Abstract
Objectives
Higher CSF antiretroviral concentrations may be associated with better control of HIV replication and neurocognitive performance, but only the unbound fraction of antiretrovirals is available to inhibit HIV. Therefore, the objective of this study was to determine total and unbound darunavir concentrations in CSF and compare findings with plasma concentrations as well as the wild-type HIV-1 90% inhibitory concentration (IC90).
Methods
Subjects with HIV infection were selected based on the use of darunavir-containing regimens with a twice-daily dosing schedule and availability of stored CSF and matched plasma. Total darunavir was measured by HPLC for plasma or liquid chromatography–tandem mass spectroscopy (LC/MS/MS) for CSF. Plasma unbound darunavir was measured by ultrafiltration and LC/MS/MS. CSF protein binding was determined by competitive binding exchange with radiolabelled darunavir.
Results
Twenty-nine matched CSF–plasma pairs were analysed and darunavir was detected in all CSF specimens (median total concentration 55.8 ng/mL), with a CSF unbound fraction of 93.5%. Median fractional penetrance was 1.4% of median total and 9.4% of median unbound plasma concentrations. Unbound darunavir concentrations in CSF exceeded the median IC90 for wild-type HIV in all subjects by a median of 20.6-fold, despite the relatively low fractional penetrance. Total darunavir concentrations in CSF correlated with both total and unbound darunavir concentrations in plasma.
Conclusions
Darunavir should contribute to the control of HIV replication in the CNS as a component of effective combination antiretroviral regimens.
Keywords: HIV, antiretroviral therapy, central nervous system, protein binding
Introduction
The CNS is infected early in the course of HIV infection and HIV RNA is often detected in the CSF of untreated individuals with chronic disease. CNS infection can lead to HIV-associated neurocognitive disorders (HAND), which remain common despite potent combination antiretroviral therapy (cART).1 Supporting the importance of the virus in the pathogenesis of HAND, HIV RNA concentrations in CSF are higher in patients with cognitive impairment than in those without impairment in cross-sectional and longitudinal studies,2–4 although this association has weakened since the advent of cART.5
Antiretrovirals (ARVs) differ in their distribution—or penetration—into the CNS, with some drugs resulting in CSF concentrations similar to those in plasma and others resulting in CSF concentrations that are <1% of those in plasma [e.g. protease inhibitors (PIs)]. Only ARVs that penetrate into the CNS in therapeutic concentrations will be able to reduce HIV replication in that compartment. Factors such as molecular weight, liposolubility, protein binding and affinity for efflux transporters all affect the CNS distribution of ARVs. ARVs reaching higher levels in the CSF are associated with better control of HIV replication6,7 and, often, better neurocognitive performance,8–10 although not all reports agree.7
A less well understood factor is the extent of protein binding of ARVs in the CSF. This is important, because ARVs that are bound to proteins, such as albumin or α-1 acid glycoprotein, are unavailable to inhibit HIV replication. Darunavir is a PI, a class of drugs that is generally highly bound to plasma proteins and a substrate for the efflux transporter P-glycoprotein, factors leading to limited distribution in the CNS.11 However, PIs are very potent ARVs and relatively low concentrations are required to inhibit viral replication. The objectives of this study were to determine total and unbound darunavir concentrations in CSF and compare them with matched plasma concentrations and the established in vitro 50% and 90% inhibitory concentrations (IC50 and IC90, respectively) for wild-type HIV-1.
Methods
CSF–plasma specimen pairs were selected from subjects who had HIV-1 infection and who had enrolled in observational cohort studies conducted at or coordinated by the University of California, San Diego (UCSD) between August 2006 and December 2008. These studies included the CNS HIV AntiRetroviral Therapy Effects Research, the Core Support Program in Mental Health/AIDS Research and the California NeuroAIDS Tissue Network. Selection criteria included the use of darunavir and availability of stored CSF and matched plasma obtained within 16 h of self-reported dosing. The UCSD Human Research Protections Program approved this research. Written informed consent was obtained from all subjects.
CSF was obtained by lumbar puncture performed with aseptic technique using a 22 gauge pencil-point needle by experienced operators. Plasma was obtained within 1 h of CSF by routine phlebotomy. All specimens were stored at −70°C until analysis.
Total darunavir concentrations were measured by HPLC for plasma and stable isotope liquid chromatography–tandem mass spectrometry (LC/MS/MS) for CSF. Darunavir was extracted with either acetonitrile (plasma) or methyl-tert-butyl ether (CSF), after addition of a d6-darunavir internal standard (CSF). The dynamic ranges of these assays were 3.9–2000 (plasma) and 0.39–200 ng/mL (CSF). Plasma unbound darunavir concentrations were determined by LC/MS/MS analysis of plasma ultrafiltrate (10 kDa cut-off). As CSF unbound darunavir concentrations were expected to be below the detection limit of LC/MS/MS analysis, CSF unbound darunavir concentrations were imputed from protein binding. This was derived by equilibrating the bound drug in a matrix via competitive binding exchange with the radiolabelled drug and subsequent measurement of the radiolabelled drug in the 10 kDa ultrafiltrate of the matrix.12 Recovery from all matrices was ≥96%. CSF darunavir concentrations were compared with the IC50 and IC90 for wild-type HIV-1 (1.78 and 2.43 ng/mL, respectively).13,14
HIV RNA was quantified by RT–PCR using a Roche Taqman RealTime assay (Roche Diagnostics) with a lower limit of quantification (LLQ) of 50 copies/mL. The blood T lymphocyte subsets including CD4+ were measured by flow cytometry. Adherence was self-reported and adequate adherence defined as subjects taking >95% of their scheduled ARVs over 4 days preceding the CSF and blood specimen collection.
Data were analysed with descriptive, bivariable and multivariable statistics using standard methods (JMP, SAS Institute, Cary, NC, USA). Spearman's correlation coefficient was used to assess the relationship between plasma and CSF darunavir concentrations.
Results
Twenty-nine CSF–plasma pairs were obtained from 16 subjects. When more than one specimen pair was obtained from the same subject, the interval between specimens ranged from 6 to 12 months. Seven subjects provided one pair, six subjects two pairs, two subjects three pairs and one subject four pairs. Subjects were predominantly middle-aged (median age of 48 years; IQR 45–52), white (56%) men (93%) with AIDS (100%). The median CD4+ T lymphocyte count at the time of sampling was 213 cells/mm3 (IQR 108–413). The median nadir CD4+ T lymphocyte count was 5 cells/mm3 (IQR 2–57). Disease severity based on the 1993 CDC classification system was categorized as stage C in 93% and stage B in 7% of subjects. HIV RNA was below the LLQ in 59% of plasma and 86% of CSF specimens.
Median darunavir use was 9.4 months (IQR 3.6–14.7). Darunavir was dosed as 600 mg twice daily with 100 mg of ritonavir twice daily in all subjects. Concurrent ARVs included one to three nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) in all subjects, one non-NRTI in 7%, one integrase inhibitor in 25%, one cell-entry inhibitor in 14% and one fusion inhibitor in 7%. Adherence was >95% for 87% of the sampling timepoints. The last darunavir dose was taken with food prior to sampling in 83% of the sampling timepoints.
Darunavir plasma and CSF concentrations as well as sampling information are displayed in Figure 1 and aggregate data are summarized in Table 1. Post-dose sampling intervals (PDSIs) were evenly distributed over the dosing interval, with median PDSIs of 7.2 h (IQR 3.8–12.9) for CSF and 6.7 h (IQR 3.4–12.4) for plasma. Darunavir was detected in all but one (3%) CSF specimen. CSF fractional penetrance (i.e. the fraction of plasma drug reaching the CSF compartment) was 1.4% of the median total and 9.4% of the median unbound plasma concentrations. Darunavir unbound fractions were 97.2% (CSF) and 6.5% (plasma). The CSF-to-total and CSF-to-unbound plasma concentration ratios did not change significantly over the dosing interval. Correlations between total darunavir in CSF and either total (ρ = 0.60, P = 0.0008) or unbound (ρ = 0.66, P = 0.0001) plasma concentrations were similar and statistically significant (Figure 2). There was no statistically significant correlation between PDSIs and total CSF darunavir concentrations (r = −0.35, P = 0.07) or total plasma concentrations (r = −0.29, P = 0.13). However, unbound plasma concentrations (r = −0.43, P = 0.02) did decline significantly over the dosing interval. There were no statistically significant correlations between CSF total protein or CSF/plasma total protein ratio and either total or unbound darunavir CSF concentrations. The results were not substantially different when the analyses were performed with only one timepoint (the earliest by convention) per subject.
Figure 1.
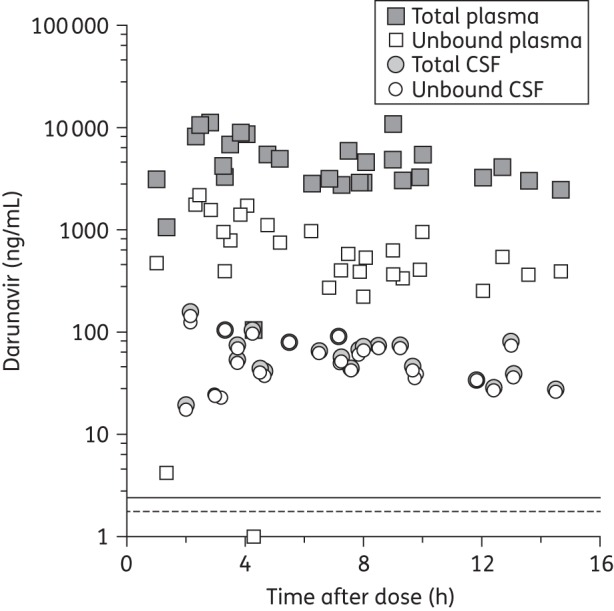
Total and unbound plasma and CSF darunavir concentrations with post-dose sampling time. The horizontal broken line shows the median IC50 (1.78 ng/mL). The horizontal continuous line shows the median IC90 (2.43 ng/mL). The LLQs were 0.39 ng/mL (CSF) and 3.9 ng/mL (plasma).
Table 1.
Summary of darunavir concentrations, protein binding and relationships with median IC50 and IC90
| Variable | Median | IQR | Range |
|---|---|---|---|
| CSF (total) (ng/mL) | 55.8 | 39.5–79.1 | 19.4–159.6 |
| CSF (unbound) (ng/mL) | 50.2 | 35.0–72.6 | 0–143.8 |
| Plasma (total) (ng/mL) | 4094 | 2993–6411 | 104–11 298 |
| Plasma (unbound) (ng/mL) | 538 | 369–968 | 1–2206 |
| CSF-to-total plasma ratio | 0.014 | 0.009–0.018 | 0.003–0.026 |
| Unbound CSF-to-unbound plasma ratio | 0.085 | 0.062–0.127 | 0.029–4.124 |
| Plasma protein binding | 97.2% | 96.1%–98.0% | 88.0%–99.7% |
| CSF protein binding | 6.5% | 3.0%–9.3% | 0%–10.2% |
| Unbound CSF-to-median IC50 ratio | 28.1 | 19.7–40.8 | 0–80.8 |
| Unbound CSF-to-median IC90 ratio | 20.6 | 14.4–29.9 | 0–59.2 |
Figure 2.
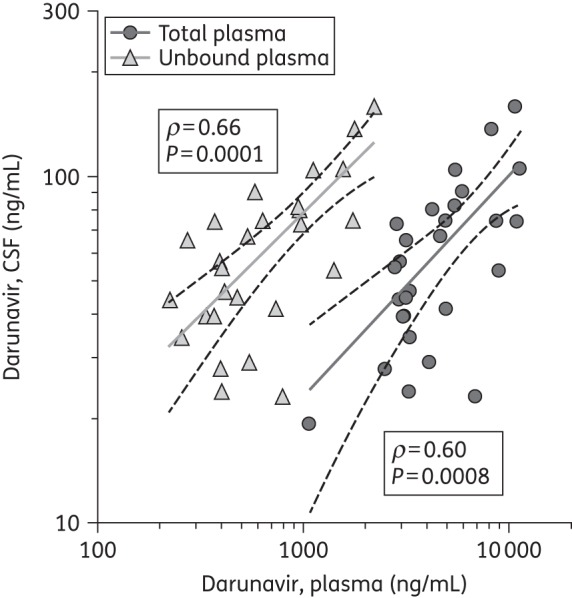
Correlations between CSF and total plasma concentrations and CSF and unbound plasma concentrations in log-transformed values with linear fit (continuous lines) and 95% CIs (broken lines). Three pairs have been excluded from the graph: two plasma concentrations <100 ng/mL and corresponding CSF concentrations; and one CSF concentration below the LLQ and the corresponding plasma concentration.
All unbound darunavir concentrations in CSF (excluding the single value below the LLQ) exceeded the median IC50 for wild-type HIV-1 by a median of 28.1-fold and the median IC90 by a median of 20.6-fold. Higher darunavir concentrations in plasma (total or unbound) were not associated with undetectable HIV RNA levels in plasma (t = 0.95, P = 0.35 and t = 1.05, P = 0.31, respectively). Too few (14%) HIV RNA levels were detectable in CSF to reliably assess any correlation with CSF darunavir concentrations.
Discussion
This is the first report examining darunavir concentrations in CSF along with CSF protein binding. Unbound darunavir plasma concentrations and the degree of plasma protein binding were consistent with prior reports.15 Excluding the single value below the LLQ, all unbound darunavir CSF concentrations exceeded the wild-type HIV median IC50 and IC90, the lowest value observed being 8.0-fold higher than the IC90. The subject with the undetectable CSF darunavir concentration had the lowest plasma darunavir concentration, had one of the highest plasma HIV RNA values and the highest CSF HIV RNA level among all subjects, all suggesting potential cART non-adherence. IC50 and IC90 were used in this report as they are generally less variable and are commonly referenced in clinical resistance testing reports compared with the 90% effective concentration (EC90). In addition, since the EC90 is protein corrected, it may not provide an accurate estimate of inhibitory concentrations in a low-protein environment, such as the CSF compartment.
The distinctly different degrees of protein binding in CSF and plasma may be explained in part by the much lower concentration of binding proteins in CSF compared with in plasma (100–1000-fold lower), including albumin and α-1 acid glycoprotein.16,17 These findings are consistent with a pharmacokinetic study measuring bound and unbound indinavir in plasma and CSF and showing a CSF protein binding of 1.4%.18 The concentrations of darunavir in CSF are ∼10-fold lower than predicted by plasma unbound concentrations (assuming all unbound drug is distributed into the CNS). The lower than expected CSF fractional penetrance indicates that limiting factors other than protein binding restrict the distribution of darunavir into the CSF, similar to other PIs.19–21 P-glycoprotein and other efflux transporter affinity may be one of those barriers.22,23 Compared with other PIs, darunavir appears to have intermediate distribution into the CNS based on fractional penetrance (higher than atazanavir, lopinavir and amprenavir, and lower than indinavir). Likewise, darunavir's CSF-to-IC50 ratio is numerically higher than those of atazanavir, lopinavir and amprenavir, and is in the same range as indinavir, but the clinical value of this measure has not been demonstrated.19–21,24 Despite those limitations, darunavir achieved therapeutic CSF concentrations based on the wild-type HIV-1 in vitro IC50 and IC90. In addition, the relatively low proportion of CSF specimens with detectable HIV RNA (often when HIV RNA was detectable in plasma) may be supportive of darunavir's antiviral effectiveness in the CNS. Since all subjects took cART, HIV control cannot be solely attributed to darunavir. However, subjects as a group had excellent control (the majority of detectable plasma HIV RNA levels were <500 copies/mL), considering how advanced their immune suppression had been in the past, a factor known to favour compartmentalization of HIV in the CNS.25,26 Four samples had detectable HIV RNA in CSF despite all CSF darunavir concentrations being above the wild-type HIV-1 IC90, except for the subject who had the darunavir concentration below the LLQ and also the highest CSF HIV RNA level (3150 copies/mL). All the other three samples had CSF HIV RNA levels just above the LLQ (range 54–75 copies/mL). The findings in those three samples with marginally detectable CSF HIV RNA may suggest suboptimal plasma viral suppression (one sample with very high plasma HIV RNA) or drug resistance (compartmental or systemic; two samples with undetectable or marginally detectable plasma HIV RNA) to darunavir or other ARVs in the subjects' regimens.
The lack of correlation between PDSIs and total CSF and plasma darunavir concentrations may be related to a longer half-life in this population than the terminal elimination half-lives reported.27,28 The reason for a decline in unbound plasma darunavir with PDSIs with relatively stable total plasma darunavir is not readily apparent and plasma unbound darunavir pharmacokinetic data have not been published. The median total CSF darunavir concentration (55.8 ng/mL) and fractional penetrance based on the total darunavir median concentration (1.4%) reported in this analysis are comparable to those recently reported in two smaller studies using twice-daily dosing schedules (34.2–38.2 ng/mL and 0.9%).29,30 The correlation between total darunavir in CSF with total and unbound darunavir concentrations in plasma suggests that plasma concentrations could be an informative qualitative estimate of CSF darunavir concentrations and that interventions focused on increasing concentrations in plasma might also result in higher concentrations in the CNS. Given the recent findings by Calcagno et al.,30 those observations would require validation in ARV-inexperienced HIV-infected individuals using a darunavir once-daily dosing schedule. Given the likely affinity of darunavir for efflux transporters such as P-glycoprotein, agents inhibiting both the cytochrome P-450 system and P-glycoprotein (e.g. ritonavir) would be more advantageous in terms of CNS delivery than agents that only inhibit the cytochrome P450 system (e.g. cobicistat) when combined with PIs such as darunavir. The study limitations include the small sample size, the concurrent administration of other ARVs and adherence self-reporting, although the plasma concentrations reported are in the same range as reported in other darunavir pharmacokinetic studies.27,28
In summary, our findings provide evidence that darunavir is largely unbound to protein in the CSF, that, when using a twice-daily dosing schedule, darunavir concentrations in CSF are within the estimated therapeutic range and that total and unbound plasma darunavir concentrations could be used to qualitatively estimate concentrations in CSF. Darunavir should contribute to the control of HIV replication in the CNS as a component of cART.
Funding
This work was supported by an investigator-initiated research grant from Tibotec and by the National Institutes of Health via the following awards: N01 MH22005, HHSN271201000030C and HHSN271201000036C.
Transparency declarations
R. J. E. received consultant fees from NeurogesX and is funded by NIH grants R01MH058076, U01MH83506, P30MH62512, R01MH83552, P50DA26306, R01MH095621 and 2U01NS32228. D. B. C. is supported by NIH grants NS32228, AI69495, MH22005, DA022137, MH058076 and 3857-53187. He has also received support from Pfizer, NeurogesX and Biogen. In addition, he has provided scientific advisory or consulting to Biogen Idec, Elan, Roche, Genentech, GlaxoSmithKline, Janssen, Millennium, Bristol-Myers Squibb, Genzyme, Wyeth and Pfizer. A. C. C. had the following disclosures: research support from Boehringer-Ingelheim (past), Gilead Sciences (past), Koronis (past), Merck & Company (current), Schering-Plough (past) and Tibotec-Virco (past); former member of a Data, Safety and Monitoring Board for a Merck-sponsored study; attendee at Advisory Board Meetings for GlaxoSmithKline (past) and Pfizer (past); and stock ownership (personal/immediate family member) with Abbott Laboratories, Bristol-Myers Squibb, Johnson and Johnson, and Pfizer. B. B. G. receives support from NIH grants NS072005 and MH79886. C. M. M. receives research support from the NIH (NINDS and NIMH). She receives royalties from Lippincott Williams and Wilkins and from UptoDate. J. M. receives support from N01 MH22005. J. A. M. authors chapters on HIV for the Merck Manual and receives related research funding from NIH P30 MH62512, NIH U01 MH83506, NIH/Centers for Disease Control and Prevention (CDC) U2G PS00623, NIH U01 AI69432, NIH N01 MH22005, NIH K30 RR22681, NIH R01 MH58076 and NIH U13 MH81676. D. M. S. receives research support from the NIH (NINDS and NIMH). He provided consultancy to GlaxoSmithKline and Gilead. I. G. receives ongoing research support from NIH P30 MH62512, NIH P50 DA26306, NIH P01 DA12065, NIH N01 MH22005, NIH U01 MH83506, NIH R01 MH78748, NIH R01 AG15301, NIH R01 MH83552 and NIH/University of Nebraska P01 DA026146. He has also received honoraria from Abbott Pharmaceuticals as part of their Educational Speaker Program. The salary of S. L. was funded by NIH research awards, including N01 MH22005, R01 MH58076, R01 MH92225, P50 DA26306 and P30 MH62512. He has received support for research projects from Abbott, Merck, Tibotec and GlaxoSmithKline. He has consulted for Gilead Sciences, GlaxoSmithKline, Merck and Tibotec, and has received lecture honoraria from Abbott and Boehringer-Ingelheim. The remaining authors have none to declare.
Author contributions
D. C. was involved in data collection and analysis, was involved in discussion of the results, wrote up the manuscript and was the primary author of the manuscript. S. S. R. contributed to the analytic method development and performance for CSF and plasma specimens. B. M. B. and E. C. were involved in data analysis and discussion of the results. R. J. E. was involved in the conception and design of the study, data collection and analysis, and discussion of the results. D. B. C., A. C. C., B. B. G., C. M. M., J. M., J. A. M., S. M. and D. M. S. were involved in data collection and analysis, and discussion of the results. I. G. was involved in the conception and design of the study, data collection and analysis, and discussion of the results. S. L. was involved in the conception and design of the study, data analysis, discussion of the results and manuscript preparation.
Disclaimer
The views expressed in this article are those of the authors and do not reflect the official policy or position of the US Government.
Acknowledgements
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with the Johns Hopkins University, Mount Sinai School of Medicine, University of California, San Diego (UCSD), University of Texas, Galveston, University of Washington, Seattle and Washington University, St Louis. CHARTER is headquartered at UCSD and includes Igor Grant (UCSD, Director), Ronald J. Ellis (UCSD, Co-Director), Scott L. Letendre (UCSD, Co-Director), Ian Abramson (UCSD, Co-Investigator), Muhammad Al-Lozi (Washington University, Co-Investigator), J. Hampton Atkinson (UCSD, Co-Investigator), Edmund Capparelli (UCSD, Co-Investigator), David Clifford (Washington University, Site Principal Investigator), Ann Collier (University of Washington, Site Co-Principal Investigator), Christine Fennema-Notestine (UCSD, Core Co-Principal Investigator), Anthony C. Gamst (UCSD, Core Principal Investigator), Benjamin Gelman (University of Texas, Site Principal Investigator), Robert K. Heaton (UCSD), Thomas D. Marcotte (UCSD, Core Principal Investigator), Christina Marra (University of Washington, Site Co-Principal Investigator), J. Allen McCutchan (UCSD, Site Principal Investigator), Justin McArthur (Johns Hopkins, Site Principal Investigator), Susan Morgello (Mount Sinai, Site Co-Principal Investigator), David Simpson (Mount Sinai, Site Co-Principal Investigator), Davey M. Smith (UCSD, Core Principal Investigator), Michael J. Taylor (UCSD, Core Co-Principal Investigator), Rebecca Theilmann (UCSD, Imaging Physicist), Florin Vaida (UCSD, Co-Investigator) and Steven Paul Woods (UCSD, Co-Investigator). The study coordinators are Terry Alexander (UCSD, Neuromedical Coordinator), Clint Cushman (UCSD, Data Manager), Matthew Dawson (UCSD, Neurobehavioral Coordinator), Donald Franklin Jr (UCSD, Center Manager), Eleanor Head (University of Texas, Site Coordinator), Trudy Jones (University of Washington, Site Coordinator), Kristen Lewis (Johns Hopkins, Site Coordinator), Letty Mintz (Mount Sinai, Site Coordinator), Mengesha Teshome (Washington University, Site Coordinator) and Will Toperoff (UCSD, Site Coordinator).
References
- 1.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis RJ, Hsia K, Spector SA, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol. 1997;42:679–88. doi: 10.1002/ana.410420503. [DOI] [PubMed] [Google Scholar]
- 3.Ellis RJ, Moore DJ, Childers ME, et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 2002;59:923–8. doi: 10.1001/archneur.59.6.923. [DOI] [PubMed] [Google Scholar]
- 4.McArthur JC, McClernon DR, Cronin MF, et al. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42:689–98. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- 5.Sevigny JJ, Albert SM, McDermott MP, et al. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63:2084–90. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- 6.Letendre SL, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–66. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cysique LA, Vaida F, Letendre S, et al. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73:342–8. doi: 10.1212/WNL.0b013e3181ab2b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Letendre SL, McCutchan JA, Childers ME, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol. 2004;56:416–23. doi: 10.1002/ana.20198. [DOI] [PubMed] [Google Scholar]
- 10.Tozzi V, Balestra P, Salvatori MF, et al. Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr. 2009;52:56–63. doi: 10.1097/qai.0b013e3181af83d6. [DOI] [PubMed] [Google Scholar]
- 11.Kis O, Robillard K, Chan GNY, et al. The complexities of antiretroviral drug–drug interactions: role of ABC and SLC transporters. Trends Pharmacol Sci. 2009;31:22–35. doi: 10.1016/j.tips.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Dow N. Determination of compound binding to plasma proteins. In: Enna SJ, Williams M, editors. Current Protocols in Pharmacology. Wiley Online Library; 2006. pp. 7.5.1–15. [DOI] [PubMed] [Google Scholar]
- 13.Koh Y, Hirotomo N, Kenji M, et al. Novel bis-tetrahydrofuranyl-urethane-containing nonpeptidic protease inhibitor (PI) UIC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro. Antimicrob Agents Chem. 2003;47:3123–9. doi: 10.1128/AAC.47.10.3123-3129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh AK, Chapsal BD, Weber IT, et al. Design of HIV protease inhibitors targeting protein backbone: an effective strategy for combating drug resistance. Acc Chem Res. 2008;41:78–86. doi: 10.1021/ar7001232. [DOI] [PubMed] [Google Scholar]
- 15.Rittweger M, Arasteh K. Clinical pharmacokinetics of darunavir. Clin Pharmacokinet. 2007;46:739–56. doi: 10.2165/00003088-200746090-00002. [DOI] [PubMed] [Google Scholar]
- 16.Adam P, Sobek O, Taborsky L, et al. CSF and serum orosomucoid (α-1-acid glycoprotein) in patients with multiple sclerosis: a comparison among particular subgroups of MS patients. Clin Chim Acta. 2003;334:107–10. doi: 10.1016/s0009-8981(03)00229-8. [DOI] [PubMed] [Google Scholar]
- 17.Abbott NJ, Patabendige AA, Dolman DE, et al. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 18.Haas DW, Johnson B, Nicotera J, et al. Effects of ritonavir on indinavir pharmacokinetics in cerebrospinal fluid and plasma. Antimicrob Agents Chemother. 2003;47:2131–7. doi: 10.1128/AAC.47.7.2131-2137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Best BM, Letendre SL, Brigid E, et al. Low atazanavir concentrations in cerebrospinal fluid. AIDS. 2009;23:83–7. doi: 10.1097/QAD.0b013e328317a702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capparelli EV, Holland D, Okamoto C, et al. Lopinavir concentrations in cerebrospinal fluid exceed the 50% inhibitory concentration for HIV. AIDS. 2005;19:949–52. doi: 10.1097/01.aids.0000171409.38490.48. [DOI] [PubMed] [Google Scholar]
- 21.Letendre SL, Capparelli EV, Ellis RJ, et al. Indinavir population pharmacokinetics in plasma and cerebrospinal fluid. The HIV Neurobehavioral Research Center Group. Antimicrob Agents Chemother. 2000;44:2173–5. doi: 10.1128/aac.44.8.2173-2175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bachmeier CJ, Spitzenberger TJ, Elmquist WF, et al. Quantitative assessment of HIV-1 protease inhibitor interactions with drug efflux transporters in the blood–brain barrier. Pharm Res. 2005;22:1259–68. doi: 10.1007/s11095-005-5271-y. [DOI] [PubMed] [Google Scholar]
- 23.Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood–CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82:A99–109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Croteau D, Letendre S, Best BM, et al. Therapeutic amprenavir concentrations in cerebrospinal fluid. Antimicrob Agents Chemother. 2012;56:1985–9. doi: 10.1128/AAC.05098-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis RJ, Gamst AC, Capparelli E, et al. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000;54:927–36. doi: 10.1212/wnl.54.4.927. [DOI] [PubMed] [Google Scholar]
- 26.Wiley C, Schrier R, Nelson J, et al. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci USA. 1986;83:7089–93. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Back D, Sekar V, Hoetelmans RM. Darunavir: pharmacokinetics and drug interactions. Antivir Ther. 2008;13:1–13. [PubMed] [Google Scholar]
- 28.Boffito M, Jackson A, Amara A, et al. Pharmacokinetics of once-daily darunavir-ritonavir and atazanavir-ritonavir over 72 hours following drug cessation. Antimicrob Agents Chemother. 2011;55:4218–23. doi: 10.1128/AAC.01747-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yilmaz A, Izadkhashti A, Price RW, et al. Darunavir concentrations in cerebrospinal fluid and blood in HIV-1-infected individuals. AIDS Res Hum Retroviruses. 2009;25:457–61. doi: 10.1089/aid.2008.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calcagno A, Yilmaz A, Cusato J, et al. Determinants of darunavir cerebrospinal fluid concentrations: impact of once daily dosing and pharmacogenetics. AIDS. 2012;26:1529–33. doi: 10.1097/QAD.0b013e3283553619. [DOI] [PubMed] [Google Scholar]


