics, whereby the MIC and MBC values were equal to or greater than the Cmax achievable during treatment in humans, when utilized alone. However, different drug combinations demonstrated synergistic, bactericidal activities against M. xenopi in vitro. We added an ex vivo study to validate the choice of our antibiotic combinations. After evaluation of four different strains of mice (BALB/c, C57BL/6, beige and nude), nude mice appear highly susceptible to M. xenopi aerosol infection and the impact of antibiotic regimens might be assessed in the nude mouse model. Thus, our findings represent a significant contribution to the study of M. xenopi chemotherapy.
Our work also highlights the influence of experimental conditions on MIC determination, as illustrated by the discrepant MIC values obtained for the rifamycins and clarithromycin on agar or broth media. The MIC of clarithromycin was further affected by the pH of the solid media, with the MIC value being lower on Mueller–Hinton agar than on the more acidic 7H11 agar, confirming previous findings.19 In addition, M. xenopi exhibits dysgonic growth in vitro. Consequently, results from in vitro assays could reflect an artefact of the dysgonic growth and not be predictive of a clinical response.
The mechanism by which drug combinations including ethambutol exhibit synergy remains mysterious. It has been demonstrated that ethambutol inhibits arabinogalactan synthesis in Mycobacterium smegmatis, thus disrupting the cell wall.30 Thus, it is possible that subinhibitory concentrations of ethambutol induce M. xenopi cell wall damage that allows increasing bactericidal activity of combined regimens. It is also possible that the cell wall damage permits or enhances penetration of other antibiotics into the mycobacterial cell.11 Unfortunately, the usual lack of correlation between in vitro and in vivo data for drug combination activities against NTM lung diseases precludes excessively optimistic expectations.31,32
To our knowledge, we report here the first comparison study of different mouse models of M. xenopi infection, and also the first study of M. xenopi aerosol infection in mice; two previous studies utilized an intravenous route of infection.13,14 Our study indicates that nude mice (congenital athymic nu/nu mice) are more susceptible to M. xenopi aerosol infection than the immune-competent BALB/c and C57BL/6 mouse strains, as well as the immune-compromised beige mice. Importantly, the impact of clarithromycin treatment could clearly be demonstrated in the M. xenopi-infected nude mice, thus suggesting that this model can be utilized for chemotherapy studies.
Contrary to the mouse model of TB, in which untreated mice infected by aerosol with >3.5 log10 cfu of M. tuberculosis die 4–6 weeks after infection, none of the mice infected by aerosol with >4.5 log10 cfu of M. xenopi died by 23 weeks post-infection, despite impressive gross lung pathology. These findings reflect the low intrinsic virulence of M. xenopi, which is an opportunistic pathogen in patients made vulnerable by underlying disease.
We used the nude mouse model to test the in vivo effect of different antibiotic combinations on M. xenopi infection. Despite the relatively low SIT and SBT values in our ex vivo studies (Table 3), amikacin-containing regimens were the most efficacious against M. xenopi in the nude mice. While the clarithromycin and moxifloxacin combination had the lowest MIC values in vitro, this drug pairing was not the most active in vivo. However, no antagonism was observed between clarithromycin and moxifloxacin in the mice. In this model system, clarithromycin and moxifloxacin exhibited the same activity when combined with rifampicin/ethambutol or rifampicin/ethambutol/amikacin. The moxifloxacin/clarithromycin regimen presented at least the same activity as the combination of moxifloxacin, rifampicin and ethambutol.
In conclusion, clarithromycin and moxifloxacin had the lowest MIC values in vitro, and all tested drug combinations exhibited synergism against M. xenopi, with amikacin-containing regimens having the greatest synergistic activities. The ethambutol/rifampicin combination with clarithromycin or moxifloxacin had significant bactericidal activity against M. xenopi. The nude mouse, being highly susceptible to M. xenopi, can be utilized for in vivo chemotherapy studies for this infection. In this model, amikacin-containing regimens were the most effective, and no differences were observed between clarithromycin- or moxifloxacin-containing regimens in vivo. This observation should be examined in humans, comparing clarithromycin or moxifloxacin with ethambutol and rifampicin.
Funding
This work was supported by the National Institutes of Health contract AI400007 and by a scholarship from the French College of Respiratory Diseases (Collège des Enseignants de Pneumologie).
Transparency declarations
None to declare.
Acknowledgements
We thank Timothy Singer, a colleague of Dr Jacques Grosset at Johns Hopkins University, for formatting the manuscript. These results were presented in part as abstracts at the American Thoracic Society Conference in May 2011 (Abstract A19410) (in vitro data) and at the European Respiratory Society Conference in September 2011 (Poster P2725) (mouse models).
References
- 1.Andréjak C, Lescure FX, Pukenyte E, et al. Mycobacterium xenopi pulmonary infections: a multicentric retrospective study of 136 cases in North-East France. Thorax. 2009;64:291–6. doi: 10.1136/thx.2008.096842. [DOI] [PubMed] [Google Scholar]
- 2.Andréjak C, Thomsen VØ, Johansen IS, et al. Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med. 2010;181:514–21. doi: 10.1164/rccm.200905-0778OC. [DOI] [PubMed] [Google Scholar]
- 3.Smith MJ, Citron KM. Clinical review of pulmonary disease caused by Mycobacterium xenopi. Thorax. 1983;38:373–7. doi: 10.1136/thx.38.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 5.Research Committee of the British Thoracic Society. First randomised trial of treatments for pulmonary disease caused by M avium intracellulare, M malmoense, and M xenopi in HIV negative patients: rifampicin, ethambutol and isoniazid versus rifampicin and ethambutol. Thorax. 2001;56:167–72. doi: 10.1136/thorax.56.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruf B, Schürmann D, Mauch H, et al. Effectiveness of the macrolide clarithromycin in the treatment of Mycobacterium avium complex infection in HIV-infected patients. Infection. 1992;20:267–72. doi: 10.1007/BF01710792. [DOI] [PubMed] [Google Scholar]
- 7.Grosset J, Ji B. Prevention of the selection of clarithromycin-resistant Mycobacterium avium-intracellulare complex. Drugs. 1997;54(Suppl 2):23–7. doi: 10.2165/00003495-199700542-00006. [DOI] [PubMed] [Google Scholar]
- 8.Wright J. Current strategies for the prevention and treatment of disseminated Mycobacterium avium complex infection in patients with AIDS. Pharmacotherapy. 1998;18:738–47. [PubMed] [Google Scholar]
- 9.Rapp RP, McCraney SA, Goodman NL, et al. New macrolide antibiotics: usefulness in infections caused by mycobacteria other than Mycobacterium tuberculosis. Ann Pharmacother. 1994;28:1255–63. doi: 10.1177/106002809402801109. [DOI] [PubMed] [Google Scholar]
- 10.Bakker-Woudenberg IA, van Vianen W, van Soolingen D, et al. Antimycobacterial agents differ with respect to their bacteriostatic versus bactericidal activities in relation to time of exposure, mycobacterial growth phase, and their use in combination. Antimicrob Agents Chemother. 2005;49:2387–98. doi: 10.1128/AAC.49.6.2387-2398.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banks J, Jenkins PA. Combined versus single antituberculosis drugs on the in vitro sensitivity patterns of non-tuberculous mycobacteria. Thorax. 1987;42:838–42. doi: 10.1136/thx.42.11.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heifets LB. Synergistic effect of rifampin, streptomycin, ethionamide, and ethambutol on Mycobacterium intracellulare. Am Rev Respir Dis. 1982;125:43–8. doi: 10.1164/arrd.1982.125.1.43. [DOI] [PubMed] [Google Scholar]
- 13.Lounis N, Truffot-Pernot C, Bentoucha A, et al. Efficacies of clarithromycin regimens against Mycobacterium xenopi in mice. Antimicrob Agents Chemother. 2001;45:3229–30. doi: 10.1128/AAC.45.11.3229-3230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klemens SP, Cynamon MH. Activities of azithromycin and clarithromycin against nontuberculous mycobacteria in beige mice. Antimicrob Agents Chemother. 1994;38:1455–9. doi: 10.1128/aac.38.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangadharam PR, Edwards CK, 3rd, Murthy PS, et al. An acute infection model for Mycobacterium intracellulare disease using beige mice: preliminary results. Am Rev Respir Dis. 1983;127:648–9. doi: 10.1164/arrd.1983.127.5.648. [DOI] [PubMed] [Google Scholar]
- 16.Collins FM, Stokes RW. Mycobacterium avium-complex infections in normal and immunodeficient mice. Tubercle. 1987;68:127–36. doi: 10.1016/0041-3879(87)90028-6. [DOI] [PubMed] [Google Scholar]
- 17.Torreles JB, Ellos D, Osborne T, et al. Characterization of virulence, colony, morphotype and the glycopeptidolipid of Mycobacterium avium strain 104. Tuberculosis. 2002;82:293–300. doi: 10.1054/tube.2002.0373. [DOI] [PubMed] [Google Scholar]
- 18.Lorian V. Antibiotics in Laboratory Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 19.Truffot-Pernot C, Ji B, Grosset J. Effect of pH on the in vitro potency of clarithromycin against Mycobacterium avium complex. Antimicrob Agents Chemother. 1991;35:1677–8. doi: 10.1128/aac.35.8.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gradelski E, Kolek B, Bonner DP, et al. Activity of gatifloxacin and ciprofloxacin in combination with other antimicrobial agents. Int J Antimicrob Agents. 2001;17:103–7. doi: 10.1016/s0924-8579(00)00317-4. [DOI] [PubMed] [Google Scholar]
- 21.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 22.Niemi M, Backman JT, Fromm MF, et al. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin Pharmacokinet. 2003;42:819–50. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 23.Chu S, Wilson DS, Deaton RL, et al. Single- and multiple-dose pharmacokinetics of clarithromycin, a new macrolide antimicrobial. J Clin Pharmacol. 1993;33:719–26. doi: 10.1002/j.1552-4604.1993.tb05613.x. [DOI] [PubMed] [Google Scholar]
- 24.Garnham JC, Taylor T, Turner P, et al. Serum concentrations and bioavailability of rifampicin and isoniazid in combination. Br J Clin Pharmacol. 1976;3:897–902. doi: 10.1111/j.1365-2125.1976.tb00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keung A, Eller MG, McKenzie KA, et al. Single and multiple dose pharmacokinetics of rifapentine in man: part II. Int J Tuberc Lung Dis. 1999;3:437–44. [PubMed] [Google Scholar]
- 26.Kirby WM, Clarke JT, Libke RD, et al. Clinical pharmacology of amikacin and kanamycin. J Infect Dis. 1976;134(Suppl):S312–5. doi: 10.1093/infdis/135.supplement_2.s312. [DOI] [PubMed] [Google Scholar]
- 27.Lee CS, Gambertoglio JG, Brater DC, et al. Kinetics of oral ethambutol in the normal subject. Clin Pharmacol Ther. 1977;22:615–21. doi: 10.1002/cpt1977225part1615. [DOI] [PubMed] [Google Scholar]
- 28.Stalker DJ, Jungbluth GL. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinet. 2003;42:1129–40. doi: 10.2165/00003088-200342130-00004. [DOI] [PubMed] [Google Scholar]
- 29.Stass H, Dalhoff A, Kubitza D, et al. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother. 1998;42:2060–5. doi: 10.1128/aac.42.8.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takayama K, Kilburn JO. Inhibition of synthesis of arabinogalactan by ethambutol in Mycobacterium smegmatis. Antimicrob Agents Chemother. 1989;33:1493–9. doi: 10.1128/aac.33.9.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baugnée PE, Pouthier F, Delaunois L. Pulmonary mycobacteriosis due to Mycobacterium xenopi “in-vitro” sensitivity to classical antitubercular agents and clinical development. Acta Clin Belg. 1996;51:19–27. [PubMed] [Google Scholar]
- 32.Jenkins PA, Campbell IA, Banks J, et al. Clarithromycin vs ciprofloxacin as adjuncts to rifampicin and ethambutol in treating opportunist mycobacterial lung diseases and an assessment of Mycobacterium vaccae immunotherapy. Thorax. 2008;63:627–34. doi: 10.1136/thx.2007.087999. [DOI] [PubMed] [Google Scholar]


