Abstract
INTRODUCTION
Little is published about the local resection of oesophageal cancers. We adopted the principles of rectal cancer surgery, ie standard surgical dissection techniques as well as standard pathological processing and reporting, and assessed the feasibility of applying them to oesophagogastric junction (OGJ) cancer.
METHODS
Over a two-year period consecutive patients with invasive cancers of the OGJ were studied. Following staging and neoadjuvant chemotherapy (NAC), a standard dissection defined as a total adventitial resection of the cardia (TARC) was performed. Standard histopathological processing involved external inking, photographing, transverse slicing and mounting of cut samples on megablocks. Hospital morbidity and mortality as well as survival at five years' follow-up were assessed.
RESULTS
Forty consecutive patients had a TARC for OGJ carcinoma. Of these, 32 were offered NAC. Introducing TARC did not result in increased morbidity or mortality. Twenty-seven patients (68%) had an R0 resection that was directly related to the tumour stage and significantly related to a response to chemotherapy. Sixteen patients (42%) were alive five years after their TARC operation.
CONCLUSIONS
Although the adventitia of the OGJ is not as well developed as that of the rectum, TARC can be performed safely as a standardised resection for OGJ cancers. Whereas the R0 rate for early stage tumours is very high, it remains disappointingly low for T3N1 tumours despite NAC. Improved long-term survival for these advanced tumours will only be achieved with better neoadjuvant and adjuvant therapies.
Keywords: Esophagogastric cancer, Resection margins, Histopathology, Chemotherapy, Survival
The aim of an oesophagectomy for cancer is long-term cure because of the availability of effective non-surgical ways of palliating dysphagia.1 Long-term survival is affected negatively by the N, T and R0 stages.2 The oncological principle underlying curative resection of solid tumours is complete local clearance. Surprisingly little is published about the local resection of oesophageal cancers yet there is much debate about the optimal surgical approach to the oesophagus, ie whether it should be done via the right chest, left chest, transhiatal or laparoscopic route.3,4
Altorki and Skinner described good long-term survival after an en bloc oesophagectomy in which he combined a radical local resection with a 2–3 field block dissection of lymph nodes.5 By focusing on the local resection of the primary tumour rather than the lymphadenectomy in rectal cancer, total mesorectal resection of the rectum has been shown by studies replicated across the world to be associated with superior local recurrence rates and long-term survival compared with other surgical techniques.6
Furthermore, the pathological processing of resected rectums with external inking and slicing the specimens transversely for assessment on large histopathology slides have helped both in setting standards for histopathology reporting and also in assessing the quality of surgical resection. An adequately performed total mesorectal resection of the rectum (judged both surgically and histopathologically) is now considered the optimal operation for patients with rectal cancer.7 We adopted the principles of rectal cancer surgery (ie standard surgical dissection techniques of the primary tumour as well as standard pathological processing and reporting) and assessed the feasibility of applying them to oesophagogastric junction (OGJ) cancer.
Methods
Patients
A prospectively gathered database of consecutive oesophagogastrectomies performed by the same surgeon for OGJ tumours over a two-year period ending in May 2005 was analysed. All patients underwent pre-operative spiral computed tomography (CT) to stage their disease according to the TNM (tumour, lymph node, metastasis) classification. In many instances, endoscopic ultrasonography staging was also performed. All cases were discussed in a multidisciplinary meeting involving upper gastrointestinal surgeons, physicians, radiologists, gastrointestinal histopathologists, oncologists and nurse specialists. All patients staged as T3 or N1 were offered neoadjuvant chemotherapy (NAC) consisting of epirubicin, cisplatin and fluorouracil. CT was repeated to monitor the response to treatment. All patients were followed up until death or for a minimum of five years.
Surgical approach
The surgical approach was based on tumour location and surgeon's choice. The methods used consisted of the Ivor–Lewis approach with a right thoracotomy, the transhiatal approach with a left neck incision, an extended total gastrectomy with a left thoracotomy and a left thoracotomy only. A D2 lymphadenectomy (ie extending to the regional lymph nodes outside the perigastric area) was performed routinely in the abdomen. No block dissection of lymph nodes was performed in the chest or neck apart from in those patients who had an Ivor–Lewis oesophagectomy where the paraoesophageal and subcarinal lymph nodes were removed. The main focus of each operation was the local dissection of the tumour at the OGJ, defined as a total adventitial resection of the cardia (TARC).
Total adventitial resection of the cardia
The steps for TARC are shown in Figure 1. The dissection was started anteriorly in the midline with a horizontal 3cm incision in the diaphragm at the level of the inferior phrenic vein. The plane between the pericardium and the prepericardial fat pad was developed and dissected laterally in both directions until the pleura was entered. The dissection was continued downwards by excising a 0.5cm cuff of diaphragmatic crus with an inferior pleural attachment until the anterior surface of the aorta was reached. The remaining posterior crural fibres (of the left crus) were divided anterior to the aorta and the adventitial tissue anterior to the aorta was dissected off en bloc.
Figure 1.
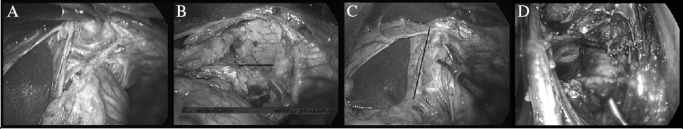
Total adventitial resection of the cardia: Starting the crural dissection anteriorly, enter the mediastinum between the pericardium and pericardial fat pad (A). The dissection anterior to the pericardial fat pad is continued laterally in both directions until the pleura is entered (B). The line of dissection through the right crus and lower mediastinal pleura is shown (C). Having divided the posterior fibres of the left crus, the preaortic plane is entered and the preaortic fascia is elevated en bloc with the specimen (D).
The mediastinal pleura was then incised longitudinally in a cranial direction, anteriorly at the level of the pericardial fat pad and posteriorly at the anterior border of the aorta. The anterior and posterior pleural incisions on both sides converge where the pericardial fat pad peters out, approximately 8cm above the hiatus and below the level of the inferior pulmonary veins. Care was taken not to remove too much crus on the right where it is close to the inferior vena cava or on the left where excessive crural resection may give rise to post-operative herniation of the small and large bowel. The anterior tendinous part of the resected hiatus was repaired with a continuous size 1 nylon suture. Bilateral transhiatal chest drains were inserted. The result was an en bloc resection of a cuff of hiatus, the pericardial fat pad, the lower mediastinal pleura and the preaortic adventitia (Fig 2).
Figure 2.
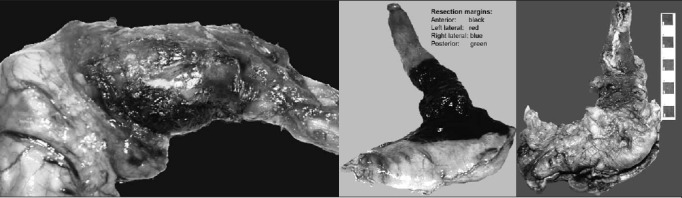
Three different oesophagogastrectomy specimens with intact adventitia after total adventitial resection of the cardia: Two specimens have been inked in the pathology department with different colours for the anterior, posterior, left and right lateral margins prior to being fixed and cut up.
Histopathological assessment
The resected specimen was placed in formalin and examined within 24 hours of surgery by a consultant histopathologist. The gastric staple line running along the greater curvature side was used to orientate the specimen. The four quadrants (anterior, posterior, left and right) of the oesophageal circumferential resection margin were marked in four different colours (Fig 2). A longitudinal incision was made in the oesophagus 2cm above the estimated (by palpation) upper limit of the tumour or, if no tumour was palpable externally, 5cm above the OGJ. The gastric resection margin was opened along one side of the staples. The specimen was washed in cold water. A paper towel wick was threaded through the OGJ and the specimen was left to fix overnight.
The following day the inked external anterior and posterior surfaces were photographed. The portions of oesophagus and stomach included were measured. The OGJ was defined externally as where the tubular oesophagus flares out into the stomach and internally as the proximal limit of the gastric rugal folds. Transverse slices were made through the region of the tumour, in the oesophagus, the OGJ and the proximal stomach (Fig 3). The slices were photographed and examined.
Figure 3.
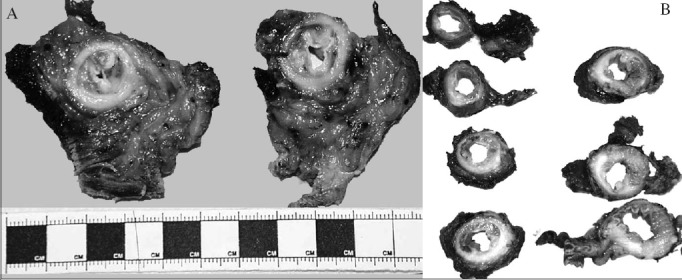
Pathology specimens of the oesophagogastric junction (OGJ) cut transversely showing varying amounts of intraluminal tumour, muscle wall infiltration and adventitia: a Siewert type I tumour containing mostly intraluminal tumour with a large amount of perioesophageal adventitia (A) and a Siewert type II tumour infiltrating the muscle wall and containing relatively sparce adventitia (B)
The tumour was described and measured, and its distance from the proximal and distal resection margins measured. If it was known, or presumed, to be an adenocarcinoma, it was categorised as Siewert types I, II or III. For a Siewert type I tumour, the bulk of the tumour is situated 1–5cm above the OGJ. In type II, the tumour bulk is 1cm above to 2cm below the OGJ and in type III it is 2–5cm below the OGJ. The presence of a columnar cell-lined oesophagus and the appearance of the background oesophageal and gastric mucosa were noted.
It is important to define what constitutes the circumferential margin at the OGJ from both a surgical and histopathological viewpoint. Immediately external to the muscularis propria lies a connective tissue layer. Where this is covered in mesothelium, it is termed serosa and forms a natural surface. This term encompasses the visceral pleura in the thoracic cavity, which covers the lateral aspects of the intrathoracic oesophagus, and the visceral peritoneum in the abdominal cavity, which covers the anterior aspect of the intra-abdominal oesophagus and the stomach.8
Where the connective tissue layer is not covered in mesothelium, a natural surface is lacking and it is termed adventitia. The adventitia of the lower thoracic oesophagus merges anteriorly with the prepericardial fat pad and posteriorly with the preaortic fascia where there is no serosal covering. Furthermore, the distribution of the adventitial fat is not always concentric and it may be concentrated more anteriorly and posteriorly (Fig 1B). The TARC procedure is therefore more closely related to the new ‘cylindrical’ operation for low rectal and anal cancer than to total mesorectal excision for cancers of the upper and mid-rectum. The basic anatomical difference is that the upper and mid-rectum have a well defined fascial plane at the edge of the mesorectal tissue whereas the low rectum, anal canal and oesophagus do not.9
Microscopic examination of the sample was performed to assess the extent of the resection margin. An R0 resection required complete proximal, distal and circumferential margin clearance. A total circumferential margin resection was defined as >1mm adventitial clearance where there was no serosal covering. An R1 resection implied macroscopic tumour clearance but microscopic margin involvement. If there was macroscopic evidence of a tumour at the resection margin, it was considered to be an R2 resection.
For microscopic examination the specimen was sampled as follows:
〉 A continuous strip of stomach as close as possible to the gastric staple line (distal resection margin).
〉 The proximal oesophageal resection margin, taken circumferentially.
〉 Up to three of the transverse slices were embedded intact (including attached pericardium, pleura, diaphragm, etc).
〉 Further sections of the tumour were taken as required, for example to show the closest approach of the tumour to the oesophageal adventitial resection margin or oesophageal (below the diaphragm) or gastric serosa.
〉 Sections were taken of the background oesophagus and stomach including any other abnormalities.
〉 All the lymph nodes were harvested from the specimen and separated into paraoesophageal, gastric lesser curvature and gastric greater curvature (if any) groups.
A histological response to chemotherapy was scored according to the Mandard criteria: the amount of residual tumour is scored relative to fibrosis on a scale from 1 (complete regression) to 5 (no regression).10 Other features related to chemotherapy were noted, eg evidence of tumour necrosis, mucinous change in the tumour with large extracellular lakes of mucin, increased nuclear atypia of tumour cells and the tumour becoming poorly differentiated and consisting of single cells rather than forming glands. This was reported as either a complete response (Mandard 1), partial response (Mandard 2–4) or no response (Mandard 5) to chemotherapy.
Statistical analysis
Data were analysed utilising GraphPad Instat® 3 (GraphPad Software Inc, La Jolla, CA, US). Long-term survival was defined as patients actually surviving to five years after the date of their surgical resection. The independent variables age, sex, type of operation, tumour type, Siewert type, response to chemotherapy, pT stage, pN stage and R0 resection were tested by multiple regression for their effect on long-term survival. A chi-square test was used to test the relation between a response to chemotherapy and an R0 resection margin. Differences with a p-value of <0.05 were deemed statistically significant.
Results
In total, 40 patients underwent resection of an OGJ cancer during the 2-year study period. The median age was 63 (range: 35–81) and 36 of the patients (90%) were male (Table 1). An Ivor–Lewis oesophagogastrectomy was performed for 19 patients, a transhiatal resection for 16, an extended total gastrectomy for 4 and a left thoracotomy for 1 patient.
Table 1.
Demographic data and outcome of the 40 patients who underwent an oesophagectomy
| Median age in years (range) | 63 (35–81) |
| Sex (male-to-female ratio) | 34:6 |
| Histology (adeno-to-squamous ratio) | 36:4 |
| Neoadjuvant chemotherapy advised | 32 (80%)* |
| Pathological T stage: | |
| T0 | 1 (2.5%) |
| T1 | 10 (25%) |
| T2 | 9 (23%)† |
| T3 | 16 (40%) |
| T4 | 4 (10%) |
| Pathological stage N1 | 22 (55%) |
| Hospital mortality | 1 (2.5%) |
| Respiratory complications | 9 (23%) |
| Clinically significant anastomotic leak | 2 (5%) |
| Return to theatre | 1 (2.5%) |
| Median length of hospital stay in days (range) | 15 (10–91) |
Two patients (pT3N1 and pT3N0) declined neoadjuvant chemotherapy.
One pT2N0 patient died in hospital.
Introducing TARC did not require any additional surgical instruments. It did result in all patients receiving bilateral chest drains routinely whereas previously only right-sided chest drains were placed. One patient had a post-operative transhiatal herniation of the colon into the left chest requiring reoperation. This was thought to be due to the left crural dissection. In another patient a puncture was made in the inferior vena cava during the right crural dissection and this was repaired with interrupted sutures. There was no increase in any other intraoperative or post-operative complications. Nine patients suffered respiratory complications in the form of a pleural effusion or an infection requiring antibiotics. Two patients suffered clinically significant anastomotic leaks; one required a reoperation. The median hospital stay was 15 days (range: 10–91 days). One patient with a pT2N0 tumour died in hospital as a consequence of a peri-operative bleed unrelated to the TARC.
Inking oesophagogastrectomy specimens to assess lateral resection margins required no new training or equipment since it was already standard laboratory practice for other cancers. Similarly, slicing oesophagogastrectomy specimens transversely and the use of megablocks was similar to our laboratory practice for rectal cancer and required no additional training or equipment.
Twenty-seven patients (68%) had R0 resections (Table 2). This included eight patients whose initial staging revealed T1/2N0 tumours. These eight patients all underwent primary surgical intervention and all had an R0 resection. Thirty-two patients, whose initial staging suggested more advanced tumours, were advised to have NAC and 59% had R0 resections. Two patients declined NAC: one had an R0 resection of a pT3N1 tumour and agreed to six cycles of adjuvant chemotherapy while the other had an R1 resection of a pT3N0 tumour but declined any adjuvant therapy.
Table 2.
R0 resection rate and 5-year survival in the 8 patients who had an early initial stage tumour and were advised not to have neoadjuvant chemotherapy (NAC), and the 32 patients who were deemed to have advanced initial stage tumours and were advised to have NAC. The pathological N stage distribution for the different T stages is also shown
| Number of patients | pN0 | R0 | 5-year survival | |
|---|---|---|---|---|
| Pathological T stage of patients advised not to have NAC (n=8): | ||||
| T0 | 0 | – | – | – |
| T1 | 7 | 5 (71%) | 7 (100%) | 7 (100%) |
| T2 | 1 | 1 (100%) | 1 (100%) | –* |
| T3 | 0 | – | – | – |
| T4 | 0 | – | – | – |
| Pathological T stage of patients advised to have NAC (n=32): | ||||
| T0 | 1 | 1 (100%) | 1 (100%) | 1 (100%) |
| T1 | 3 | 2 (66%) | 3 (100%) | 2 (66%) |
| T2 | 9 | 3 (33%) | 7 (78%) | 3 (38%)† |
| T3 | 16 | 6 (38%) | 8 (50%) | 3 (19%) |
| T4 | 4 | 0 (0%) | 0 (0%) | 0 (0%) |
This patient died in hospital.
One pT2N1 patient was lost to follow-up.
The remaining 30 patients received NAC as per the protocol and 18 (60%) had an R0 resection (Table 3). One patient had complete pathological tumour regression and had an R0 resection. Nineteen patients (63%), staged pT1–4, had a partial histological response to chemotherapy (Mandard 2–4). Of these, 15 (79%) had R0 resections. Ten patients (33%), staged pT2–4, showed no histological response to chemotherapy (Mandard 5). Only two of these had an R0 resection (χ2=4.35, p≤0.001). The 13 R1 resections were due to tumour at the inked circumferential margin in 8 specimens, <1mm from the circumferential margin in 4 specimens and <1mm from the longitudinal margin in 1 specimen.
Table 3.
R0 and 5-year survival rates for the 30 patients who received neoadjuvant chemotherapy (NAC). The patients were divided into those who had a histological response to their NAC (Mandard 1–4) and those who did not (Mandard 5). The pathological T and N stage distributions of the two groups are also shown
| pT0 | pT1 | pT2 | pT3 | pT4 | pN0 | R0 | 5-year survival | |
|---|---|---|---|---|---|---|---|---|
| Partial/complete response to NAC (Mandard 1–4; n=20) | 1 | 4 | 4 | 10 | 1 | 10 | 16 (80%) | 7 (37%)* |
| No response to NAC (Mandard 5; n=10) | 0 | 0 | 3 | 4 | 3 | 2 | 2 (20%)† | 1 (10%) |
One pT2N1 patient, who had a response to NAC, was lost to follow up.
p<0.001, R0 rate in patients who showed a response to NAC vs R0 rate in those who did not
One patient with a pT2N1 tumour moved abroad and was lost to follow up. Of the remaining 38 patients who left hospital, 16 (42%) were alive at 5 years after the surgical resection. Five-year survival was significantly related to the T stage, N stage and R0 rate but it was not related to the patients' age, sex, tumour type or type of surgical procedure for access. Of the lymph node positive patients who were pTxN1, 21 were followed up and 4 (19%) survived 5 years. Of the patients who had R1 resections, 12 were followed up and 1 (8%) survived 5 years.
Discussion
Although each cancer patient is treated on an individual basis with a personal care plan, evidence-based practice and teamworking have resulted in significant parts of cancer treatment becoming standardised.11 An ‘optimal cancer resection’ can be defined as an operation that has the best chance of achieving local clearance of the primary tumour, the lowest local recurrence, the best long-term survival and the lowest morbidity and mortality rates. Standardised or ‘optimal’ operations have been described for breast, melanoma, sarcoma and rectal cancer.7,12–14
Unfortunately, despite these ‘optimal’ cancer resections even in the best hands positive resection margins (R1 resections) and local recurrence of these cancers do occur. Operations for OGJ cancers are not as standardised, and the reported R1 and local recurrence rates are much higher and greater variations are reported than in rectal cancer surgery.2,5,15–17 In our study we attempted to define the ‘optimal cancer resection’ of OGJ tumours as total adventitial resection of the cardia (TARC). We furthermore adopted histopathology techniques developed in rectal cancer to standardise reporting of circumferential and longitudinal resection margins.
Skinner published five-year survival rates of 40% in patients after primary en bloc oesophagectomies for cancer.5 He described his operation as a combination of: a radical resection of the OGJ including the posterior wall of the pericardium; an en bloc lymphadenectomy that included the thoracic duct and upper mediastinal lymph nodes; and a cervical lymphadenectomy.
Our definition of TARC relates only to the radical resection of the primary tumour but, unlike Skinner, we did not include a pericardectomy. We also deliberately did not include the extent of lymph node resection in the definition of TARC because we believe that dealing with the lymph nodes in OGJ cancer should be investigated as a separate oncological issue, similar to gastric cancer.18,19 The optimal surgical treatment of local, regional and distant lymph nodes has been researched extensively and debated in breast, melanoma, rectal and gastric cancer.18–21 In breast cancer and melanoma surgery, routine radical block dissection of lymph nodes has made way for more targeted and optimal node resections.22,23 Our one-field lymphadenectomy is a more limited lymph node dissection than that described by Skinner's group. The other difference is that most of our patients received NAC.
TARC is a challenging surgical technique. We have shown that it can be introduced safely. If too much crus is removed, particularly on the left, an anterior repair of the tendinous part of the hiatus should be performed with non-absorbable sutures. On the right the inferior vena cava should be protected carefully. More radical surgery than TARC may increase morbidity without providing oncological benefit. This principle has been demonstrated in melanoma and breast cancer surgery where excessive resection has been replaced by ‘optimal resection’ that maximises the rate of clear resection margins, reduces local recurrence and improves survival. In rectal cancer ‘optimal cancer surgery’ results in clear resection margins in over 95% of patients.6
Our R0 resection rate of 68% in OGJ cancers despite ‘optimal surgery’ partly explains the high local recurrence and early death rate. We have yet to show that TARC reduces local recurrence or improves long-term survival beyond that achieved by routine resections. We felt it was inappropriate to compare resection margins in our pre-TARC patients because pathological processing was only standardised when TARC was introduced. Standardised pathological processing is crucial for accurate assessment of resection margins, particularly circumferential margins.
NAC has been shown in some studies to improve survival for patients after surgery for breast and oesophagogastric cancer.24–26 The survival benefit seems to be achieved by those patients who have a significant response to chemotherapy compared with those who have no response.27 It has been hypothesised that NAC downstages sensitive oesophagogastric cancers when compared with adjuvant chemotherapy.25,26 Assessment of the response to NAC can be by clinical, radiological or histological criteria but it is not clear which of these parameters best reflects long-term survival.28 In this study we used only histology to assess the response to chemotherapy.
Overall, 68% of patients had either a partial or complete histological response to chemotherapy. The R0 rate in the T3N1 NAC group was 59% (82% in the responders compared with 9% in the non-responders). Previous studies have reported a wide range of 50–90% clear circumferential resection margins following oesophagectomies.16,28,29 The variation in these figures is likely to reflect variation in surgical technique, pathological processing and reporting, and the stage of the resected tumours. We were unable to relate a partial response to NAC to T stage reduction. Whereas the tumour bulk appeared to decrease in patients with a partial chemotherapy response, cancerous cells were present in all the histological layers including the adventitia. Any survival advantage achieved from NAC is unlikely to be solely attributable to its effects on the primary tumour.
Our data demonstrate that optimising surgical techniques and standardising pathological processing are achievable objectives in OGJ tumours. An R0 resection rate of 92% is achieved when early stage (T1–2N0) OGJ cancer is managed with TARC. These early stage tumours are likely to be cured by TARC with or without chemotherapy.
Patients staged with advanced but ‘operable’ T2–3N1 tumours of the OGJ have a 59% chance of undergoing a ‘curative’ resection with NAC and TARC. This is largely due to the 66% of patients who have a response to chemotherapy. For the 33% of patients who have no response to chemotherapy, the outlook is very poor and it raises the question whether they will have been better served with primary surgery followed by adjuvant therapy.
Conclusions
In the future improved neoadjuvant strategies such as early assessment of tumour sensitivity, stopping ineffective drugs and availability of more effective drugs may improve the R0 resection rate for advanced stage tumours. The survival of advanced OGJ tumours is only likely to improve substantially when neoadjuvant and/or adjuvant therapies become more effective.
Acknowledgments
The material in this paper was presented as a poster at the annual conference of the Association of Upper Gastrointestinal Surgeons held in Oxford, September 2010.
References
- 1.Sreedharan A, Harris K, et al. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev. 2009;4 doi: 10.1002/14651858.CD005048.pub2. CD005048. [DOI] [PubMed] [Google Scholar]
- 2.Siewert JR, Stein HJ, et al. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg. 2001;234:360–367. doi: 10.1097/00000658-200109000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omloo JM, Lagarde SM, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg. 2007;246:992–1,000. doi: 10.1097/SLA.0b013e31815c4037. [DOI] [PubMed] [Google Scholar]
- 4.Hamouda AH, Forshaw MJ, et al. Peri-operative outcomes after transition from conventional to minimally invasive Ivor–Lewis esophagectomy in a specialized center. Surg Endosc. 2010;24:865–869. doi: 10.1007/s00464-009-0679-9. [DOI] [PubMed] [Google Scholar]
- 5.Altorki N, Skinner D. Should en bloc esophagectomy be the standard of care for esophageal carcinoma? Ann Surg. 2001;234:581–587. doi: 10.1097/00000658-200111000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlsen E, Schlichting E, et al. Effect of the introduction of total mesorectal excision for the treatment of rectal cancer. Br J Surg. 1998;85:526–529. doi: 10.1046/j.1365-2168.1998.00601.x. [DOI] [PubMed] [Google Scholar]
- 7.Quirke P, Steele R, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821–828. doi: 10.1016/S0140-6736(09)60485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinnatamby CS. Last's Anatomy. 10th edn. Oxford: Churchill Livingstone; 1999. p. 189. 201–202, 242–243. [Google Scholar]
- 9.Guillem JG, Minsky BD. Extended perineal resection of distal rectal cancers: surgical advance, increased utilization of neoadjuvant therapies, proper patient selection or all of the above? J Clin Oncol. 2008;26:3,481–3,482. doi: 10.1200/JCO.2007.15.6646. [DOI] [PubMed] [Google Scholar]
- 10.Mandard AM, Dalibard F, et al. Pathologic assessment of tumor regression after pre-operative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2,680–2,686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Peeters KC, van de Velde CJ. Quality assurance of surgery in gastric and rectal cancer. Crit Rev Oncol Hematol. 2004;51:105–119. doi: 10.1016/j.critrevonc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Renton SC, Gazet JC, et al. The importance of the resection margin in conservative surgery for breast cancer. Eur J Surg Oncol. 1996;22:17–22. doi: 10.1016/s0748-7983(96)91253-6. [DOI] [PubMed] [Google Scholar]
- 13.Hauschild A, Rosien F, Lischner S. Surgical standards in the primary care of melanoma patients. Onkologie. 2003;26:218–222. doi: 10.1159/000071616. [DOI] [PubMed] [Google Scholar]
- 14.Khanfir K, Alzieu L, et al. Does adjuvant radiation therapy increase loco-regional control after optimal resection of soft-tissue sarcoma of the extremities? Eur J Cancer. 2003;39:1,872–1,880. doi: 10.1016/s0959-8049(03)00426-x. [DOI] [PubMed] [Google Scholar]
- 15.Pennathur A, Zhang J, Chen H, Luketich JD. The ‘best operation’ for esophageal cancer? Ann Thorac Surg. 2010;89:S2,163–S2,167. doi: 10.1016/j.athoracsur.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerut T, Coosemans W, et al. Is there a role for radical esophagectomy. Eur J Cardiothorac Surg. 1999;16:S44–S47. doi: 10.1016/s1010-7940(99)00185-2. [DOI] [PubMed] [Google Scholar]
- 17.Dresner SM, Griffin SM. Pattern of recurrence following radical oesophagectomy with two-field lymphadenectomy. Br J Surg. 2000;87:1,426–1,433. doi: 10.1046/j.1365-2168.2000.01541.x. [DOI] [PubMed] [Google Scholar]
- 18.Bonenkamp JJ, Songun I, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745–748. doi: 10.1016/s0140-6736(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 19.Cushieri A, Fayers P, et al. Post-operative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. Lancet. 1996;347:995–999. doi: 10.1016/s0140-6736(96)90144-0. [DOI] [PubMed] [Google Scholar]
- 20.Havenga K, Enker WE, et al. Improved survival and local control after total mesorectal excision or D3 lymphadenectomy in the treatment of primary rectal cancer: an international analysis of 1411 patients. Eur J Surg Oncol. 1999;25:368–374. doi: 10.1053/ejso.1999.0659. [DOI] [PubMed] [Google Scholar]
- 21.Quan ML, McCready D. The evolution of lymph node assessment in breast cancer. J Surg Oncol. 2009;99:194–198. doi: 10.1002/jso.21201. [DOI] [PubMed] [Google Scholar]
- 22.van Akkooi AC, Voit CA, Verhoef C, Eggermont AM. New developments in sentinel node staging in melanoma: controversies and alternatives. Curr Opin Oncol. 2010;22:169–177. doi: 10.1097/CCO.0b013e328337aa78. [DOI] [PubMed] [Google Scholar]
- 23.Stamatakos M, Stefanaki C, Kontzoglou K, Safioleas M. Sentinel lymph node biopsy in breast cancer: a systematic review. Onkologie. 2010;33:121–126. doi: 10.1159/000277142. [DOI] [PubMed] [Google Scholar]
- 24.Wong Wong Keet A, Al-Rafae M, et al. Long-term outcome after neo-adjuvant chemotherapy for breast cancer in BRCA1/2 carriers. Int J Cancer. 2009;125:2,236–2,238. doi: 10.1002/ijc.24596. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham D, Allum WH, et al. Peri-operative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 26.Allum WH, Stenning SP, et al. Long-term results of a randomized trial of surgery with or without pre-operative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5,062–5,067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 27.Rubbia-Brandt L, Giostra E, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 28.Forshaw MJ, Gossage JA, Mason RC. Neoadjuvant chemotherapy for oesophageal cancer: the need for accurate response prediction and evaluation. Surgeon. 2005;3:373–382. doi: 10.1016/s1479-666x(05)80047-2. [DOI] [PubMed] [Google Scholar]
- 29.Dexter SP, Sue-Ling H, et al. Circumferential resection margin involvement: an independent predictor of survival following surgery for oesophageal cancer. Gut. 2001;48:667–670. doi: 10.1136/gut.48.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]


