Abstract
Context
Emotion regulation deficits figure prominently in generalized anxiety disorder (GAD), as well as other anxiety and mood disorders. Research examining emotion regulation and top-down modulation has implicated reduced coupling of the amygdala with prefrontal and anterior cingulate cortex (ACC), suggesting altered frontolimbic white matter connectivity in GAD.
Objective
To investigate structural connectivity between ventral prefrontal/ACC areas and the amygdala in GAD, and to assess associations with functional connectivity between those areas.
Design
Participants underwent diffusion tensor imaging (DTI) and functional magnetic resonance imaging (fMRI) scans.
Setting
University magnetic resonance imaging facility.
Participants
Forty-nine GAD patients and 39 healthy volunteers, including a subset of 21 patients without comorbid Axis I diagnoses and 21 healthy volunteers matched for age, sex, and education.
Main Outcome Measure
Mean fractional anisotropy (FA) values in the left and right uncinate fasciculus, as measured by tract-based analysis for DTI data.
Results
Lower mean FA values in bilateral uncinate fasciculus indicated reduced frontolimbic structural connectivity in GAD. This reduction in uncinate fasciculus integrity was most pronounced for patients without comorbidity and was not observed in other white matter tracts. Across all subjects, higher FA values were associated with more negative functional coupling between the pregenual ACC and amygdala during the anticipation of aversion.
Conclusions
Decreased frontolimbic structural connectivity suggests a neural basis for emotion regulation deficits in GAD. The functional significance of these structural differences is underscored by decreased functional connectivity between the ACC and amygdala in subjects with reduced structural integrity of the uncinate fasciculus.
Introduction
Anxiety disorders are the most common class of mental disorders, and the prevalence of generalized anxiety disorder (GAD) is as high as 5.7%.1 A hallmark feature of GAD is excessive, uncontrollable worry. As the emphasis of worry is on adverse events that may occur in the future, theoretical models of GAD have emphasized aberrant anticipatory processing.2–4 A prominent model of GAD has proposed that worry serves as an implicit strategy for avoiding negative emotional experiences.5 In this way worry may serve as a compensatory mechanism for deficits in productive emotion regulation strategies in GAD.5–8
Neuroimaging research provides a promising avenue for investigating these components of GAD phenomenology. In particular, a growing number of functional imaging studies on GAD emphasize neural correlates of emotion regulation deficits in the disorder. Amygdala hyperactivity has been observed in a number of studies4,9–12 but not in others.13–15 Ventral regions of the prefrontal cortex (PFC), including the ventrolateral PFC and anterior cingulate cortex (ACC), have also shown abnormal patterns of activation in multiple studies.9,11–13 These areas have been prominently featured in research on emotion regulation and top-down modulation in healthy populations,16–24 with the ventral PFC/ACC areas presumed to modulate amygdala responses to aversion and threat.20,21,23,25–30 A recent study found aberrant functional connections between ventral PFC/ACC regions and the amygdala in GAD,9 suggesting a neural basis for regulatory deficits in the disorder. Reduced connectivity may be associated with reduced down-regulation of the amygdala, such that the elevated anxiety observed in GAD may be a direct manifestation of amygdala hyperactivity. In addition, two recent studies4,15 found that increased ACC activity prior to treatment was associated with better outcomes following an 8-week medication trial, suggesting improved prognoses for those patients with relatively preserved regulatory functions of the ACC.
It is not known whether GAD is accompanied by alterations in white matter connectivity between PFC/ACC regions and the amygdala. Reductions in the neuronal connections linking PFC/ACC regions to the amygdala may be responsible for the emotion regulation deficits and functional imaging findings described above for GAD. A primary candidate for testing such structural connections is the uncinate fasciculus, the major white matter tract that directly connects the amygdala to ventral regions of the PFC and ACC.31–37 Recent studies using diffusion tensor imaging (DTI) indicate a promising role for the uncinate fasciculus as a candidate marker of regulation deficits in GAD, as reduced structural integrity of the uncinate fasciculus has been implicated in social anxiety disorder,38 bipolar disorder,39 trait anxiety,40 and individuals with low-expressing 5-HTTLPR alleles.41 The sole prior report on white matter in GAD42 did not specifically investigate the uncinate fasciculus and used a different measure of diffusion than in the above reports.38–41
The primary focus of the present study was to investigate whether GAD patients exhibited reduced structural integrity of the uncinate fasciculus – operationalized as lower fractional anisotropy values, a common measure of DTI data – in line with the research reviewed above implicating regulatory deficits and corresponding functional abnormalities in GAD.4,6–13,15 In addition, symptom-relevant functional consequences of uncinate fasciculus structure were investigated using fMRI data from the same scan session in all participants for a task that targeted anticipatory abnormalities in GAD.2–4,43 In analyses directly comparing DTI and fMRI data, we used a multiple regression approach to relate individual differences in uncinate fasciculus structure to functional connectivity between the amygdala and PFC/ACC regions. We predicted that increased structural integrity of the uncinate fasciculus would be associated with more negative coupling between those regions20,27–30,44,45 in all participants, reflecting enhanced anticipatory regulatory function in those subjects with the most robust frontolimbic structural connectivity. Finally, based on prior reports linking anxiety and the uncinate fasciculus to common polymorphisms affecting serotonin and brain-derived neurotrophic factor (BDNF)41,46–53, we also tested whether structural connectivity of the uncinate fasciculus was reduced in S/LG carriers relative to LA homozygotes for 5-HTTLPR,41 and in Met carriers relative to Val homozygotes for the BDNF Val66Met polymorphism,53 although interactions with diagnostic groups were possible.54
Methods
Subjects
DTI scans were collected from a total of 88 volunteers, who were recruited through newspaper and e-mail advertisements. All subjects were right-handed (Edinburgh Handedness Inventory) and underwent a Structured Clinical Interview for DSM-IV (SCID),55 administered by trained doctorate-level clinicians. There were 49 patients diagnosed with GAD (30 women; Table 1). Thirteen patients had no history of other psychopathology, as determined by the SCID, while an additional 8 had no other current disorder (of these 8, 4 were diagnosed with past major depressive disorder [MDD], 3 with past MDD and substance abuse, and 1 with past substance abuse). The other 28 patients met criteria for a current comorbid anxiety or mood disorder (10 with MDD only, 5 with social anxiety disorder [SAD] only, 10 with MDD and SAD, and 3 with SAD and past MDD). Control subjects were 39 volunteers with no history of psychopathology (19 women). In addition to primary analyses on the full sample, we conducted ancillary analyses on a matched sample of the 21 patients with no other current diagnosis (12 women) and 21 healthy control subjects (matched for age, sex, and education).
Table 1.
Demographic, genotypic and symptom information of healthy control sample and GAD patients
| Full Sample
|
Matched Sample
|
|||
|---|---|---|---|---|
| Control (n=39) | GAD (n=49) | Control (n=21) | GAD (n=21) | |
| Demographics | ||||
| Age, Mean (SD) | 23.85 (6.86) | 27.10 (10.61) | 23.14 (5.66) | 24.05 (6.60) |
| Women, n (%) | 19 (49) | 30 (61) | 12 (57) | 12 (57) |
| Education, Mean (SD) | 16.38 (2.34) | 15.73 (1.71) | 16.29 (2.45) | 16.24 (1.81) |
|
| ||||
| Ethnic Background, n | ||||
| Europe | 30 | 39 | 16 | 14 |
| Africa | 2 | 3 | 2 | 2 |
| Far East Asia | 6 | 5 | 2 | 4 |
| Undeclared | 1 | 2 | 1 | 1 |
|
| ||||
| BDNF grouping, n (%) | ||||
| ValVal | 24 (62) | 27 (55) | 13 (62) | 10 (48) |
| Met Carrier | 13 (33) | 16 (33) | 7 (33) | 7 (33) |
| MetMet | 4 (10) | 2 (4) | 1 (5) | 0 (0) |
| ValMet | 9 (23) | 14 (29) | 6 (29) | 7 (33) |
| Missing | 2 (5) | 6 (12) | 1 (5) | 4 (19) |
|
| ||||
| 5-HTTLPR Grouping, n (%) | ||||
| LALA | 9 (23) | 12 (25) | 5 (24) | 5 (24) |
| S/LG carrier | 28 (72) | 33 (67) | 15 (72) | 15 (72) |
| SASA | 7 (18) | 8 (16) | 6 (29) | 5 (24) |
| SALG | 1 (3) | 2 (4) | 0 (0) | 1 (5) |
| SALA | 18 (46) | 20 (41) | 9 (43) | 7 (33) |
| LGLA | 2 (5) | 3 (6) | 0 (0) | 2 (10) |
| Missing | 2 (5) | 4 (8) | 1 (5) | 1 (5) |
|
| ||||
| Symptom measures, mean (SD) | ||||
| Ham-A | 1.64 (1.61) | 18.90 (7.53) | 1.52 (1.37) | 17.62 (7.33) |
| Ham-D | 2.62 (2.60) | 27.73 (11.19) | 2.05 (2.04) | 22.86 (12.31) |
| GAD-Q | 1.55 (1.30) | 10.24 (1.98) | 1.44 (1.44) | 9.54 (1.61) |
| PSWQ | 33.66 (8.30) | 63.29 (9.24) | 32.45 (6.94) | 61.33 (9.82) |
Note. Ham-A = Hamilton Rating Scale for Anxiety. Ham-D = Hamilton Rating Scale for Depression. GAD-Q = Generalized Anxiety Disorder Questionnaire. PSWQ = Penn State Worry Questionnaire.
Table 1 provides scores for the Hamilton Rating Scales for Anxiety (Ham-A)56 and Depression (Ham-D),57 Generalized Anxiety Disorder Questionnaire (GAD-Q),58 and Penn State Worry Questionnaire (PSWQ),59 which were administered after the SCID at the screening session. Current medication was an exclusion criterion for this study (past medication history was only collected for the final 14 patients, of whom 6 reported no past medications, 7 had taken antidepressant or anti-anxiety medications [Zoloft, Paxil, Prozac, Wellbutrin, clonazepam, Xanax] for periods ranging from 2 months to 1 year, and 1 had tried a brief trial of a sleeping medication [name not recalled] approximately one year prior to participation). Informed consent was obtained from all participants prior to the experiment in accordance with study approval by the Institutional Review Board of the University of Wisconsin School of Medicine and Public Health. All subjects were paid for their participation.
Data acquisition
DTI images were collected using a 3.0 Tesla GE SIGNA Scanner with a quadrature birdcage head coil. A vacuum pillow was used to minimize distortion due to head movement. Scans were performed using a diffusion weighted MRI (cardiac-gated, 2D echo planar sequence, repetition time [TR] = ~10–12 sec [dependant upon heart rate], echo time [TE] = 72 ms, flip angle α = 90°, field of view [FOV] = 24 × 24 cm, matrix = 128×128 [interpolated to 256×256], slice thickness = 3 mm, 39 axial slices, 12 optimum non-collinear encoding directions, b=1000 s/mm2 with a single b=0 image, number of excitations [NEX] = 3). Field maps for correcting geometric distortions in the DTI data were also obtained (see Author Supplemental Methods). DTI allows for research of white matter integrity in vivo by measuring magnitude and orientation of water diffusion. Dense white matter tracts have highly anisotropic diffusion of water oriented parallel to the fiber bundle, while gray matter has predominately isotropic water diffusion. A common diffusion measure, fractional anisotropy (FA), describes the directional variance of water diffusion and is high in white matter regions with dense, well-myelinated, and parallel axon bundles. Another measure often used is mean diffusivity (MD), which describes the average diffusivity in all directions, and is sensitive to the overall density of tissue membranes. Thus, FA and MD reflect complimentary aspects of tissue microstructure (coherence versus density).60–63
Whole-brain anatomical and functional images were acquired from all subjects in the same scanning session (see Author Supplemental Methods for pulse sequences). The functional paradigm implemented was an emotional anticipation task.4,23,64,65 Participants viewed cues that were followed by a 2- to 8-s jittered interstimulus interval (ISI) and subsequent aversive and neutral pictures (see Author Supplemental Methods for full details).
Image analysis
Distortions in the diffusion weighted images caused by eddy currents, magnetic field inhomogeneities and head motion were corrected using affine co-registration and geometrically unwarping the EPI images using the FSL toolbox.66 The FA and MD maps were calculated using the Diffusion Toolkit (trackvis.org).
Deterministic tractography was the primary method employed for assessing whether patients have abnormalities in white matter integrity. The actual shape of the white matter fiber tracts, or large bundles of axons connecting distal brain regions, can be deduced by visualizing the water diffusion as tensors and then plotting lines through those tensors.63 Tractography uses the principal direction of the tensor to reconstruct the white matter tracts of interest, and analyses are conducted on the identified tracts in their entirety (e.g., uncinate fasciculus, corpus callosum).67
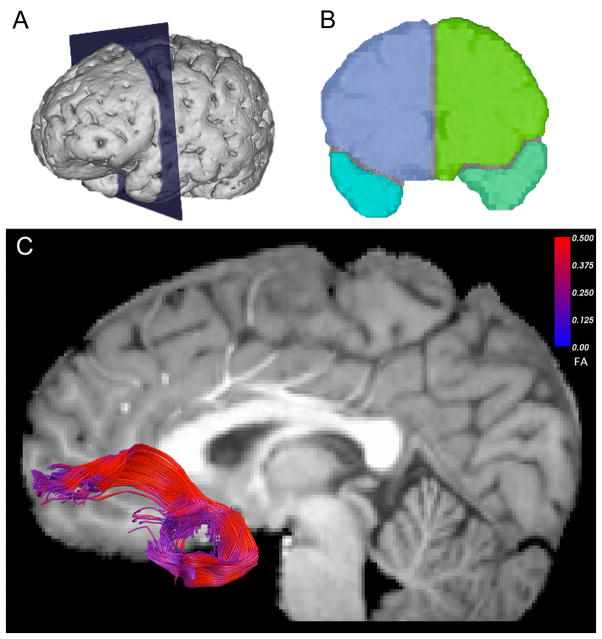
To estimate the fiber tracts, a line originating at a seed voxel was propagated following the tensor direction. This was accomplished using CAMINO software,68 which applies a tensor deflection (TEND) algorithm for deterministic tractography.69 Fiber trajectories were terminated at voxels with FA values less than 0.15, or when the dot product between the previous and the current direction was less than 0.7. TrackVis (trackvis.org) was used to visualize the identified tracts and to manually delineate the uncinate fasciculus and three control regions – cingulum, inferior fronto-occipital fasciculus and corpus callosum – in each subject using ROI-based axonal tracking methods70 (Figure 1; eVideo 1). Mean FA and MD values were exported for each of these structures, as well as for the whole brain. One patient had missing data for the cingulum, and one for the corpus callosum. The manual delineation was executed by D.T. and two trained students (kappas = 0.83).
Figure 1.
Region of interest (ROI) placement for delineation of bilateral uncinate fasciculus. Bilateral frontal and temporal lobe seed ROIs (a) were manually drawn in the most posterior coronal slice that showed clear separation of the frontal and temporal lobes bilaterally, as depicted in (b). The Boolean ‘AND’ term was used to select only fibers that crossed through both the temporal and frontal seed ROIs for tract-based analysis. (c) Uncinate fasciculus tracts overlaid on anatomical T1-weighted image for a single subject. For 3D rendering, see eVideo 1 online.
In an attempt to provide converging evidence for the results obtained using the aforementioned tract-based analysis, the FA maps were co-registered to anatomical images using an optimized nonlinear registration method (DARTEL71) and voxelwise, whole-brain analysis of FA maps was conducted in SPM8 (fil.ion.ucl.ac.uk/spm/) (see Author Supplemental Methods).
To assess whether differences in structural integrity of the uncinate fasciculus were associated with differences in functional connectivity, we implemented context-dependent correlation analysis, or psychophysiological interaction (PPI).72 PPI allows for the identification of brain regions in which functional coupling with a seed region is modulated by the task manipulation. Briefly, we defined the bilateral amygdala anatomically and averaged the two amygdalae, extracted amygdala time series data, and examined the relationship of these data with preprocessed, whole-brain fMRI data. This analysis identified regions showing differential functional coupling with the amygdala during aversive vs. neutral anticipation (see Author Supplemental Methods for full details). Two subjects (1 patient) were missing fMRI data and were excluded from analysis.
Genetic Materials
Buccal cells were collected from participants by having them rinse with 10 ml of commercial mouthwash for 1 minute. Genotyping methods were adapted from published reports investigating variants of the serotonin transporter gene (5-HTTLPR S and L alleles; rs25531 LA and LG alleles)73 and the BDNF SNP rs626574 (see Author Supplemental Methods). Participants with S and LG alleles were grouped together given evidence that these alleles are functionally equivalent.75 Additionally, due to the relative infrequency of the Met allele, analyses compared subjects with the Val/Val genotype to Met allele carriers (Val/Met and Met/Met).53 Six subjects (4 patients) were missing data for 5-HTTLPR, and 8 subjects (6 patients) were missing data for the BDNF Val66Met polymorphism (Table 1).
Statistical Analysis
The GAD and control groups did not differ in age (F(1,86)=2.75, p=0.10), sex (F(1,86)=1.37, p=0.25), education (F(1,86)=2.28, p=0.14), or whole-brain FA (F(1,86)=0.04, p=0.84). Accordingly, findings were highly similar for all analyses on FA values for the uncinate fasciculus regardless of whether sex, age, education, and whole-brain FA were included as covariates. Unless otherwise indicated, analyses included all 4 covariates in order to specify these sources of variance in the model rather than leaving them unspecified in the error term.76
All data for tract-based analyses were analyzed using SPSS (version 18). A Group (GAD, Control) × Hemisphere (Left, Right) ANCOVA tested group differences in mean FA values for the left and right uncinate fasciculus. To assess the specificity of findings to FA, two additional analyses were conducted: 1) identical ANCOVA except that MD values for the left and right uncinate fasciculus and whole-brain MD were also included as covariates, and 2) analogous ANCOVA testing group differences in mean MD values. A Group (GAD only, GAD comorbid, Control) × Hemisphere (Left, Right) ANCOVA compared GAD subjects with and without current comorbid diagnoses. An ancillary Group × Hemisphere ANCOVA was conducted for the subsample of 21 GAD patients without current comorbid diagnoses and 21 healthy controls matched for sex, age, and education. For this ANCOVA, only whole-brain FA was used as a covariate since these groupings were matched on the 3 demographic variables.
Two different analytic approaches were used for testing the specificity of findings to the uncinate fasciculus. First, ANCOVAs identical to the primary analysis above were conducted separately for the cingulum, inferior fronto-occipital fasciculus, and corpus callosum. Second, for the voxelwise whole-brain DTI data, two-sample t tests comparing the two groups were performed using SPM8.
To directly relate DTI findings to functional connectivity data, multiple regression analyses were implemented in AFNI77 (version 2) to identify PFC/ACC regions in which functional coupling with the amygdala was correlated with uncinate fasciculus FA values. The primary analysis was for average uncinate fasciculus FA values; ancillary analyses were conducted for left and right uncinate fasciculus FA values separately. The dependent variable for these analyses was the standardized PPI coefficient at each voxel in the anatomically defined PFC for the contrast of aversive vs. neutral anticipation. Independent variables used to predict these PPI coefficients were Group, Uncinate Fasciculus FA values, and the Group × Uncinate Fasciculus interaction term (using the same covariates indicated above). Analyses focused on the two predictors involving Uncinate Fasciculus FA values in order to identify a direct relationship between individual differences in frontolimbic structural and functional connectivity. The Uncinate Fasciculus FA predictor identified voxels in which FA values were related to functional coupling with the amygdala across all subjects, whereas the interaction term identified voxels in which the two groups differed in the relationship between FA values and functional coupling with the amygdala. Although not central to study hypotheses on the association of structural and functional connectivity, the Group predictor identified voxels that showed a difference in functional coupling with the amygdala between the two groups (controlling for FA values). Small-volume correction for multiple comparisons using an uncorrected p threshold of .01 resulted in a minimum cluster size of 264 mm3 in order to meet a threshold of p < .05 (corrected).
Associations of the DTI data with genetic polymorphisms (5-HTTLPR, BDNF Val66Met) were assessed with Genotype × Group × Hemisphere ANCOVAs. Finally, Pearson correlation coefficients were calculated within each group separately to assess associations between FA values for the uncinate fasciculus and symptom measures: Ham-A, Ham-D, GAD-Q, and PSWQ. An alpha level of .05 was used for all statistical tests.
Results
Group differences in frontolimbic structural connectivity
For a Group (GAD, Control) × Hemisphere (Left, Right) ANCOVA, a Group main effect indicated that the 49 GAD patients had lower FA values in bilateral uncinate fasciculus than the 39 healthy controls (F(1,82)=5.773, p=0.02; Figure 2). No other effects were significant (ps>0.19). The Group main effect for FA remained significant when MD values for the left and right uncinate fasciculus and whole-brain MD were also included as covariates (F(1,79)=6.632, p=0.01). For the analogous ANCOVA on MD values for the uncinate fasciculus, no effects involving Group were observed (ps>0.26).
Figure 2.
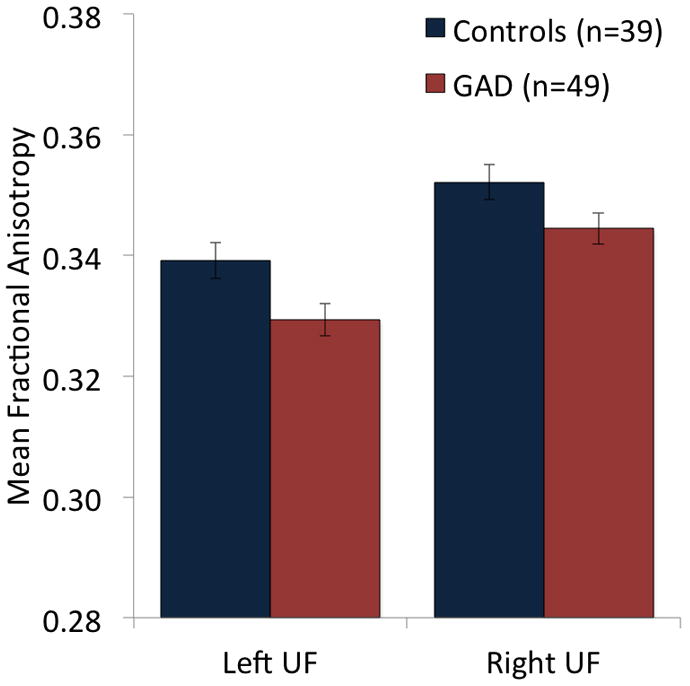
Patients with generalized anxiety disorder (GAD) showed reduced mean fractional anisotropy (FA) values for the uncinate fasciculus (UF) relative to healthy control subjects, as indicated by a Group main effect for a Group × Hemisphere ANCOVA with covariates of age, sex, education, and whole-brain FA.
To further explore the relationship between GAD pathology and uncinate fasciculus microstructure, we conducted additional analyses on various groupings of GAD patients in our sample. For analyses separating GAD subjects with (n=21) and without (n=28) current comorbid diagnoses, a main effect of Group (F(2,81)=4.065, p=0.02) in the absence of effects for Hemisphere or Group × Hemisphere (ps>0.78) indicated that the GAD patients without comorbidity had lower FA in the uncinate fasciculus than the healthy controls (t(57)=2.29, p=0.01), whereas the comorbid patients did not differ from either of these groups (ps>0.23). Consistent with this finding, an additional analysis conducted on the matched sample of 21 patients without current comorbid Axis I disorders and 21 healthy volunteers also revealed a Group main effect (F(1,39)=7.998, p=0.007; Author Supplemental Figure 1) and again, no effects of Hemisphere or Group × Hemisphere (ps>0.79). Of note, the Group effect was also observed for the 13 patients with no current or past comorbidities and 13 matched healthy controls (F(1,23)=13.36, p=0.001).
Tract-based analyses conducted on 3 control regions – the corpus callosum, cingulum, and inferior fronto-occipital fasciculus – revealed that group differences were largely specific to the uncinate fasciculus. ANCOVAs analogous to those conducted above for the uncinate fasciculus indicated no effects involving Group for any of the 3 structures in the full sample (all ps>0.18) or matched sample (all ps>0.06).
Voxelwise, whole-brain analyses yielded confirmatory evidence of lower uncinate fasciculus FA in GAD patients than controls. In the full sample, this effect was observed for the left uncinate fasciculus at p<0.01 (uncorrected) and for the right at p<0.02 (uncorrected; see Author Supplemental Table 1). The reduction in bilateral uncinate fasciculus FA was observed at a more stringent threshold of p=0.005 (uncorrected) for the sample of 21 patients without current comorbid diagnoses and matched healthy volunteers. Consistent with above tract-based analyses on the 3 control regions, the whole-brain analysis on the full sample indicated an absence of reliable group differences outside the uncinate fasciculus, whereas the analysis on the matched sample revealed group differences in the fornix, internal capsule, and arcuate fasciculus.
Associations between frontolimbic structural connectivity and functional connectivity
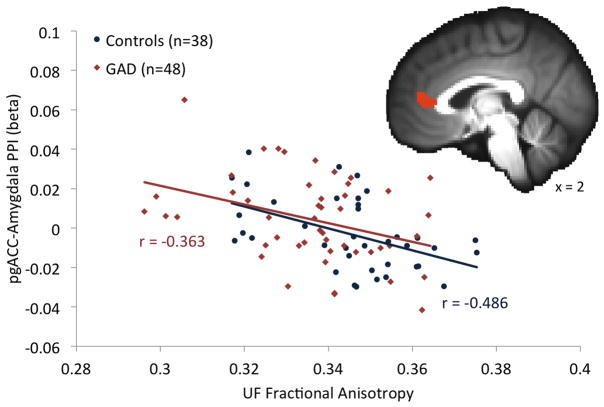
For analyses investigating whether individual differences in uncinate fasciculus FA values were related to condition-specific functional coupling between the PFC/ACC and the amygdala, the pregenual ACC showed the predicted association between higher FA values and increased negative coupling with the amygdala across all subjects (Figure 3). This effect was observed in the full sample at p<0.05 (corrected) for the average of the right and left uncinate fasciculus and for regressions of PPI coefficients on each uncinate fasciculus separately (Author Supplemental Table 2). An overlapping pregenual ACC cluster showed the same association with average uncinate fasciculus FA values for the matched sample. A left dorsolateral PFC region showed the same pattern of greater negative coupling with the amygdala for subjects with higher FA values in both the full and matched samples (Author Supplemental Table 2). No effects were observed for the Group × Uncinate Fasciculus FA interaction, indicating an absence of group differences in the relationship between FA values and functional coupling with the amygdala. Although not central to study hypotheses relating structural and functional connectivity, Group main effects were also examined (Author Supplemental Table 2). There was no effect of Group in the pregenual ACC, but a group difference was seen in the left ventrolateral PFC, suggesting that this region’s reduced functional connectivity with the amygdala in the patients was independent of group differences in uncinate fasciculus strength.
Figure 3.
(a) Participants with higher uncinate fasciculus fractional anisotropy (FA) values showed greater negative coupling between the pregenual anterior cingulate cortex (pgACC) and amygdala during the anticipation of aversive vs. neutral pictures. (b) This relationship was seen in both patients with generalized anxiety disorders (red squares) and healthy controls (blue diamonds), as evidenced by a significant pgACC cluster for the FA predictor but not the Group or Group × FA predictors. Note that r values are presented for illustrative purposes only and are not intended as depicting additional statistical tests.78,79
Frontolimbic structural connectivity associations with genomic and symptom measures
For 5-HTTLPR, the Genotype (S/LG carrier, LA/LA) × Group (GAD, Control) × Hemisphere (Left, Right) ANCOVA failed to identify effects for Genotype (F(1,74)=0.583, p=0.45), Group × Genotype (F(1,74)=2.80, p=0.10), or any other effects involving Genotype (all ps>0.49). Decomposition of the marginally significant Group × Genotype interaction indicated that for the patients, S/LG carriers had lower FA values than LA/LA subjects (F(1,39)=4.11, p=0.049), whereas no effect was observed for the healthy controls (F(1,31)=0.37, p=0.55). The analogous ANCOVA for the BDNF Val66Met polymorphism with Genotype (Met carrier, Val/Val) failed to identify effects for Genotype (F(1,72)=0.379, p=0.54), Group × Genotype (F(1,72)=0.005, p=0.95), or any other effects involving Genotype (all ps>0.15). The Genotype effect was also not observed for analyses conducted on each group separately (both ps>0.23).
Correlations calculated within each group separately revealed no reliable associations between uncinate fasciculus FA and symptom measures, including the GAD-Q, PSWQ, Ham-A, and Ham-D (all ps>0.16, Bonferroni corrected).
Discussion
Using new tract-based analysis for DTI to assay structural connectivity in GAD and healthy controls, we observed bilaterally reduced FA of the uncinate fasciculus, a prominent white matter pathway connecting ventral PFC and ACC regions to the amygdala and other limbic areas. This effect was observed in the full sample of 49 GAD patients with and without current comorbid Axis I conditions and 39 healthy controls, and was particularly pronounced for patients with no comorbidities. These uncinate fasciculus findings suggest a structural basis for emotion regulation deficits in GAD6–9 and are consistent with previous functional imaging reports of abnormal activation patterns in the amygdala and ACC.4,7–13 Analyses conducted across imaging modalities elucidated the functional significance of the structural differences in the uncinate fasciculus. Across all subjects, lower FA values were associated with reduced negative coupling between the ACC and amygdala, precisely the relationship expected for poorer regulatory function. Decreased structural integrity of the uncinate fasciculus in patients with GAD may have detrimental functional consequences for emotion regulation, thereby contributing to heightened anxiety.
These DTI findings are the first report of uncinate fasciculus abnormalities in GAD. Of note, a recent DTI study of GAD did not report on FA for the uncinate fasciculus, but instead used a method assessing apparent diffusion coefficient (ADC), which is equivalent to MD, for circular ROIs in each of the four major brain lobes and the corpus callosum, and found no group differences for either the frontal or temporal lobe.42 The uncinate fasciculus findings here provide complementary support for past fMRI studies in GAD that have noted hyperactivity of the amygdala relative to healthy controls while participants are involved in processes such as implicit emotion regulation and conflict monitoring,9 the anticipation of emotional (and non-emotional) images,4 and viewing emotional faces.10,11 One interpretation of the amygdala hyperactivity that has frequently been observed in GAD is that patients fail to effectively recruit prefrontal circuitry that serves to regulate amygdala responses. Strong support for this hypothesis comes from the work of Etkin and colleagues,9 who demonstrated decreased coupling of the pregenual ACC and amygdala during the implicit regulation of emotional conflict in GAD. Such decreased coupling may be due to reductions in the integrity of the uncinate fasciculus, which is the primary white matter pathway connecting ventral prefrontal cortex with limbic structures, including the amygdala.31,36
By relating DTI data for the uncinate fasciculus to fMRI data on a disorder-relevant task of anticipatory function, findings here provide evidence that reduced microstructural integrity of this pathway is likely to have functional consequences for prefrontal-limbic communication. This builds on two important earlier reports that investigated relations between DTI and fMRI data related to anxiety and mood disorders.39,40 Our analytic procedure reduced a multi-step procedure for examining relations among DTI, fMRI, and psychopathology criterion (bipolar disorder39, trait anxiety40) to a single step that incorporates all three domains and allows for the simultaneous assessment of uncinate fasciculus structural integrity and diagnostic group (and their interaction) in predicting context-dependent functional connectivity with the amygdala.
Our data suggest that uncinate fasciculus integrity may be central to the previous observation in GAD of reduced functional connectivity between the pregenual ACC and amygdala.9 Indeed, in an analysis analogous to that conducted by Etkin et al.9 with group as the sole predictor of context-dependent connectivity, GAD patients showed reduced connectivity between the pregenual ACC and the amygdala (Author Supplemental Figure 2). This group main effect was not significant in our primary model that included both group and uncinate fasciculus values as predictors, reflecting the substantial overlap between those individuals with GAD and those with the lowest FA values. Findings from both of these studies demonstrate decreased connectivity between the pregenual ACC and amygdala in GAD, with the current report emphasizing the importance of structural contributions. In addition, the current report extends previous findings9 of altered functional connectivity to the domain of anticipatory processing. This replication across experimental paradigms provides evidence that altered pregenual ACC – amygdala circuitry may be central to the pathology of GAD.
These structural and functional imaging studies point to a neurobiological basis for deficient emotion regulation abilities in individuals with GAD. Studies examining voluntary emotion regulation frequently report activation in many regions of the PFC and ACC, which is often inversely related to amygdala activation.25,26,28,29,43,80,81 During the anticipation of aversive images, we identified negative functional coupling of the pregenual ACC and amygdala only in those subjects with the highest uncinate fasciculus values. Despite the lack of explicit task instructions, it seems likely that participants nevertheless enacted preparatory regulatory strategies during the anticipation period. Our data suggest that decreased uncinate fasciculus integrity in GAD may interfere with this prefrontal regulation of amygdala activation, adding to a growing literature on altered prefrontal-amygdala communication in GAD.4,9,12 In addition to these pregenual ACC findings, subjects with higher uncinate fasciculus values also showed greater negative coupling between the dorsolateral PFC and amygdala during the anticipation of aversion. Of note, there are robust connections between the amygdala and ventral portions of the PFC/ACC,36,82,83 while more dorsal portions of the PFC project weakly or not at all to the amygdala.36,84,85 It may be that ventral portions of the PFC/ACC serve as critical nodes in facilitating communication between dorsal PFC regions and the amygdala during regulation of emotional responses.29,30,86 Future research could test the hypothesis that deficient performance in GAD on an explicit emotion regulation task previously shown to engage the dorsolateral PFC25,26 is mediated by reduced integrity of the uncinate fasciculus.
Specificity of the findings was addressed in three ways. First, the group differences were anatomically specific to the uncinate fasciculus, as indicated by the absence of group differences elsewhere in the brain. This was determined using tract-based analyses in the cingulum, corpus callosum and inferior fronto-occipital fasciculus, as well as voxelwise, whole-brain analyses.
Second, the group differences for the uncinate fasciculus were strongest for the GAD patients without comorbidities, suggesting some degree of specificity for GAD. This observation stands in contrast to the identification of reduced uncinate fasciculus FA in social anxiety disorder,38 trait anxiety,40 and bipolar disorder.39 Indeed, the accumulating positive findings across different studies suggest that decreased integrity of the uncinate fasciculus may be a general risk factor for affective pathology. Future research investigating questions of comorbidity and specificity might focus in particular on uncinate fasciculus structure in unipolar depression, as 23 of 28 subjects in our comorbid group had a current or past diagnosis of MDD.
Third, group differences were observed for FA but not MD. The null findings for MD in the uncinate fasciculus are consistent with the only previously published MD findings for GAD.42 Although the precise biological characteristics associated with different DTI measures are not fully known,63 FA and MD likely quantify complementary aspects of brain microstructure. Differences in FA may reflect alterations in either myelination or axonal density, whereas MD reflects the overall density of tissue membranes irrespective of fiber orientation.63 Accordingly, findings here for FA implicate a difference in the microstructural components that have directional dependence due to myelination or axonal density. Of note, the evidence for minimal axonal plasticity in the adult brain87,88 is relevant to findings for the present sample, which included a broad age range. The role of uncinate fasciculus structure in the development and course of GAD and other affective disorders are important topics for future investigations.
Of potential relevance to the etiology of GAD, ancillary analyses examined relations between uncinate fasciculus integrity and common genetic polymorphisms linked to anxiety. We did not replicate recent findings of reduced uncinate fasciculus FA in healthy volunteers for the low-expressing 5-HTTLPR allele,41 although this pattern was observed for the patients. We also failed to replicate the finding of reduced uncinate fasciculus FA for the BDNF Met allele.53 Further research is needed to determine the replicability of that original finding for the BDNF Met allele53 and to clarify whether the effects of this polymorphism on anxiety52 and fear extinction51,53 are mediated by the uncinate fasciculus or a separate mechanism. A critical consideration is that while the sample of 88 subjects is large for a neuroimaging patient study, and larger than for many published neuroimaging genetics studies, the sample size is insufficient for detecting the smaller effect sizes that are typical of genetics studies; thus, these mainly negative genetics findings are not conclusive.50
In summary, using DTI tract-based analysis, we identified evidence of reduced integrity of the uncinate fasciculus, a crucial white matter pathway linking ventral PFC and ACC to limbic regions, in GAD. These results indicate that altered structure of a neural pathway involved both in normative emotion regulation and fear extinction processes may contribute to atypical emotional processing in GAD. The group differences in uncinate fasciculus structural connectivity, together with the observed association with functional connectivity, support a model positing emotion regulation deficits in GAD6–8 and suggest weak top-down control of amygdala reactivity. Further research is needed to determine how worry, the hallmark feature of GAD, affect the neurobiology identified here, but its presumed function in avoiding negative emotional experiences may actually sensitize amygdala activity, resulting in a generalized state of heightened anxiety.89 Finally, the identification of a relationship between measures of structural and functional connectivity in a circuit highly relevant for emotion regulation and anxiety disorders underscores the potential and promise for new discovery that can come about through the integration of independent modalities of imaging data.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institute of Mental Health [R01-MH74847, K02-MH082130, and K08-MH63984 to JBN; T32-MH018931 for DWG; T32-GM007507 and R25-GM083252 for PJH; P50-MH84051 to ALA]; National Science Foundation Graduate Research Fellowship to DWG; and by a core grant to the Waisman Center from the National Institute of Child Health and Human Development [P30-HD03352].
We gratefully acknowledge Nagesh Adluru, Michael Anderle, Ron Fisher, Andrew Fox, Danielle Green, Estrella Montoya, Frank Prado, Amber Sartori, and Erik Wing for their contributions to this project.
Footnotes
Presented in part at American College of Neuropsychopharmacology, 2009, and Anxiety Disorders Association of America, 2010, and Society for Neuroscience, 2010.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Barlow DH. Anxiety and its Disorders. New York: Guilford Press; 2004. [Google Scholar]
- 3.Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am Psychologist. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- 4.Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, Kalin NH. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borkovec TD, Alcaine O, Behar E. Avoidance theory of worry and generalized anxiety disorder. In: Heimberg RG, Turk CL, Mennin DS, editors. Generalized Anxiety Disorder: Advances in Research and Practice. New York: Guilford; 2004. [Google Scholar]
- 6.Mennin DS, Heimberg RG, Turk CL, Fresco DM. Applying an emotion regulation framework to integrative approaches to generalized anxiety disorder. Clin Psychol-Sci Pr. 2002;9:85–90. [Google Scholar]
- 7.Mennin DS, Heimberg RG, Turk CL, Fresco DM. Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behav Res Ther. 2005;43:1281–1310. doi: 10.1016/j.brat.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Mennin DS, Holaway RM, Fresco DM, Moore MT, Heimberg RG. Delineating components of emotion and its dysregulation in anxiety and mood psychopathology. Behav Res Ther. 2007;38:284–302. doi: 10.1016/j.beth.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 11.McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, Fromm S, Charney DS, Leibenluft E, Ernst M, Pine DS. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 12.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Blair RJ, Chen G, Charney DS, Ernst M, Pine DS. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 14.Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, McCaffrey D, Vythilingam M, Finger E, Mondillo K, Jacobs M, Charney DS, Blair RJ, Drevets WC, Pine DS. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: Evidence for separate disorders. Am J Psychiatry. 2008;165:1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Alexander AL, Davidson RJ, Kalin NH. A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry. 2008;63:858–863. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 17.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 19.Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 20.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 21.Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2008;18:1475–1484. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- 22.Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: A clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci. 2009;10:141–152. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- 23.Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, Nitschke JB. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb Cortex. 2010;20:929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarinopoulos I, Dixon GE, Short SJ, Davidson RJ, Nitschke JB. Brain mechanisms of expectation associated with insula and amygdala response to aversive taste: Implications for placebo. Brain Behavior and Immunity. 2006;20:120–132. doi: 10.1016/j.bbi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrides M, Pandya DN. Association pathways of the prefrontal cortex. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. Oxford: Oxford University Press; 2002. [Google Scholar]
- 32.Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- 33.Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: Anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and meyer’s loop of the optic radiation. AJNR Am J Neuroradiol. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- 34.Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 35.Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Lozano AM, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malykhin N, Concha L, Seres P, Beaulieu C, Coupland NJ. Diffusion tensor imaging tractography and reliability analysis for limbic and paralimbic white matter tracts. Psychiatry Res. 2008;164:132–142. doi: 10.1016/j.pscychresns.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, Arfanakis K. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry. 2009;66:691–694. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, Tie K, Gong G, Shah MP, Jones M, Uderman J, Constable RT, Blumberg HP. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66:516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pacheco J, Beevers CG, Benavides C, McGeary J, Stice E, Schnyer DM. Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. J Neurosci. 2009;29:6229–6233. doi: 10.1523/JNEUROSCI.0896-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brambilla P, Como G, Isola M, Taboga F, Zuliani R, Goljecszek S, Ragogna M, Brondani G, Baiano M, Perini L, Ferro A, Bazzocchi M, Zuiani C, Balestrieri M. White-matter abnormalities in the right posterior hemisphere in generalized anxiety disorder: a diffusion imaging study. Psychol Med. 2011 doi: 10.1017/S0033291711001255. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 43.Simmons A, Strigo I, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiat. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 44.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J Cognitive Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 45.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobio. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjami J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 47.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stein MB, Schork NJ, Gelernter J. Gene-by-environment (serotonin transporter and childhood maltreatment) interaction for anxiety sensitivity, an intermediate phenotype for anxiety disorders. Neuropsychopharmacol. 2008;33:312–319. doi: 10.1038/sj.npp.1301422. [DOI] [PubMed] [Google Scholar]
- 49.Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, Weiss RD, Farrer L, Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66:1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flint J, Greenspan R, Kendler K. How Genes Influence Behavior. Oxford: Oxford University Press; 2010. pp. 76–95. [Google Scholar]
- 51.Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU, Glover G, Ballon DJ, Liston C, Teslovich T, Van Kempen T, Lee FS, Casey BJ. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lau JYF, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C, Monk CS, Nelson EE, Shen PH, Pine DS, Ernst M. Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biol Psychiatry. 2009;65:349–355. doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.First MG, Gibbon M, Spitzer R, Williamns J. User’s Guide for the Structured Clinical Interview for the DSM-IV Axis I Disorders - Research Version. New York: Biometrics Research Department; 1996. [Google Scholar]
- 56.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 57.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newman MG, Zuellig AR, Kachin KE, Constantino MJ, Przeworski A, Erickson T. Preliminary reliability and validity of the generalized anxiety disorder questionnaire-IV: A revised self-report diagnostic measure of generalized anxiety disorder. Behavior Therapy. 2002;33:215–233. [Google Scholar]
- 59.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the penn state worry questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 60.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 61.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. MRM. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 63.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. NeuroImage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 65.Mackiewicz KL, Sarinopoulos I, Cleven KL, Nitschke JB. The effect of anticipation and the specificity of sex differences in amygdala and hippocampus function in emotional memory. P Natl Acad Sci USA. 2006;103:14200–14205. doi: 10.1073/pnas.0601648103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 67.Mori S, Crain B, Chacko VP, van Zijl PCM. Three dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 68.Cook PA, Bai Y, Nedjati-Gilani S, Seunarine KK, Hall MG, Parker GJ, Alexander DC. Camino: Open-source diffusion-MRI reconstruction and processing. 14th Scientific Meeting of the International Society for Magnetic Resonance in Medicine; 2006. p. 2759. [Google Scholar]
- 69.Lazar M, Weinstein DM, Tsuruda JS, et al. White Matter Tractography Using Tensor Deflection. Hum Brain Mapp. 2003;18:306–321. doi: 10.1002/hbm.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, Pearlson GD, Melhem ER, Solaiyappan M, Raymond GV, Moser HW, van Zijl PC. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med. 2002;47:215–223. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- 71.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;15:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 72.Friston KJ, Buchel C, Fink GR, Morris J, Rolls E, Dolan R. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 73.Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 74.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Myakishev MV, Khripin Y, Hu S, Hamer DH. High-throughput SNP genotyping by allele-specific PCR with universal energy-transfer-labeled primers. Genome Res. 2001;11:163–169. doi: 10.1101/gr.157901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miller GA, Chapman JA. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- 77.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 78.Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci. 2009:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- 79.Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 81.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 83.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 84.Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 85.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hartley CA, Phelps EA. Changing fear: The neurocircuitry of emotion regulation. Neuropsychopharm Rev. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bevalier D, Levi DM, Roger WL, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;10:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharm Rev. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.