Abstract
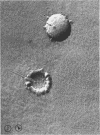
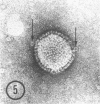
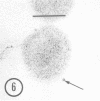
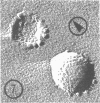
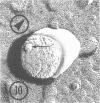
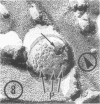
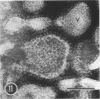
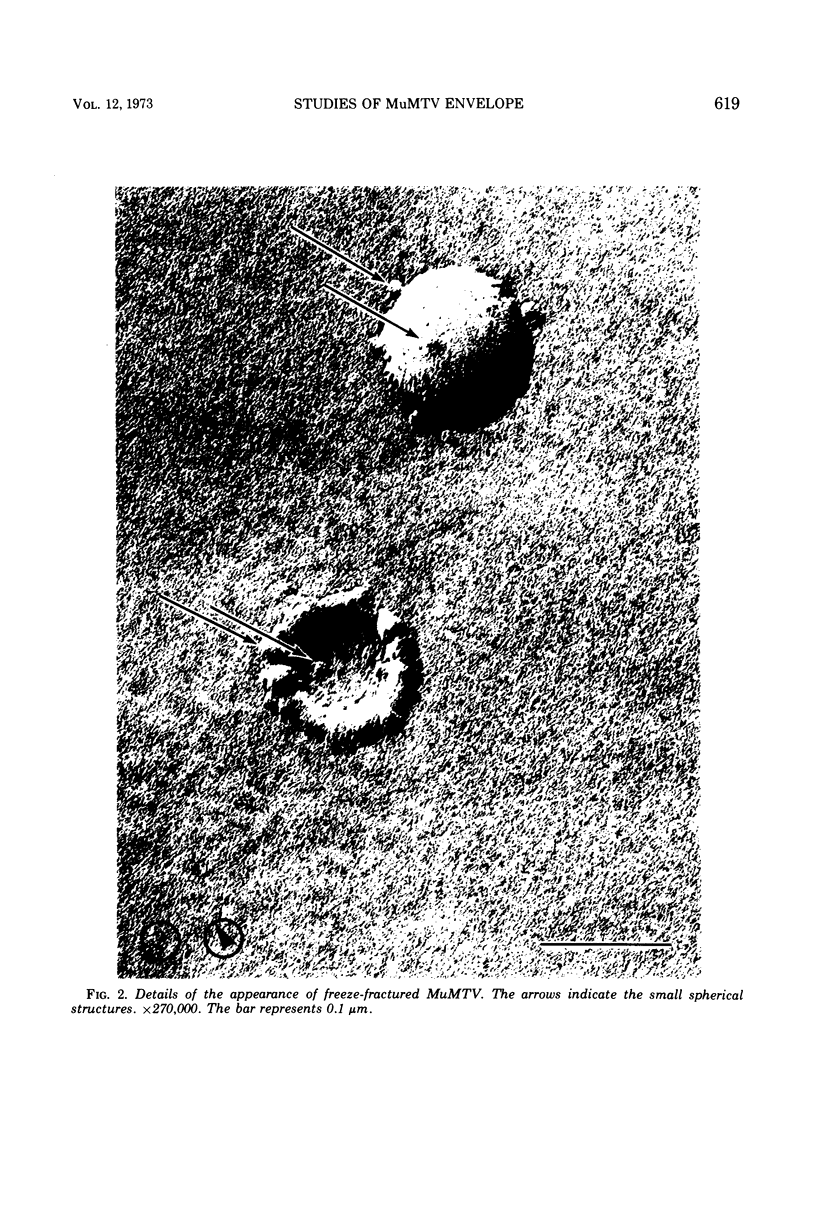
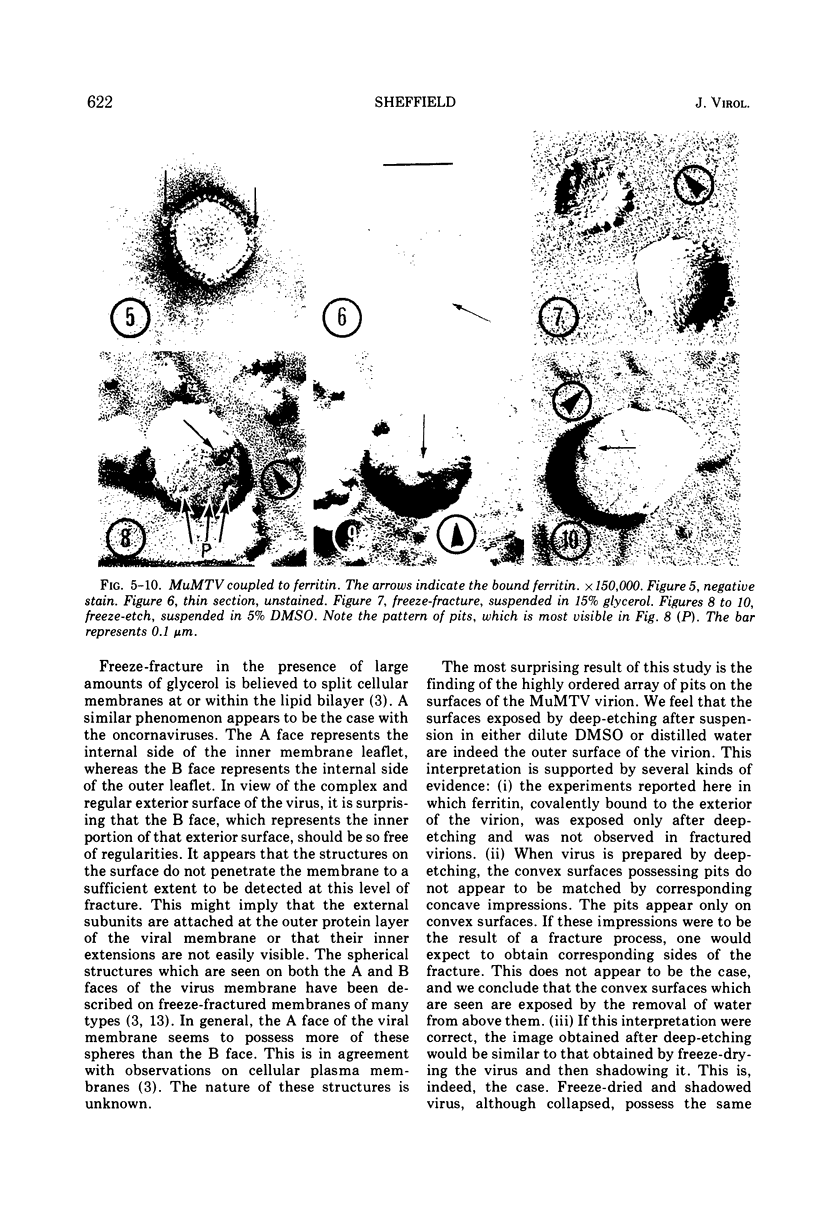
As part of a study of the cell surface changes associated with the production of murine mammary tumor virus, the structure of the envelope of this virus has been examined by using freeze-fracture techniques. Both fracture and deep-etch surfaces were examined. The fracture faces contain 10-nm spheres comparable to those observed on fractured plasma membranes, although fewer in number. Surfaces exposed by etching possess a highly regular hexagonal array of pits 25 nm apart. By examining freeze-fracture and freeze-etch preparations of virus with ferritin covalently bound to its surface, it has been determined that the surface exposed by etching is the outer surface of the virus. The pitted exterior surface of the mammary tumor virus appears to be a unique surface structure.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Bouteille M. Ultrastructural localization of antibody by antigen label with peroxidase. Exp Cell Res. 1968 Oct;53(1):166–176. doi: 10.1016/0014-4827(68)90364-9. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Bauer H., Gelderblom H., Hüper G. Polypeptides of avian RNA tumor viruses. IV. Components of the viral envelope. Virology. 1972 Mar;47(3):551–566. doi: 10.1016/0042-6822(72)90545-4. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Strauss J. H., Jr Glycopeptides of the membrane glycoprotein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):449–466. doi: 10.1016/0022-2836(70)90314-1. [DOI] [PubMed] [Google Scholar]
- Calafat J., Hageman P. C. Binding of Concanavalin A to the envelope of two murine RNA tumour viruses. J Gen Virol. 1972 Jan;14(1):103–106. doi: 10.1099/0022-1317-14-1-103. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Branton D. Fracture planes in an ice-bilayer model membrane system. Science. 1967 Nov 3;158(3801):655–657. doi: 10.1126/science.158.3801.655. [DOI] [PubMed] [Google Scholar]
- Dunlop W. F., Robards A. W. Some artifacts of the freeze-etching technique. J Ultrastruct Res. 1972 Aug;40(3):391–400. doi: 10.1016/s0022-5320(72)90109-8. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A., Revel J. P. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol. 1970 May;45(2):272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAIRSTONE M. A., SHEFFIELD J. B., MOORE D. H. STUDY OF B PARTICLES IN THE MAMMARY TUMORS OF DIFFERENT MOUSE STRAINS. J Natl Cancer Inst. 1964 Nov;33:825–836. doi: 10.1093/jnci/33.5.825. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Differences between the envelope glycoproteins and glycopeptides of avian tumor viruses released from transformed and from nontransformed cells. Virology. 1972 Nov;50(2):359–372. doi: 10.1016/0042-6822(72)90387-x. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Weinstein R. S. The ultrastructure of the nexus. A correlated thin-section and freeze-cleave study. J Cell Biol. 1970 Dec;47(3):666–688. doi: 10.1083/jcb.47.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V., Frank H. Fine structure of influenza A2 (Singapore) as revealed by negative staining, freeze-drying and freeze-etching. J Gen Virol. 1971 Jan;10(1):37–51. doi: 10.1099/0022-1317-10-1-37. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Frank H., Schäfer W. Properties of mouse leukemia viruses. 3. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze-drying and freeze-etching. Virology. 1972 Aug;49(2):345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- Park R. B. Freeze etching: a classical view. Ann N Y Acad Sci. 1972 Jun 20;195:262–272. [PubMed] [Google Scholar]
- Park R. B., Pfeifhofer A. O. Ultrastructural observations on deep-etched thylakoids. J Cell Sci. 1969 Jul;5(1):299–311. doi: 10.1242/jcs.5.1.299. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane splitting in freeze-ethching. Covalently bound ferritin as a membrane marker. J Cell Biol. 1970 Jun;45(3):598–605. doi: 10.1083/jcb.45.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar N. H., Charney J., Moore D. H. Mammary-tumor virion structures in mouse milk fractions. J Natl Cancer Inst. 1969 Dec;43(6):1275–1288. [PubMed] [Google Scholar]
- Shigematsu T., Dmochowski L. Studies on the acid mucopolysaccharide coat of viruses and transformed cells. Cancer. 1973 Jan;31(1):165–174. doi: 10.1002/1097-0142(197301)31:1<165::aid-cncr2820310123>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Marchesi V. T. Demonstration of the outer surface of freeze-etched red blood cell membranes. J Cell Biol. 1970 Jun;45(3):649–653. doi: 10.1083/jcb.45.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartiovaara J., Branton D. Visualization of ribosomes by freeze-etching. Exp Cell Res. 1970 Aug;61(2):403–406. doi: 10.1016/0014-4827(70)90464-7. [DOI] [PubMed] [Google Scholar]
- Wrigglesworth J. M., Packer L., Branton D. Organization of mitochondrial structure as revealed by freeze-etching. Biochim Biophys Acta. 1970;205(2):125–135. doi: 10.1016/0005-2728(70)90243-4. [DOI] [PubMed] [Google Scholar]