Abstract
Objectives
We aim to develop antibacterial peptide mimics resistant to protease degradation, with broad-spectrum activity at sites of infection.
Methods
The bactericidal activities of LL-37, ceragenins CSA-13, CSA-90 and CSA-92 and the spermine-conjugated dexamethasone derivative D2S were evaluated using MIC and MBC measurements. Gingival fibroblast counting, interleukin-8 (IL-8) and lactate dehydrogenase (LDH) release from keratinocytes (HaCat) were used to determine effects on cell growth, pro-inflammatory response and toxicity.
Results
All tested cationic lipids showed stronger bactericidal activity than LL-37. Incubation of Staphylococcus aureus with half the MIC of LL-37 led to the appearance of bacteria resistant to its bactericidal effects, but identical incubations with CSA-13 or D2S did not produce resistant bacteria. Cathelicidin LL-37 significantly increased the total number of gingival fibroblasts, but ceragenins and D2S did not alter gingival fibroblast growth. Cationic lipids showed no toxicity to HaCat cells at concentrations resulting in bacterial killing.
Conclusions
These data suggest that cationic lipids such as ceragenins warrant further testing as potential novel antibacterial agents.
Keywords: oral infection, microbiological assay, ceragenins, D2S
Introduction
Antimicrobial peptides produced in the oral cavity and upper respiratory tract play a key role in maintaining homeostasis of this complex environment. Antimicrobial peptides could have potential therapeutic use for prevention of dental caries and treatment of topical infection, periodontal diseases and certain systemic diseases.1 The molecular pattern that determines the activity of natural cationic antibacterial peptides can be a model for the design of new mimics, such as synthetic cationic lipids. Since the pioneering description of human cathelicidin (hCAP-18) in 1995,2 various functions have been considered for LL-37, a peptide derived from the C terminus of hCAP-18. LL-37 functions as an antimicrobial agent against a broad spectrum of Gram-positive and Gram-negative bacteria3 and as a cell-penetrating peptide that can deliver nucleic acids into host cells.4 As a result of its effects on host cells, LL-37 might also be directly involved in healing processes, especially cutaneous wound angiogenesis and cell growth.5 LL-37 kills bacteria by mechanisms involving electrostatic interactions with bacterial surfaces and disruption of bacterial membranes.6 The hypothesis that facial amphiphilicity rather than specific biochemical interactions determines host cationic antibacterial peptide (CAP) activity has motivated efforts to design and synthesize cationic steroid antibiotics (CSAs) that mimic the activity of CAPs. CSAs display positive charges arranged on one face and hydrophobic residues on the other, are relatively easy to prepare and are stable on epithelial surfaces and in most body fluids due to protease resistance.7 Ceragenin CSA-13 displays a broad range of antimicrobial activity.8 It also shows low toxicity in animal studies, supporting this compound's possible application in human treatment.9 Disubstituted dexamethasone–spermine (D2S), a cationic corticosteroid derivative initially identified as a by-product of dexamethasone–spermine synthesis for the purpose of improving cellular gene delivery, displays strong antibacterial activity with a low lytic effect on host plasma membranes10 and inhibits the inflammatory response induced by bacterial wall products in vitro.11 The anti-inflammatory activity of D2S is at least partly mediated through direct glucocorticoid receptor activation.11
In this study we describe high-potency antibacterial activities of LL-37, CSA-13, CSA-90, CSA-92 and D2S against bacterial strains that are associated with infections in the upper respiratory tract and oral cavity.
Material and methods
Materials
LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) peptide was purchased from Peptide2.0 (Chantilly, VA, USA). Pseudomonas isolation agar, Luria–Bertani broth, Mueller–Hinton broth and tryptic soy broth were purchased from Difco (Sparks, MD). Brain heart infusion agar was from Emapol (Gdańsk, Poland). Dulbecco's Modified Eagle's Medium (DMEM) and Minimum Essential Medium (MEM Alpha) were from Gibco (Grand Island, NY). Protease inhibitor cocktail (S8820-20TAB) was from Sigma. Fetal bovine serum was from Hyclone (Logan, UT). P. aeruginosa strain Xen 5, engineered by conjugation and transposition of a plasmid carrying transposon Tn5 luxCDABE, was purchased from Caliper Life Science Inc. (CA, USA). Ceragenins were prepared as previously described12 and were characterized by NMR and liquid chromatography/mass spectrometry. Collection of saliva and dental plaque biofilms from 21 healthy subjects was performed in the Department of Prosthetic Dentistry, Medical University of Białystok, in accordance with a protocol approved by the Medical University of Białystok Ethics Committee for Research on Humans and Animals and written consent was obtained from all subjects.
Antimicrobial testing
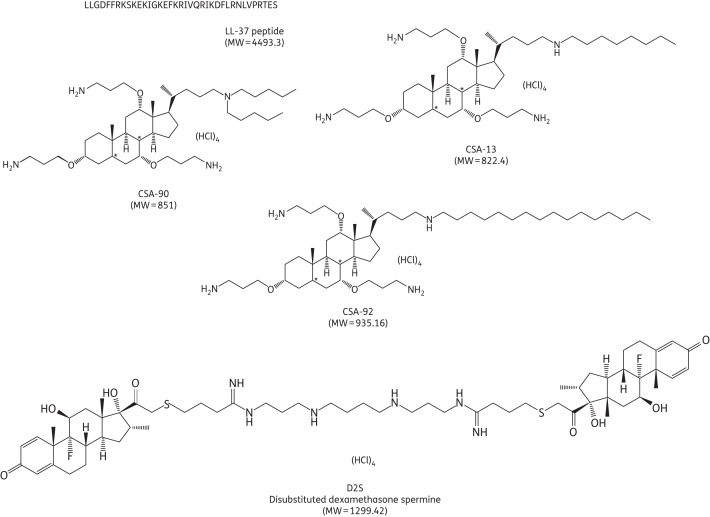
The MICs and MBCs of the antibacterial agents (Figure 1) against different clinical isolates, including Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus salivarius, Streptococcus mutans, Enterococcus faecalis and Helicobacter pylori (∼105 cfu/mL), were determined using bacteria at the logarithmic phase of growth as previously described.11 Antibacterial activity against H. pylori was measured as described previously.13 In addition to 20 different bacterial strains for which MBC and MIC values were evaluated, 4 clinical isolates (S. salivarius, S. mutans, E. faecalis and H. pylori) were used to evaluate the antibacterial activities of LL-37, CSA-13, CSA-90, CSA-92 and D2S in an experimental setting in which these strains were added to human saliva (combined collection from 21 volunteers, filtered through a 0.45 μm membrane) and premixed with dental plaque (combined collection from the same subjects from whom saliva was collected), followed by antibacterial treatment. The working solution of saliva/dental plaque was made by adding 9 g of saliva to 1 g of dental plaque. This mixture was free of E. faecalis and H. pylori as indicated by lack of E. faecalis outgrowth and negative serological tests for H. pylori antigen. Detection of H. pylori antigens was based on the monoclonal antibody test used for H. pylori antigen detection in the stool (Amplified IDEIA TM Hp StAR™, Oxoid, Ely, UK) as described previously.13 Endogenous S. salivarius and S. mutans were absent. Selective agar medium MS-agar (Difco, Detroit, USA) was used for isolation of cocci of the genus Streptococcus. Clinical isolates of S. salivarius, S. mutans and H. pylori were grown in microaerophilic conditions and E. faecalis was grown in an aerobic atmosphere. This experiment was designed to determine whether saliva or dental plaque contained factors that inhibit antibacterial agents. The concentration of agent that prevents visible bacterial growth (MIC) and that which eradicates tracer bacteria (MBC) were determined at 10 and 30 min and 18 h. At 18 h, cfu counts were obtained for all antibacterial peptide concentrations to assess the cumulative effect of bacterial killing.14 Proteolytic degradation of CAPs represents the most likely mechanism that bacteria use to prevent their destruction. The ability of bacteria to develop resistance to LL-37, CSA-13 and D2S was measured using successive generations of three different clinical isolates of S. aureus (up to eight passages) that were grown from a suspension subjected to treatment with half the MIC of antibacterial agents. Proteolytic activity was assessed using Mueller–Hinton agar plates with the addition of fat-free milk (10%).15 When required, MIC values were evaluated in buffer containing a protease inhibitor cocktail.
Figure 1.
Structures of cathelicidin LL-37 and cationic lipid molecules. For amino acids, the one-letter codes are used.
Cell culture and cell growth
Human gingival fibroblasts were obtained from the gingiva surrounding extraction sites from patients (14–18 years) attending the dental clinic at the Hospital for Sick Children (Toronto, ON). Explant cultures were produced from 1 mm3 pieces and were cultured in MEM supplemented with 10% fetal bovine serum at 37°C with 5% CO2 and were cultured for three to six passages before experiments were performed. Cultured cells were seeded and then allowed to spread for 24–72 h. Human immortalized keratinocytes (HaCat cells, passage 44) were grown in DMEM supplemented with 10% fetal bovine serum and 2 mM glutamine. When required, gingival fibroblasts and HaCat cells were treated with LL-37, CSA-13, CSA-90, CSA-92 or D2S (0.5–50 μM) for 2–24 h before cell culture medium was collected or cell counting was performed (Leica DM IRBE microscope).
Evaluation of IL-8 concentration and LDH activity in cell culture media
To assess interleukin-8 (IL-8) and lactate dehydrogenase (LDH) release, HaCat cells were grown to confluence in 24-well plates. In all experiments, medium was changed to serum-free and phenol red-free DMEM ∼2 h prior to cell treatment. Cell culture medium samples were centrifuged (10 min, 5000 rpm at room temperature) and the supernatant was stored at −80°C. IL-8 and LDH were determined using an IL-8 ELISA kit (Thermo Scientific) and an LDH cytotoxicity assay kit (BioVision Inc.), respectively.
Statistical analysis
Data are reported as mean ± SD from three to six repeats. Differences between means were evaluated using the unpaired Student's t-test and differences with P < 0.05 were considered as statistically significant.
Results
Bactericidal activity
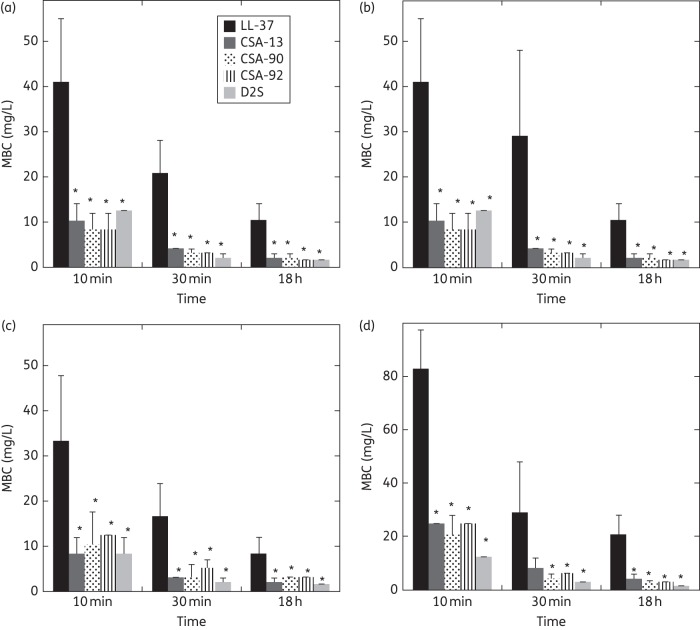
Table 1 shows MIC and MBC values determined for LL-37, CSA-13, CSA-90, CSA-92 and D2S against 20 different bacterial strains. The bactericidal activity of LL-37 and all tested cationic lipids against Lactobacillus casei ssp. casei, a bacterial strain present in the digestive tract, was lower than activites for other strains. This observation indicates that some commensal strains of bacteria might be less susceptible to host antibacterial agents than pathogenic ones. Additionally, the similar potencies of the four cationic lipids across all strains (Table 1) suggest that they work by the same molecular mechanism [all four cationic lipids are either highly potent (e.g. S. aureus) or relatively impotent (e.g. Lactobacillus)]. In agreement with our previous observation showing a decrease of P. aeruginosa Xen 5 chemiluminescence during the first minute after CSA-13 addition,16 we observed that MBC values did not differ significantly after 30 min or 18 h (Figure 2). As shown in Figure 2 and Table 2, cationic lipids had significantly stronger antibacterial activity than LL-37. A concentration of cationic lipid even at one-tenth of MBC values could eradicate 19%–82% of bacteria added to a saliva/dental plaque mixture.
Table 1.
Antibacterial activity of LL-37 peptide and different cationic lipids against pathogens associated with oral infections
| Bacterial strain | Antibacterial agents (MBC/MIC mg/L) |
|||||
|---|---|---|---|---|---|---|
| LL-37 | CSA-13 | CSA-90 | CSA-92 | D2S | AMC MIC (mg/L) | |
| Staphylococcus aureus ATCC 29213 | 28/14 | 1.4/0.7 | 2.8/0.7 | 0.75/0.75 | 2.1/2.1 | 0.3 |
| Streptococcus salivarius ATCC 13419 | 28/14 | 1.4/0.7 | 1.4/0.7 | 3 /1.5 | 2.1/1.1 | 0.037 |
| Streptococcus sanguinis ATCC 10556 | 28/14 | 0.7/0.7 | 1.4/1.6 | 3/1.5 | 2.1/1.1 | 0.037 |
| Streptococcus mutans ATCC 35668 | 28/28 | 1.4/0.7 | 1.4/0.7 | 1.5/0.75 | 1.1/1.1 | 0.037 |
| Staphylococcus epidermidis* | 56/56 | 0.7/0.35 | 1.4/0.7 | 3/3 | 2.1/2.1 | ND |
| Streptococcus pneumoniae* | 14/14 | 0.7/0.35 | 1.4/0.7 | 1.5/1.5 | 1.1/0.55 | 0.015 |
| Streptococcus pyogenes* | 14/14 | 0.7/0.7 | 1.4/0.35 | 0.75/0.75 | 2.1/01.1 | 0.007 |
| Haemophilus influenzae* | 56/28 | 0.7/0.35 | 1.4/0.7 | 1.5/0.7 | 1.1/1.1 | ND |
| Enterococcus faecalis ATCC 29212 | 56/28 | 2.8/2.8 | 2.8/1.4 | 3/8 | 2.1/1.1 | 0.3 |
| Moraxella catarrhalis ATCC 23246 | 28/28 | 1.4/1.4 | 1.4/0.7 | 1.5/0.35 | 2.1/1.1 | 0.075 |
| Helicobacter pylori* | 28/14 | 0.7/0.7 | 1.4/0.7 | 1.5/0.75 | 1.1/0.55 | 0.075 |
| Peptostreptococcus anaerobius ATCC 27337 | 224/224 | 5.6/5.6 | 22.4/22.4 | 11.7/5.8 | 32.4/16.2 | 1.2 |
| Porphyromonas gingivalis ATCC 33277 | 224/224 | 5.6/5.6 | 11.2/11.2 | 23.4/11.7 | 16.2/16.2 | 1.2 |
| Fusobacterium nucleatum ATCC 25586 | 224/224 | 22.4/11.2 | 11.2/11.2 | 23.4/11.7 | 32.4/16.2 | 2.4 |
| Lactobacillus casei ssp. casei ATCC 393 | 224/224 | 44.8/22.4 | 44.8/44.8 | 46.8/46.8 | 64.8/32.4 | 2.4 |
| Tannerella forsythensis ATCC 43037 | 224/224 | 22.4/22.4 | 44.8/44.8 | 46.8/46.8 | 32.4/16.2 | 2.4 |
| Staphylococcus aureus Xen 29 (pleural fluid isolate NCTC8532) | 28/28 | 2.8/1.4 | 2.8/1.4 | 5.8/2.9 | 4.05/4.05 | 0.15 |
| Pseudomonas aeruginosa Xen 5 (derived from P. aeruginosa strain ATCC 19660) | 448/224 | 11.2/5.6 | 22.4/22.4 | 23.4/11.7 | 8.1/8.1 | 2.4 |
| Neisseria meningitidis (B) | 56/28 | 0.7/0.7 | 1.4/1.4 | 5.8/2.9 | 8.1/2.1 | ND |
| Neisseria meningitidis (C) | 112/28 | 1.4/0.7 | 1.4/1.4 | 5.8/2.9 | 4.05/2.1 | ND |
Clinical strains are indicated with asterisks. AMC, amoxicillin/clavulanic acid 2 : 1; ND, not determined.
Figure 2.
Bactericidal activity of LL-37, CSA-13, CSA-90, CSA-92 and D2S against clinical isolates of S. salivarius (a), S. mutans (b), E. faecalis (c) and H. pylori (d) (∼105 cfu/mL), evaluated after their addition to a suspension of dental plaque in saliva. After the addition of antibacterial molecules, at different timepoints (10 min, 30 min and 18 h), bacteria were spotted on agar plates. *Significantly different from LL-37's effect at the same timepoint.
Table 2.
Cumulative percentage of bacterial killing after 18 h of incubation with different concentrations of antibacterial agents, then addition to saliva/dental plaque mixture. Each percentage value was calculated based on the average cfu obtained from three individual measurements
| Test organism/agent concentration (mg/L) | Cumulative killing (%) |
||||
|---|---|---|---|---|---|
| LL-37 | CSA-13 | CSA-90 | CSA-92 | D2S | |
| Streptococcus salivarius* (1.1 × 105 cfu/mL) | |||||
| 0.4 | 9.1 | 10.9 | 8.2 | 9.1 | 16.4 |
| 0.8 | 19.1 | 49.1 | 37.3 | 19.1 | 39.1 |
| 1.6 | 27.3 | 71.8 | 74.6 | 30 | 79.1 |
| 3.125 | 46.4 | 100 | 100 | 59.1 | 100 |
| 6.25 | 80 | 100 | |||
| 12.5 | 100 | ||||
| 25 | |||||
| Streptococcus mutans (1.5 × 105 cfu/mL) | |||||
| 0.4 | 14.7 | 20.7 | 12 | 21.3 | 32.7 |
| 0.8 | 32 | 54.7 | 40 | 46 | 65.4 |
| 1.6 | 54 | 85.4 | 84 | 66 | 100 |
| 3.125 | 81.3 | 100 | 100 | 100 | |
| 6.25 | 96.6 | ||||
| 12.5 | 100 | ||||
| 25 | |||||
| Enterococcus faecalis (2.3 × 105 cfu/mL) | |||||
| 0.4 | 9.6 | 7.8 | 4.3 | 6.5 | 13.9 |
| 0.8 | 25.7 | 16.9 | 31.3 | 17.4 | 56.1 |
| 1.6 | 34.8 | 42.6 | 69.1 | 47.4 | 83.1 |
| 3.125 | 42.6 | 80 | 100 | 70 | 100 |
| 6.25 | 64.8 | 100 | 100 | ||
| 12.5 | 85.7 | ||||
| 25 | 100 | ||||
| Helicobacter pylori* (1.8 × 105 cfu/mL) | |||||
| 0.4 | 10 | 11.1 | 16.7 | 8.3 | 32.8 |
| 0.8 | 16.1 | 50.5 | 49.5 | 21.1 | 82.2 |
| 1.6 | 38.9 | 83.3 | 87.8 | 55 | 100 |
| 3.125 | 50 | 100 | 100 | 87.2 | |
| 6.25 | 71 | 100 | |||
| 12.5 | 93.9 | ||||
| 25 | 100 | ||||
Clinical strains are indicated with asterisks.
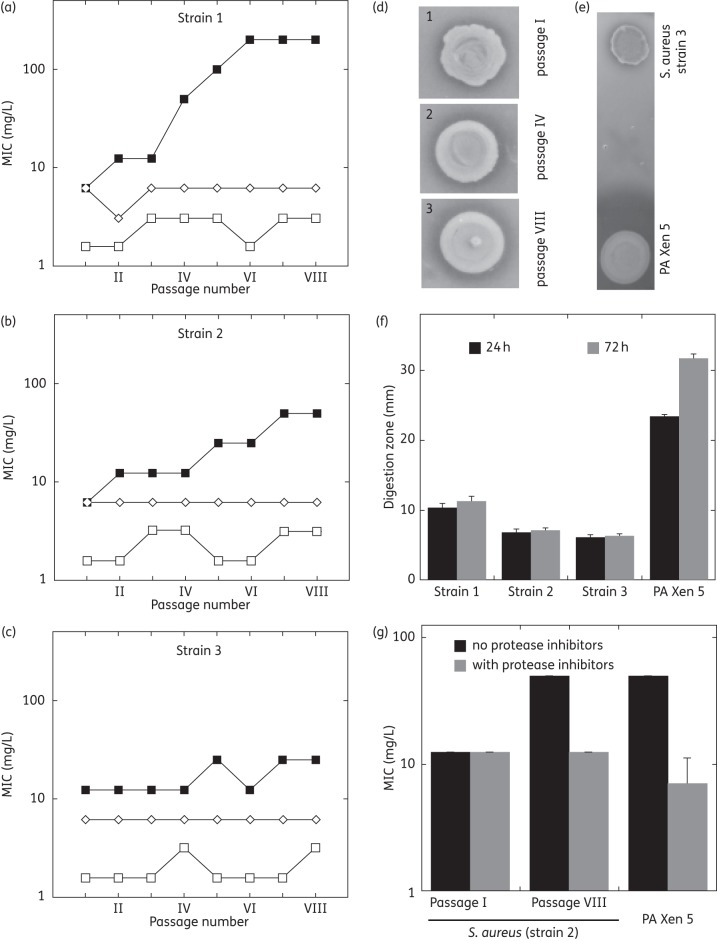
Susceptibility of Staphylococcus aureus to LL-37 peptide or cationic lipids after repeated incubations
After eight consecutive passages of three clinical strains of S. aureus incubated with half the MIC of CSA-13 or D2S, there was no loss of susceptibility to these cationic lipids (Figure 3a–c). In contrast, during three passages totalling ∼60 h of incubation, two strains developed increased resistance to LL-37. This increase in MIC might be caused by increased protease production as indicated by increased proteolytic activity (Figure 3d–f) and decreased LL-37 MIC values in the presence of protease inhibitors (Figure 3g). This interpretation is supported by data showing high production of protease by P. aeruginosa Xen 5 and a decrease of the LL-37 MIC value against this strain in the presence of protease inhibitors. However, determining the exact mechanism of the observed increase in bacterial resistance requires further investigation.
Figure 3.
(a–c) MIC values of LL-37 (filled squares), CSA-13 (open squares) and D2S (open diamonds) evaluated against three different clinical isolates of S. aureus treated with half the MIC of each molecule with eight passages (panels a, b and c represent strains 1, 2 and 3 respectively). Strains 1 and 2 were methicillin-susceptible S. aureus (MSSA), macrolide–lincosamide–streptogramin B-negative [MLSB(−)] and β-lactamase(+); strain 3 was MSSA, MLSB(+), β-lactamase(+). (d) Proteolytic activity of S. aureus (strain 2) evaluated using Mueller–Hinton agar plates with the addition of fat-free milk (10%). Each spot represents bacterial outgrowth from 50 μL inoculum (108 cfu/mL) of strain 2 from passages 1, 4 and 8 (spots 1, 2 and 3, respectively) applied on a surface controlled with a sterile cylinder of 6 mm diameter. (e and f) Comparison of proteolytic activity of S. aureus (strain 3) with proteolytic activity of P. aeruginosa Xen 5 (PA Xen 5). (g) MIC values of LL-37 against S. aureus (strain 2) and P. aeruginosa Xen 5 in the presence and absence of protease inhibitors (diluted 1 : 1000 in HEPES buffer containing 2 mM MgCl2).
Effects of antibacterial agents on cell proliferation, IL-8 production and LDH release
We observed an increased number of cultured gingival fibroblasts after LL-37 administration, suggesting that LL-37 might enhance fibroblast proliferation, which is consistent with recent data showing that histatin-2 and LL-37, at a narrow optimized concentration, induce skin fibroblast proliferation.17 The tested cationic lipids did not exhibit any significant effects on gingival fibroblast proliferation (Figure 4a) after 24 h of treatment. We observed an increase in IL-8 release from HaCat cells following LL-37 addition at a concentration comparable to that required for effective bacterial killing (∼5 μM). Among the antibacterial lipids, CSA-13 showed the strongest ability to induce IL-8 production, causing an ∼40% increase (P < 0.05) after 18 h of incubation at 5 μM (Figure 4b). CSA-13 also showed the strongest membrane activity, causing increases of ∼10% and 25% in LDH release from HaCat cells at 25 and 50 μM, respectively. Compared with LL-37, CSA-13 toxicity towards human keratinocytes did not differ significantly (Figure 4c), but CSA-13 antibacterial activity was much stronger (Tables 1 and 2).
Figure 4.
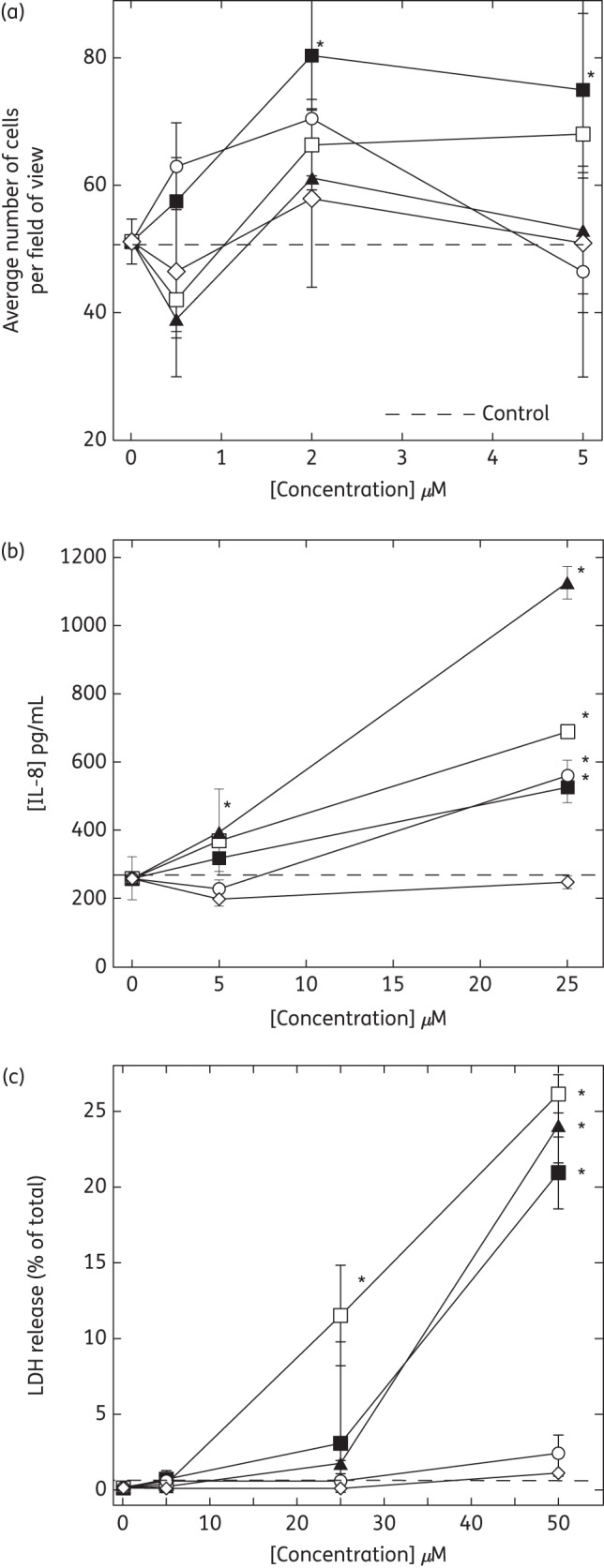
Growth of gingival fibroblasts (a) and release of IL-8 (b) and LDH (c) from keratinocytes (HaCat) under treatment with LL-37 (filled squares), CSA-13 (open squares), CSA-90 (filled triangles), CSA-92 (open circles) or D2S (open diamonds). The average number of cells per field of view was determined in gingival fibroblast cell culture 24 h after cell treatment. IL-8 and LDH concentration after HaCat cell treatment was determined in cell culture media at the 18 and 2 h timepoints, respectively. *Significantly different from control sample.
Discussion
One of the current challenges in the treatment of bacterial infections is the development of drug-resistant bacterial strains that evade the specific molecular mechanisms of antibiotic drugs. Our data indicate that novel cationic lipids, which mimic mechanisms of action of natural antibacterial agents and are usually not hindered by the development of bacterial resistance, can kill bacterial strains associated with oral and upper respiratory tract infections with no effect on cell proliferation.
All four of the tested cationic lipids were active against a broad spectrum of pathogens, including those tested for the first time, such as Neisseria meningitis B and C, Moraxella catarrhalis and Tannerella forsythensis. These studies confirmed the strong activity of LL-37 and cationic lipids against a clinical strain of S. mutans, the major aetiological agent of dental caries,18,19 and S. aureus, which often colonizes the nasopharynx.20 Since all experiments were conducted in vitro, the potential clinical use of these compounds requires further testing. LL-37 activity against Porphyromonas gingivalis, which is strongly implicated in the pathogenesis of periodontitis, was limited, potentially a result of the ability of this bacterium to produce proteolytic enzymes.21 Cationic lipids show much higher activity against P. gingivalis, likely due to protease resistance.6 This is consistent with earlier data showing high activity of CSA-13 against protease-positive Porphyromonas.8
Structure analysis indicates that the cationic lipids tested all possess elements that can interact with the negatively charged bacterial wall molecules required to dissipate membrane potential.22 The alkyl group of variable chain length in the ceragenins is attached to an amine at C24, which alters hydrophobicity and should influence partitioning into membranes. Similar bactericidal activities of CSA-13, CSA-90, CAS-92 and D2S are consistent with the ‘facial amphiphilicity concept’ used to design cationic lipids that target bacterial membranes.12,23
The inability of S. aureus strains to develop resistance after incubation with half the MIC of CSA-13 or D2S suggests that these compounds employ a general antibacterial mechanism that prevents the development of survival adaptations by the bacteria. Another advantage of cationic lipids over LL-37 is their lack of effect on host cell division.5,24 However, these activities could initiate inappropriate cell proliferation and tissue growth with increasing LL-37 concentrations. LL-37 can also bind to host DNA, thereby altering innate tolerance to self-DNA and as a consequence drive autoimmunity pathways via activation of toll-like receptor 9. This reaction provides a possible explanation for the pathological events occurring in psoriasis and systemic lupus erythematosus.25 Clinical use of LL-37 might be limited if it is associated with the development of neoplastic growth and autoimmune disorders.
Release of IL-8 from HaCat cells after addition of either LL-37, CSA-13 or CSA-90 suggests that the pleiotropic activity of antibacterial peptides on host cells26 might be mediated by their physical effects on membrane structure and not only by binding to specific membrane receptors. A lack of IL-8 release after D2S treatment likely results from D2S glucocorticoid receptor activation.11
In conclusion, we found that LL-37, CSA-13, CSA-90, CSA-92 and D2S exhibited antibacterial activity against oral and upper respiratory tract bacteria, which was maintained in saliva. The antibacterial activity was dependent on the bacterial species, and prolonged incubation of strains such as S. aureus could lead to resistance against LL-37, but not the cationic lipids. LL-37 could also stimulate growth of gingival fibroblasts under conditions where cationic lipids had no effect. Advances in our knowledge of innate immunity and the interactions of host antibacterial peptides with pathogens could help the rational design of more effective antibacterial and immunomodulatory peptide mimics.
Funding
This work was supported by NIH grants: HL67286 (to P. A. J.), HL66565 (to S. L. D.) and Medical University of Bialystok grants: 3-22574F; 123-22861F (to K. L.).
Transparency declarations
Brigham Young University has licensed ceragenin technology to N8 Medical, Inc. P. B. Savage was a paid consultant for Ceragenix Pharmaceuticals and currently consults for N8 Medical. In 2008 R. B. obtained a reimbursement from Ceragenix for attending the ICAAC Meeting. P. J. and R. B. began a sponsored research agreement with Genentech Inc. in December 2011, which is not related to the present study. This manuscript was submitted before the sponsored research agreement was initiated. All other authors: none to declare. None of the research reported in this paper was supported by any corporate entity.
References
- 1.Dale BA, Fredericks LP. Antimicrobial peptides in the oral environment: expression and function in health and disease. Curr Issues Mol Biol. 2005;7:119–33. doi: 10.1093/jac/dki103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larrick JW, Hirata M, Balint RF, et al. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–7. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leszczynska K, Namiot A, Janmey PA, et al. Modulation of exogenous antibiotic activity by host cathelicidin LL-37. APMIS. 2010;118:830–6. doi: 10.1111/j.1600-0463.2010.02667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandgren S, Wittrup A, Cheng F, et al. The human antimicrobial peptide LL-37 transfers extracellular DNA plasmid to the nuclear compartment of mammalian cells via lipid rafts and proteoglycan-dependent endocytosis. J Biol Chem. 2004;279:17951–6. doi: 10.1074/jbc.M311440200. [DOI] [PubMed] [Google Scholar]
- 5.Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai XZ, Feng Y, Pollard J, et al. Ceragenins: cholic acid-based mimics of antimicrobial peptides. Acc Chem Res. 2008;41:1233–40. doi: 10.1021/ar700270t. [DOI] [PubMed] [Google Scholar]
- 7.Malabarba A, Ciabatti R, Kettenring J, et al. Synthesis and antibacterial activity of a series of basic amides of teicoplanin and deglucoteicoplanin with polyamines. J Med Chem. 1992;35:4054–60. doi: 10.1021/jm00100a010. [DOI] [PubMed] [Google Scholar]
- 8.Isogai E, Isogai H, Takahashi K, et al. Ceragenin CSA-13 exhibits antimicrobial activity against cariogenic and periodontopathic bacteria. Oral Microbiol Immunol. 2009;24:170–2. doi: 10.1111/j.1399-302X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 9.Saha S, Savage PB, Bal M. Enhancement of the efficacy of erythromycin in multiple antibiotic-resistant gram-negative bacterial pathogens. J Appl Microbiol. 2008;105:822–8. doi: 10.1111/j.1365-2672.2008.03820.x. [DOI] [PubMed] [Google Scholar]
- 10.Fein DE, Limberis MP, Maloney SF, et al. Cationic lipid formulations alter the in vivo tropism of AAV2/9 vector in lung. Mol Ther. 2009;17:2078–87. doi: 10.1038/mt.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bucki R, Leszczynska K, Byfield FJ, et al. Combined antibacterial and anti-inflammatory activity of a cationic disubstituted dexamethasone-spermine conjugate. Antimicrob Agents Chemother. 2010;54:2525–33. doi: 10.1128/AAC.01682-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding B, Guan Q, Walsh JP, et al. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J Med Chem. 2002;45:663–9. doi: 10.1021/jm0105070. [DOI] [PubMed] [Google Scholar]
- 13.Leszczynska K, Namiot DB, Namiot Z, et al. Application of immunoassay for detection of Helicobacter pylori antigens in the dental plaque. Adv Med Sci. 2009;54:194–8. doi: 10.2478/v10039-009-0050-3. [DOI] [PubMed] [Google Scholar]
- 14.Sieprawska-Lupa M, Mydel P, Krawczyk K, et al. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother. 2004;48:4673–9. doi: 10.1128/AAC.48.12.4673-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saran S, Isar J, Saxena RK. A modified method for the detection of microbial proteases on agar plates using tannic acid. J Biochem Biophys Methods. 2007;70:697–9. doi: 10.1016/j.jbbm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Leszczynska K, Namiot A, Cruz K, et al. Potential of ceragenin CSA-13 and its mixture with pluronic F-127 as treatment of topical bacterial infections. J Appl Microbiol. 2011;110:229–38. doi: 10.1111/j.1365-2672.2010.04874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oudhoff MJ, Blaauboer ME, Nazmi K, et al. The role of salivary histatin and the human cathelicidin LL-37 in wound healing and innate immunity. Biol Chem. 2010;391:541–8. doi: 10.1515/BC.2010.057. [DOI] [PubMed] [Google Scholar]
- 18.Aas JA, Griffen AL, Dardis SR, et al. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46:1407–17. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman H, Steinberg D, Porat Y, et al. In vitro assessment of antimicrobial peptides as potential agents against several oral bacteria. J Antimicrob Chemother. 2006;58:198–201. doi: 10.1093/jac/dkl181. [DOI] [PubMed] [Google Scholar]
- 20.Pettigrew MM, Gent JF, Revai K, et al. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis. 2008;14:1584–91. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouhara K, Komatsuzawa H, Kawai T, et al. Increased resistance to cationic antimicrobial peptide LL-37 in methicillin-resistant strains of Staphylococcus aureus. J Antimicrob Chemother. 2008;61:1266–9. doi: 10.1093/jac/dkn106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epand RF, Pollard JE, Wright JO, et al. Depolarization, bacterial membrane composition, and the antimicrobial action of ceragenins. Antimicrob Agents Chemother. 2010;54:3708–13. doi: 10.1128/AAC.00380-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epand RF, Savage PB, Epand RM. Bacterial lipid composition and the antimicrobial efficacy of cationic steroid compounds (ceragenins) Biochim Biophys Acta. 2007;1768:2500–9. doi: 10.1016/j.bbamem.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Shaykhiev R, Beisswenger C, Kandler K, et al. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol. 2005;289:L842–8. doi: 10.1152/ajplung.00286.2004. [DOI] [PubMed] [Google Scholar]
- 25.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 26.Bucki R, Leszczynska K, Namiot A, et al. Cathelicidin LL-37: a multitask antimicrobial peptide. Arch Immunol Ther Exp (Warsz) 2010;58:15–25. doi: 10.1007/s00005-009-0057-2. [DOI] [PubMed] [Google Scholar]