Abstract
Objectives
To investigate the pharmacokinetics (PK) of maraviroc, a CCR5-targeted HIV-1 entry inhibitor, in rhesus macaques following vaginal administration of various maraviroc-loaded aqueous hydroxyethylcellulose (HEC) gels, and to correlate the PK data with efficacy in a single high-dose vaginal SHIV-162P3 challenge model.
Methods
Maraviroc concentrations in vaginal fluid (Weck-Cel® sponge), vaginal tissue (punch biopsy) and plasma were assessed over 72 h following single-dose vaginal application of various maraviroc-loaded HEC gels. The range of maraviroc gel concentrations was sufficiently broad (0.003%–3.3% w/w) that test gels included both fully solubilized and predominantly dispersed formulations. The efficacy of the HEC gels against a single high-dose vaginal SHIV-162P3 challenge was also measured, and correlated with the PK concentrations.
Results
Maraviroc concentrations in vaginal fluid (range 104–107 ng/mL), vaginal tissue (100–1200 ng/g) and plasma (<102 ng/mL) were highly dependent on maraviroc gel loading, irrespective of the form of the maraviroc component within the gel (solubilized versus dispersed). Fluid and plasma concentrations were generally highest 0.5 or 2 h after gel application, before declining steadily through to 72 h. Maraviroc concentrations in the various biological compartments correlated strongly with the extent of protection against vaginal SHIV-162P3 challenge. Complete protection was achieved with a 3.3% w/w maraviroc gel.
Conclusions
A high degree of correlation between PK and efficacy was observed. Based on the data obtained with the 3.3% w/w maraviroc gel, maintenance of vaginal fluid and tissue levels in the order of 107 ng/mL and 103 ng/g, respectively, are required for complete protection with this compound.
Keywords: HIV, vaginal microbicide, drug delivery
Introduction
Vaginal microbicides are a potentially useful way to prevent the sexual transmission of HIV type 1 (HIV-1) to women.1–3 The strategy involves the vaginal application of a suitably formulated antiretroviral drug(s) [ARV(s)] some time prior to intercourse. There are several methods for delivering microbicides, including the use of vaginal rings, which release the active compound(s) over a prolonged period.4–11 However, the most common method involves formulating the ARV in an aqueous mucoadhesive gel, such as those based on hydroxyethylcellulose (HEC), which are used routinely for administering drugs vaginally.12–19 Gels of this type need to be applied shortly (at most a few hours) before intercourse takes place, and so are referred to as coitally dependent formulations. Various ARVs can be used as HIV microbicides, the most studied being the nucleotide reverse transcriptase inhibitor (NRTI) tenofovir and the non-nucleoside reverse transcriptase inhibitor (NNRTI) dapivirine.6–8,11,12,16,17 A 1.0% w/w tenofovir HEC gel formulation administered both before and after intercourse has been shown to provide a moderate level of protection to women.17 However, as part of the VOICE (MTN-003) study, the same gel formulation administered once daily showed no measurable efficacy, perhaps because of limited adherence to the application protocol. A dapivirine-releasing matrix-type vaginal ring is due to enter Phase III clinical studies in 2012.
Another drug class under consideration for microbicide use is that of the small-molecule CCR5 inhibitors, exemplified by the licensed drug maraviroc, which is effective in reducing viral loads in infected people.13,14,20–23 These ‘entry inhibitor’ compounds bind to the cell surface CCR5 co-receptor and prevent its use by HIV-1 during the virus cell entry process. In a previous study, a single 4 mL vaginal administration of various maraviroc HEC gel formulations provided rhesus macaques with dose- and time-dependent protection against a single high-dose vaginal challenge with SHIV-162P3.14 The greatest degree of protection (86%, n = 7) was achieved with a 0.3% w/w maraviroc gel (equivalent to 3 mg/mL, or 6 mM as quoted in the original paper14) when the challenge virus was given 30 min after gel application. Efficacy declined to 75%, 50% and 0% when the maraviroc gel concentrations were reduced to 0.1%, 0.01% and 0.003% w/w (equivalent to 2.0, 0.2 and 0.06 mM, respectively). Protection was also dependent on the time interval between gel application and virus challenge; the efficacy of the 0.3% w/w gel fell from 86% at 30 min to 50% and 0% when the interval was extended to 4 and 12 h, respectively.
Although the aforementioned macaque study showed that a maraviroc HEC gel has the potential to protect against HIV-1 sexual transmission, it did not assess vaginal and plasma maraviroc concentrations, likely to be important correlates of protection. To facilitate a better understanding of the correlation between efficacy and pharmacokinetic (PK) parameters, we have now repeated and extended the initial study to quantify maraviroc levels in macaque vaginal fluid and plasma as a function of both maraviroc dose and time. We have also assessed the PK and efficacy of an HEC gel containing an even higher maraviroc concentration (3.3% w/w).
Materials and methods
Preparation of HEC gels
Aqueous gels containing various concentrations of maraviroc (0.003%, 0.01%, 0.03%, 0.1%, 0.3% and 3.3% w/w; equivalent to 0.06, 0.2, 0.6, 2, 6 and 66 mM, respectively; note also that 1% w/w = 10 mg/mL) (Table 1) were prepared by mixing HEC (2.2% w/w; Natrosol HX, Ashland Aqualon) with PBS by using a motorized overhead propeller stirrer for 2 h. The appropriate amount of micronized maraviroc (ViiV Healthcare, median particle size, D50 = 0.567 μm) was then stirred in, and the pH of the gel was adjusted to 7.2 (the pKa of maraviroc).5 The first five gel formulations, with maraviroc concentrations ranging from 0.003% to 0.3% w/w, were similar to those studied previously for efficacy in macaques.14 Visual inspection showed that the maraviroc component was completely dissolved in the 0.003%, 0.01% and 0.03% w/w HEC gels (solubilized gels), while the 0.1%, 0.3% and 3.3% w/w gels also contained increasing quantities of maraviroc in suspended form (suspension gels).
Table 1.
PK parameters for maraviroc concentrations measured in macaque vaginal fluid and plasma following vaginal administration of the various maraviroc HEC gels.
| Maraviroc gel conc. / dose (% w/w / total dose) | Vaginal fluid |
Plasma |
||||
|---|---|---|---|---|---|---|
| Cmax (ng/mL) | Tmax (h) | AUC (ng·h/mL) | Cmax (ng/mL) | Tmax (h) | AUC (ng·h/mL) | |
| 0.003 / 0.12 mg | 18 960 | 12 | 314 400 | 0.16 | 2 | 0.658 |
| 0.01 / 0.4 mg | 149 700 | 0.5 | 583 800 | 0.32 | 2 | 3.774 |
| 0.03 / 1.2 mg | 122 300 | 0.5 | 833 500 | 1.32 | 0.5 | 8.371 |
| 0.10 / 4.0 mg | 394 400 | 2 | 2 420 000 | 3.33 | 0.5 | 41.12 |
| 0.30 / 12 mg | 1 387 000 | 0.5 | 5 351 000 | 18.18 | 2 | 60.40 |
| 3.3 / 132 mg | 6 564 000 | 0.5 | 57 450 000 | 147.9 | 2 | 626.5 |
PK studies in rhesus macaques
A PK study in female cycling rhesus macaques (4–14 years old) was performed at the Tulane National Primate Research Center in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the NIH, and following approval from the Tulane University Institutional Animal Care and Use Committee. The animals were pre-treated with Depo-Provera 30 days before the first application of gel, to synchronize their menstrual cycles, thin their vaginal epithelia and mimic the conditions of the previous SHIV-162P3 challenge experiments.14 The various maraviroc-containing HEC gels (4 mL) were administered vaginally to anaesthetized, recumbent animals (n = 4 macaques per group; one group per maraviroc gel concentration). Vaginal fluid (Weck-Cel®), vaginal tissue (single punch biopsy) and plasma samples were collected according to methods described in detail elsewhere.5,20
Quantification of maraviroc in biological samples
Plasma, vaginal fluid and vaginal tissue maraviroc concentrations were quantified using gradient reverse-phase HPLC (Prominence, Shimadzu) coupled to a triple-quadrupole mass spectrometer (API3200, Applied Biosystems). A detailed description of the analytical method has been reported previously.5,20 Lower limits of maraviroc quantification in vaginal fluid, vaginal tissue and plasma were 50, 1.0 and 0.1 ng/mL, respectively.
Macaque challenge study
The efficacy of the vaginally applied 3.3% w/w maraviroc HEC gel against a SHIV-162P3 vaginal challenge (30 min after gel application) was tested as described previously for the lower-concentration maraviroc gels.14 Briefly, rhesus macaques (n = 4) were treated with Depo-Provera for 30 days before the atraumatic vaginal application of 4 mL of a gel. After a 30 min interval, the SHIV-162P3 challenge virus was applied in a 1 mL volume containing 500 TCID50 (50% tissue culture infectious dose). The outcome of challenge was determined by measuring plasma viral load at 7, 14, 21, 28, 42, 49, 56, 63 and 70 days after challenge with use of a commercially available branched DNA assay for quantitative viral load determination (Siemens Diagnostics). The study was approved by the Institutional Animal Care and Use Committee of the Tulane National Primate Research Center.
Statistical analyses
GraphPad Prism software was used to determine the maximum maraviroc concentrations (Cmax), times to maximum concentration (Tmax) and areas under the concentration–time curve (AUC) for both plasma and vaginal fluid. The same software was also used to calculate Spearman correlations and one-tailed P values.
Results
PK study of maraviroc-containing HEC gels
To understand the relationship between PK parameters and protection from vaginal challenge, HEC gels containing a range of maraviroc concentrations were vaginally administered to macaques as a single 4 mL dose. The maraviroc concentrations in the gels and the methods of gel preparation and delivery were chosen to mimic as closely as possible the experimental conditions used in our earlier SHIV challenge study.14 In addition, a gel containing a 10-fold higher maraviroc concentration (3.33% w/w) was tested, predicted to provide 100% protection based on extrapolation from the previous protection data obtained at lower maraviroc concentrations.14 Mean maraviroc concentrations (±SEM) in the vaginal fluid and plasma over 72 h and in the vaginal tissue at 24 h are presented in Figure 1; values for Cmax, Tmax and AUC are summarized in Table 1. The maraviroc levels in vaginal fluid and plasma were generally highest at 0.5 and 2 h, respectively, after gel application; they then declined steadily until the end of the monitoring period (72 h) (Figure 1a and b, and Table 1). Plasma maraviroc levels were typically five orders of magnitude lower than in vaginal fluid, ranging from 10−1 to 102 ng/mL and dependent on the gel loading. As expected, the greater the maraviroc concentration in the gel, the higher the Cmax and AUC values in both vaginal fluid and plasma (Table 1).
Figure 1.
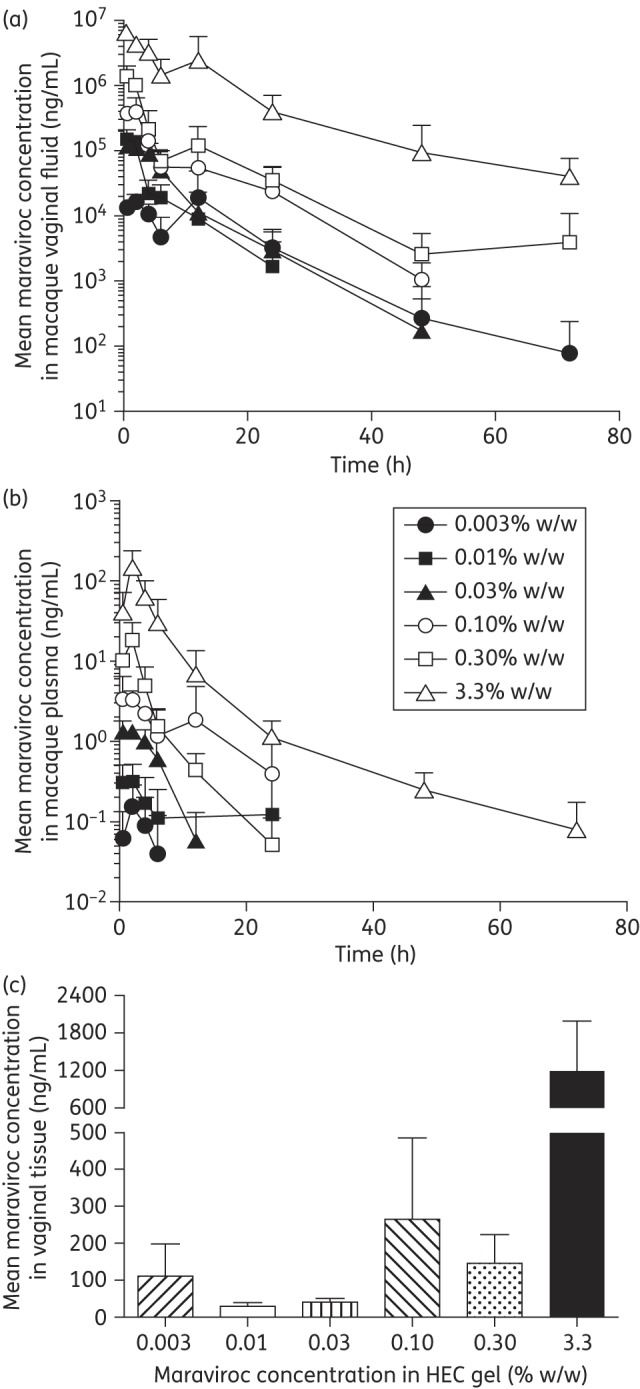
Maraviroc concentrations in macaque vaginal fluid (a), plasma (b) and vaginal tissue (c, at 24 h) following vaginal administration of HEC gels containing various maraviroc loadings. Four animals were used to test each gel formulation. Key to symbols on graph (b) also applies to graph (a). When data points are missing, maraviroc levels were below the limit of quantification for that assay.
In the previous macaque study, SHIV-162P3 challenge was performed 30 min after gel application. Here, we report strong correlations between the maraviroc concentrations in both vaginal fluid and plasma after 30 min and the quantity of the drug administered in the gel (Spearman r values = 0.9429 and 1.000, respectively) (Figure 2). At the highest applied maraviroc gel doses (0.3% and 3.3% w/w), the vaginal fluid concentrations at 30 min were 1.4 × 106 and 6.5 × 106 ng/mL, respectively (Figure 2).
Figure 2.
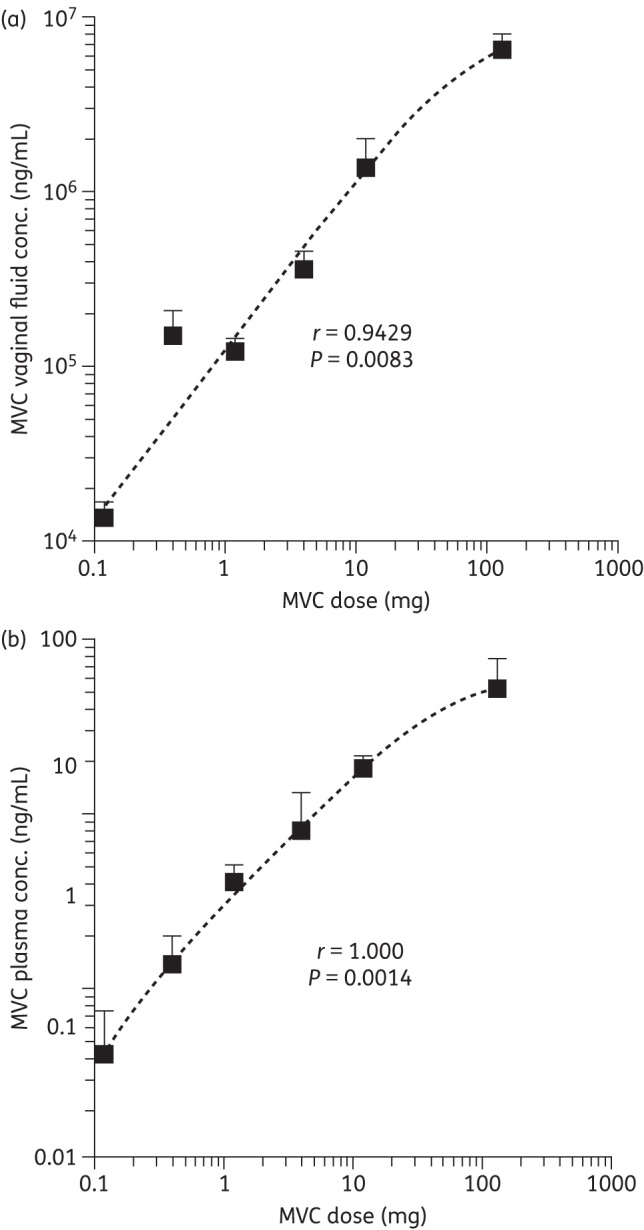
Correlation between the total maraviroc (MVC) dose administered vaginally in the HEC gels and the mean vaginal fluid (a) and plasma (b) concentrations (±SEM) measured 30 min after gel administration. The r values represent Spearman correlations; the P values are one-tailed.
The amounts of maraviroc in vaginal tissues 24 h after gel application were determined by taking a single punch biopsy sample. Although maraviroc could be detected in all the vaginal tissue samples, there was an inconsistent relationship between the amount of maraviroc applied and what was measured, although a general trend was apparent (Figure 1c). The higher maraviroc doses led to vaginal tissue concentrations that exceeded 100 ng/g and, in the case of the 3.3% w/w gel, 1200 ng/g (Figure 1c).
Complete protection from vaginal challenge by high-dose maraviroc HEC gel
The efficacy of the 3.3% w/w maraviroc HEC gel against SHIV-162P3 vaginal challenge (30 min after gel application) was also assessed using the same protocol and virus stock as for the lower maraviroc concentration gels.14 All four animals given this gel remained uninfected after challenge. Our experience with the same SHIV-162P3 challenge stock and dose is that it consistently infects control animals under the conditions of this experiment (∼95% infection rate). Hence we interpret the 0/4 infection rate as approximating to 100% protection for the 3.3% w/w maraviroc gel.
The newly derived data on the 3.3% w/w gel were pooled with those obtained previously,14 to illustrate the correlations between the maraviroc dose applied vaginally, the mean measured maraviroc vaginal fluid concentration and the extent of protection against SHIV-162P3 challenge (Figure 3).
Figure 3.
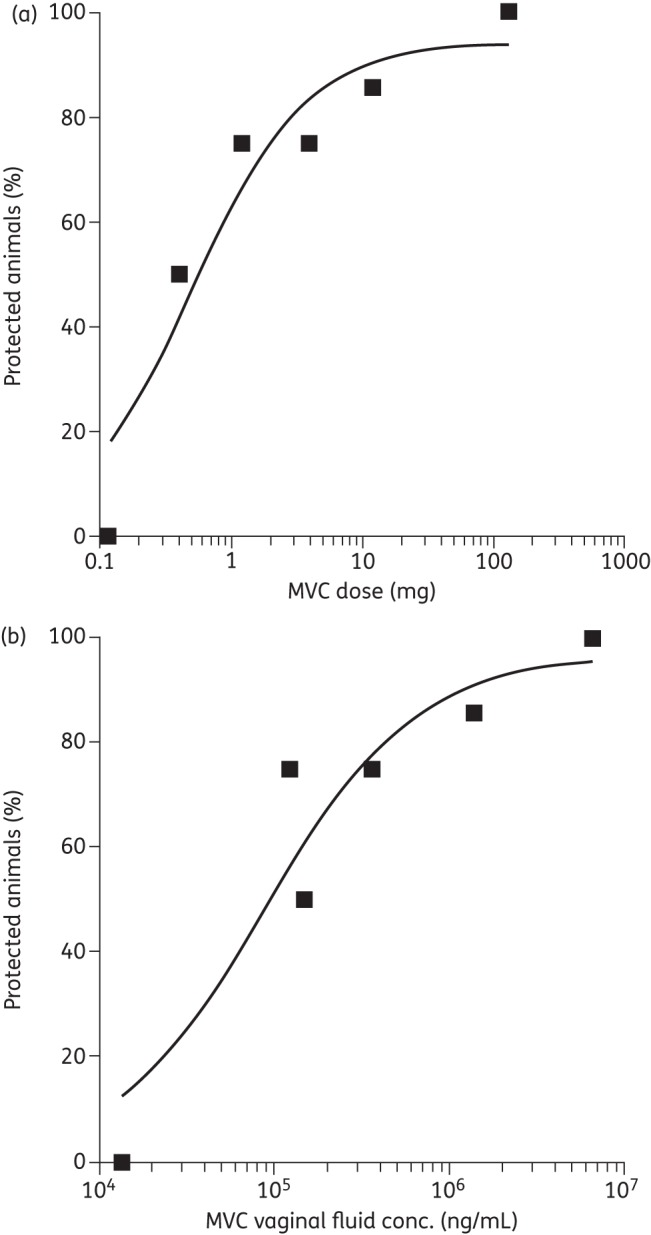
(a) Correlation between the extent of protection against SHIV-162P3 vaginal challenge 30 min after gel administration and the total maraviroc (MVC) dose administered vaginally to macaques in a 4 mL HEC gel volume. (b) Correlation between the extent of protection (as above) and the mean measured maraviroc vaginal fluid concentration as measured for the different maraviroc-loaded gels. For both graphs, the protection data for all but the highest maraviroc gel dose (132 mg, 3.3% w/w) were obtained in a previous study using four to seven animals per group.14 The protection data for the 3.3% w/w maraviroc gel and all vaginal fluid concentrations were obtained as part of this study (n = 4).
Discussion
An issue considered important in the development of microbicide gel formulations has been to ensure that the active compound is completely dissolved within the gel, based on the rationale that only solubilized drugs can have a clinical effect.12,16,24 This constraint has been particularly challenging when formulating poorly water-soluble compounds, and has invariably limited the final concentration present in water-based gels. Although surfactants and co-solvents have been used to increase the amount of dissolved microbicide,12 such strategies are best avoided as they can damage the integrity of the vaginal epithelial layer and increase HIV-1 acquisition.15,25,26
In this study, the maraviroc component was completely dissolved in the 0.003%, 0.01% and 0.03% w/w HEC gels, while the 0.1%, 0.3% and 3.3% w/w gels also contained increasing quantities of maraviroc in suspended form. The above findings are consistent with previously reported data on the aqueous solubility of maraviroc.5 Both the local (vaginal) and systemic (plasma) bioavailability of maraviroc increased proportionately with the initial maraviroc loading in the gel (Figure 2 and Table 1), despite the transition from a completely solubilized to a predominantly suspended gel form (see the Materials and methods section). The suspended maraviroc component in the higher-loading gels must be rapidly dissolved/absorbed after vaginal administration for it to contribute so effectively to the concentration profiles observed for vaginal fluid and plasma (Figure 2). One implication for formulation strategies is that the solubility of the active component in the gel base need not be the limiting factor for the amount loaded into the final product.
The HEC gel loaded with 3.3% w/w maraviroc (predominantly in the dispersed state) provided macaques with 100% protection (i.e. 0/4 infections) against a single high-dose vaginal SHIV-162P3 challenge administered 30 min later. The PK measurements indicate that this degree of protection correlates with vaginal fluid and tissue maraviroc concentrations in the order of 107 ng/mL and 103 ng/g, respectively.
Plasma maraviroc concentrations ranged from 10−1 to 102 ng/mL depending on the gel loading. The higher end of this range substantially exceeds the in vitro IC50 values for typical HIV-1 isolates, which are generally in the range 0.2–6 ng/mL (i.e. 0.1–3 nM).14,27–29 A concern therefore exists that the higher loadings of vaginally applied maraviroc gels could drive the emergence of resistance in women who use them without realizing they are HIV-1 infected. Conversely, it is also possible that the plasma concentrations of the drug could assist in protection against vaginal (or even rectal) challenge.
Maraviroc concentrations in the vaginal fluid and blood plasma of human females after a single oral dose and twice-daily oral doses over a 7 day span (300 mg/dose in each case) have been reported.29 In the single oral dose study, vaginal fluid levels ranged from 1 ng/mL (at 1 h) to 1000 ng/mL (at 12 h).29 Even the latter concentration, the highest recorded, is 10-fold lower than that achieved by the 0.003% w/w maraviroc gel (0.12 mg vaginal dose, the lowest tested) and three to four orders of magnitude less than was provided by the 3.3% w/w gel (132 mg vaginal dose, the highest tested). Thus, the vaginal fluid concentrations at the 12 h timepoint were 1.9 × 104 and 2.5 × 106 ng/mL for the 0.003% and 3.3% w/w gels, respectively, compared with 1.0 × 103 ng/mL in the human oral dosing study (Figure 1).29 The relationship between the vaginal fluid concentrations and the extent of protection from vaginal challenge (Figure 3) suggests that orally delivered maraviroc may not provide sufficiently high maraviroc concentrations in the vagina to be locally protective against vaginally deposited virus. However, the availability of the drug systemically after oral delivery may enable it to intervene successfully at non-local sites and still be protective overall. There are also quantitative limitations when extrapolating from macaque challenge data to naturally exposed human females. We note that oral delivery of a different small-molecule CCR5 inhibitor, CMPD167, to macaques did provide partial protection when daily dosing was continued after vaginal challenge.22 No PK data were obtained in that study, an omission that should perhaps be addressed using orally delivered maraviroc in a macaque PK/challenge study.
Maraviroc is a basic molecule with a widely reported pKa value of 7.2.30 Our recent experimental work has shown that maraviroc has, in fact, two pKa values, of 3.31 (triazole ring) and 7.84 (tropane ring).5 Thus, in the pH 7.2 HEC gel formulation used in the present study (selected to match the macaque vaginal pH), the triazole ring will be present in the free base form (0% ionized) while the tropane ring will be mostly ionized (∼78%). Generally, ionized drug forms have better aqueous solubility but poorer absorption compared with their non-ionized counterparts. Vaginal gels for human use are usually formulated around pH 4.5, again reflecting the normal pH of that compartment. Under these conditions, the triazole ring will be present mostly in the free base form (6% ionized) while the tropane ring will be mostly ionized (100%). It is difficult to predict how these differences in maraviroc ionization might influence its absorption and distribution following vaginal administration of a maraviroc HEC gel in women. Further studies along these lines would seem appropriate.
Vaginal ring devices containing maraviroc, either alone or in combination with dapivirine, are also being evaluated for HIV-1 prevention. Recently, we conducted a PK study in rhesus macaques with a matrix-type silicone elastomer ring containing 400 mg of maraviroc.5 The maraviroc vaginal fluid concentrations throughout the 28 day study were mostly maintained in the 106–107 ng/mL range,5 similar to those measured during the first 24 h after vaginal application of the 3.3% w/w HEC gel (Figure 1a). Whether ring and gel deliveries of maraviroc provide equivalent degrees of protection at similar vaginal fluid concentrations remains to be determined.
Funding
This work was supported by the National Institutes of Health (grant number U19 AI076982).
Transparency declarations
None to declare.
Acknowledgements
We thank John Pottage of ViiV Healthcare for providing maraviroc and Robin Shattock for his help and guidance.
References
- 1.Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med. 2008;59:455–71. doi: 10.1146/annurev.med.59.061206.112737. [DOI] [PubMed] [Google Scholar]
- 2.Hendrix CW, Cao YJ, Fuchs EJ. Topical microbicides to prevent HIV: clinical drug development challenges. Annu Rev Pharmacol Toxicol. 2010;49:349–75. doi: 10.1146/annurev.pharmtox.48.113006.094906. [DOI] [PubMed] [Google Scholar]
- 3.Shattock RJ, Rosenberg Z. Microbicides: topical prevention against HIV. Cold Spring Harb Perspect Med. 2012;2:a007385. doi: 10.1101/cshperspect.a007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolfson AD, Malcolm RK, Toner CF, et al. Potential use of vaginal rings for prevention of heterosexual transmission of HIV: a controlled-release strategy for HIV microbicides. Am J Drug Deliv. 2006;4:7–20. [Google Scholar]
- 5.Malcolm RK, Veazey RS, Geer L, et al. Sustained release of the CCR5 inhibitors CMPD167 and maraviroc from vaginal rings in rhesus macaques. Antimicrob Agents Chemother. 2012;56:2251–8. doi: 10.1128/AAC.05810-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nel A, Smythe S, Young K, et al. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:416–23. doi: 10.1097/qai.0b013e3181acb536. [DOI] [PubMed] [Google Scholar]
- 7.Romano R, Variano B, Coplan P, et al. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Human Retroviruses. 2009;25:483–8. doi: 10.1089/aid.2008.0184. [DOI] [PubMed] [Google Scholar]
- 8.Johnson TJ, Gupta KM, Fabian J, et al. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur J Pharm Sci. 2010;39:203–12. doi: 10.1016/j.ejps.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Malcolm RK, Edwards K-L, Kiser P, et al. Advances in microbicide vaginal rings. Antiviral Res. 2010;88:S30–9. doi: 10.1016/j.antiviral.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Johnson TF, Srinivasan P, Albright TH, et al. Safe and sustained vaginal delivery of pyrimidinedione HIV-1 inhibitors from polyurethane intravaginal rings. Antimicrob Agents Chemother. 2012;56:1291–99. doi: 10.1128/AAC.05721-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mesquita PMM, Rastogi R, Segarra TJ, et al. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. J Antimicrob Chemother. 2012;67:1730–8. doi: 10.1093/jac/dks097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nel AM, Coplan P, van de Wijgert JH, et al. Safety, tolerability, and systemic absorption of dapivirine vaginal microbicide gel in healthy, HIV-negative women. AIDS. 2009;23:1531–8. doi: 10.1097/QAD.0b013e32832c413d. [DOI] [PubMed] [Google Scholar]
- 13.Tsibris AMN, Pal U, Schure AL, et al. SHIV-162P3 infection of rhesus macaques given maraviroc gel vaginally does not involve resistant viruses. PLoS One. 2011;6:e28047. doi: 10.1371/journal.pone.0028047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veazey RS, Ketas TJ, Dufour J, et al. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis. 2010;202:739–44. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz JL, Rountree W, Kashuba ADM, et al. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PLoS One. 2011;6:e25974. doi: 10.1371/journal.pone.0025974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karim QA, Karim SSA, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acartürk F. Mucoadhesive vaginal drug delivery systems. Recent Pat Drug Deliv Formul. 2009;3:193–205. doi: 10.2174/187221109789105658. [DOI] [PubMed] [Google Scholar]
- 19.Andrews GP, Donnelly L, Jones DS, et al. Characterization of the rheological, mucoadhesive, and drug release properties of highly structured gel platforms for intravaginal drug delivery. Biomacromolecules. 2009;10:2427–35. doi: 10.1021/bm9003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forbes CJ, Lowry D, Geer L, et al. Non-aqueous silicone elastomer gels as a vaginal microbicide delivery system for the HIV-1 entry inhibitor maraviroc. J Control Release. 2011;156:161–9. doi: 10.1016/j.jconrel.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veazey RS, Klasse PJ, Schader SM, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus–cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 22.Veazey RS, Springer MS, Marx PA, et al. Protection of macaques from vaginal SHIV challenge by an orally delivered CCR5 inhibitor. Nat Med. 2005;11:1293–4. doi: 10.1038/nm1321. [DOI] [PubMed] [Google Scholar]
- 23.Neff CP, Kurisu T, Ndolo T, et al. A topical microbicide gel formulation of CCR5 antagonist maraviroc prevents HIV-1 vaginal transmission in humanized RAG-hu mice. PLoS One. 2011;6:e20209. doi: 10.1371/journal.pone.0020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brouwers J, Vermeire K, Grammen C, et al. Early identification of availability issues for poorly water-soluble microbicide candidates in biorelevant media: a case study with saquinavir. Antiviral Res. 2011;91:217–23. doi: 10.1016/j.antiviral.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Rustomjee R, Abdool Karim Q, Abdool Karim SS, et al. Phase I trial of nonoxynol-9 film among sex workers in South Africa. AIDS. 1999;13:1511–5. doi: 10.1097/00002030-199908200-00011. [DOI] [PubMed] [Google Scholar]
- 26.Stafford MK, Ward H, Flanagan A, et al. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:327–31. doi: 10.1097/00042560-199804010-00006. [DOI] [PubMed] [Google Scholar]
- 27.Rosario MC, Jacqmin P, Dorr P, et al. A pharmacokinetic-pharmacodynamic disease model to predict in vivo antiviral activity of maraviroc. Clin Pharmacol Ther. 2005;78:508–19. doi: 10.1016/j.clpt.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Dorr P, Westby M, Dobbs S, et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49:4721–32. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumond J, Patterson KB, Pecha AL, et al. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:546–53. doi: 10.1097/QAI.0b013e3181ae69c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker DK, Abel S, Comby P, et al. Species differences in the disposition of the CCR5 antagonist, UK- 427,857, a new potential treatment for HIV. Drug Metab Dispos. 2005;33:587–95. doi: 10.1124/dmd.104.002626. [DOI] [PubMed] [Google Scholar]


