Abstract
Contact tracing, coupled with molecular epidemiologic investigation, is especially useful for identifying an infection with few cases in the population, such as human immunodeficiency virus (HIV) infection in China. No such research is available on Chinese men who have sex with men (MSM). From 2008 to 2010 in Taizhou Prefecture in China, every newly diagnosed HIV-infected MSM was invited to participate as an “index case” in a contact tracing survey by providing contact information for up to 8 sexual contacts, who themselves were approached to receive voluntary HIV counseling and testing. Those who tested HIV-positive were then subjected to another contact tracing survey. This process was repeated until no more sexual contacts were reported or tested positive. A total of 100 HIV-infected MSM served as “index cases,” including the initial 49 cases identified through routine surveillance programs and 51 cases from the present survey. Traced MSM exhibited little willingness to receive voluntary counseling and testing. CRF01_AE (HIV type 1) was the dominant subtype. Seven of 49 independent sexual networks were deemed HIV transmission clusters. Fear of stigma or discrimination may deter Chinese MSM from receiving voluntary counseling and testing. Nonetheless, the integration of behavioral network analysis and HIV phylogenetic analysis provides enhanced evidence for developing tailored prevention strategies for HIV-infected MSM.
Keywords: contact tracing, HIV, human immunodeficiency virus, men who have sex with men, molecular epidemiology, sexual behavior, sexual networks
Contact tracing is a known strategy for controlling the spread of sexually transmitted infections (1). It is especially useful for identifying a sexually transmitted infection with few cases in the population, such as human immunodeficiency virus (HIV) in China (2, 3). With the assistance of an HIV-positive individual, contact tracing would allow for mapping of as many sexual contacts as possible and encouraging those persons to participate in HIV testing and treatment where appropriate (3–6). Each infected contact would then become the starting point for a new process of contact tracing, until no more contacts could be found.
To the best of our knowledge, there have been only 2 studies using contact tracing for HIV in China. Results of both studies were published only in the Chinese-language literature (7, 8); none have been published in the English-language literature. Unfortunately, neither existing study targeted men who have sex with men (MSM), even though the MSM population exhibits the highest incidence of HIV infection in China (9–11). It is estimated that among the 780,000 people who were living with HIV in China in 2011, 71.4% were males and 17.4% had been infected through homosexual transmission (12, 13). Contact tracing can be helpful in identifying behavioral networks and understanding disease transmission or clustering among study participants (3, 14–17). That is, an individual's risk of infection or transmission is influenced by his/her membership in a behavioral network, and an epidemic at the population level is influenced by network structures.
To improve prevention results, molecular epidemiologic investigation—a powerful tool for tracking sources and transmission patterns of a sexually transmitted infection epidemic in a geographic region—should be conducted (18). This 2-pronged approach provides enhanced evidence of transmission links between persons with sexually transmitted infections, which can be used to inform the development of tailored intervention strategies (19, 20). Therefore, in the present study, by tracing the contacts of HIV-infected MSM, we aimed to identify new HIV infections that might otherwise go unrecognized by routine practices and to elucidate transmission patterns in a sample of MSM by using HIV genotyping and phylogenetic analysis.
MATERIALS AND METHODS
Study site
This study was conducted in Taizhou Prefecture, Zhejiang Province, in eastern China. The prefecture has a population of 5.8 million (National Bureau of Statistics of China, unpublished data). By the end of 2010, a total of 656 cases of HIV infection/acquired immunodeficiency syndrome (AIDS) had been diagnosed and registered with the Chinese National Information System for AIDS Prevention and Control.
Study participants and data collection
Every MSM newly diagnosed with HIV infection from 2008 through 2010 in Taizhou Prefecture (n = 60) was invited to participate and gave informed consent to participate as an index case in an egocentric contact tracing survey. The survey requested information concerning the persons with whom the HIV-infected index case had had sex in the past 12 months. Requested information included total number of sexual contacts, gender(s) of the contact(s), nature of the relationship(s), and details on condom use. Each participant was also encouraged to provide detailed contact information for up to 8 sexual contacts, who themselves were asked to participate in this egocentric contact tracing survey and to receive voluntary HIV counseling and testing. Those who tested HIV-positive were then subjected to another egocentric contact tracing survey. This process was repeated until no more sexual contacts tested HIV-positive or no more sexual contacts were reported.
All HIV-positive participants were registered with the Chinese National Information System for AIDS Prevention and Control, which is the official entry point for an HIV/AIDS patient to receive regular follow-up and health care according to national guidelines, as well as free antiretroviral treatment where appropriate. Each participant received ¥30 (about US$5) for travel reimbursement. The study was approved by the institutional review board of Fudan University, Shanghai, China.
Voluntary HIV counseling and testing
The sexual contacts of HIV-infected MSM who were willing to participate in the study received face-to-face pretest counseling (for an average of 30–45 minutes) conducted by a public health professional, followed by a blood draw from an experienced nurse using sterilized needles and sterile tubes. Each plasma sample was coded with a unique identification number, stored at −80°C, and analyzed by 2 experienced laboratory technicians without knowledge of the personal identity of the study participants.
All plasma samples were screened for HIV antibodies using an enzyme-linked immunosorbent assay (Vironostika HIV Uni-Form II Plus O; bioMérieux, Boxtel, the Netherlands) according to the manufacturer's instructions. Participants who screened HIV-positive had their results confirmed by Western blot (Genelabs Diagnostics Pte. Ltd., Singapore, Singapore). All participants received posttest counseling.
HIV genotyping and phylogenetic analysis
RNA extraction/polymerase chain reaction/nucleotide sequencing
RNA extraction, reverse-transcriptase polymerase chain reaction (PCR) amplification, and nucleotide sequencing were performed in physically separated laboratories. Viral RNA was extracted using the QIAamp Viral RNA Mini Kit (Hoffmann-La Roche, Branchburg, New Jersey) according to the manufacturer's instructions. Extracted RNA was reverse-transcribed into cDNA (TaKaRa Biotechnology Company Ltd., Dalian, China). The cDNA was used as the template for PCR amplification of 2 HIV type 1 subgenomic regions (the env C2V3V4 region and the gag p17/p24 junction) by means of nested PCR. The primers and conditions of PCR for env and gag applied in this study were as previously described in the literature (21–25), including priority and backup primers for each amplification. The PCR reaction was carried out in 20 μL of solution containing PCR reagents (TaKaRa Biotechnology Company), primers (priority primers were used first and, if there was no amplification, backup primers were then used), and an HIV cDNA template. The thermal profile included a first-round amplification of the outer fragment involving 5 minutes at 95°C, followed by 40 cycles of 1 minute at 95°C, 1 minute at 42°C, 30 seconds at 72°C, and a final elongation step of 15 minutes at 72°C; and then a second-round amplification of the inner fragment involving 5 minutes at 95°C, followed by 40 cycles of 1 minute at 95°C, 50 seconds at 48°C, 50 seconds at 72°C, and a final elongation step of 15 minutes at 72°C.
PCR reactions and the thermal profile for gag amplification were the same as those for env. Relevant positive and negative controls were always included to avoid false-positive results in the PCR. PCR products were sequenced using the ABI PRISM 3730xl DNA Analyzer (Applied Biosystems, New York, New York).
Phylogenetic analysis
Nucleotide sequences of amplified gag and env regions from all HIV-infected participants, together with other nonclustered control sequences, were aligned using the ClustalX program in MEGA software, version 4.1 (Center for Evolutionary Medicine and Informatics, The Biodesign Institute, Tempe, Arizona). Phylogenetic and molecular evolutionary analyses were conducted using MEGA software, version 4.1. Evolutionary distances were calculated using Kimura 2-parameter modeling, excluding positions with alignment gaps in any sequence. Phylogenetic dendrograms were constructed using the neighbor-joining method with Kimura 2-parameter modeling. The reliability of each node was evaluated by means of bootstrapping with 2,000 replicates.
Statistical methods
In addition to descriptive statistics, identification of potential HIV transmission pairs was based on both behavioral and molecular connections between them. Two HIV-infected participants were determined to be a potential HIV transmission pair if they had had unprotected anal and/or vaginal sex and if they had a genetic distance of 1.5% or less between their gag and/or env gene sequences (26–28). An HIV transmission cluster was defined as a group or sexual network involving at least 1 potential HIV transmission pair. For all analyses, we set the significance level for P values at 0.05.
RESULTS
Flow of contact tracing and identification of HIV infections
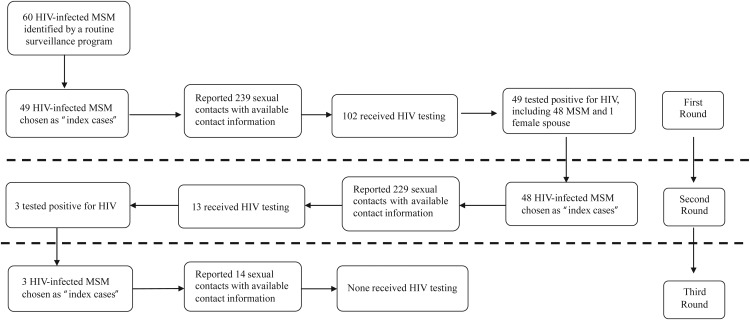
The whole process of contact tracing and identification of HIV infections is summarized in Figure 1 and Table 1. In the first round, 49 (81.7%) of 60 HIV-infected MSM who were identified from routine HIV surveillance programs reported a total of 799 persons with whom they had had anal and/or vaginal sex in the past 12 months, and they were able to provide contact information for 239 (29.9%) of those 799 sexual contacts. Of this group of 239 contacts, 102 (42.7%) received HIV testing, of whom 49 (including 1 female spouse of an MSM) tested positive for HIV. All 48 newly identified HIV-infected MSM cases served as “index cases” in the second round of the contact tracing survey. They reported having had a total of 698 sexual contacts in the past 12 months and were able to provide contact information for 229 (32.8%) of those 698 contacts. Of this group of 229 contacts, only 13 (5.7%) received HIV testing, of whom 3 (23.1%) tested positive. These 3 newly identified HIV cases served as “index cases” in the third round of the contact tracing survey, reporting a total of 37 sexual contacts and providing contact information for 14 (37.8%) of those contacts. None of them received HIV testing.
Figure 1.
Tracing of risky sexual contacts of human immunodeficiency virus (HIV)-infected men who had sex with men (MSM) in Taizhou Prefecture, eastern China, 2008–2010.
Table 1.
HIV Testing and HIV Prevalence Among Reported Sexual Contacts of HIV-infected MSM Index Cases Who Had Available Contact Information, Taizhou Prefecture, Eastern China, 2008–2010
| Type of Sexual Contact | No. of Sexual Contacts | % | First Round (Na = 49, nb = 239) |
Second Round (N = 48, n = 229) |
Third Round (N = 3, n = 14) |
Total (N = 100, n = 482) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Tested for HIV | No. Tested/No. of Sexual Contacts | HIV Prevalence, % | No. HIV-Positive/No. Tested | % Tested for HIV | No. Tested/No. of Sexual Contacts | HIV Prevalence, % | No. HIV-Positive/No. Tested | % Tested for HIV | No. Tested/No. of Sexual Contacts | HIV Prevalence, % | No. HIV-Positive/No. Tested | % Tested for HIV | No. Tested/No. of Sexual Contacts | HIV Prevalence, % | No. HIV-Positive/No. Tested | |||
| Spouse or long-term female partner | 48 | 10.0 | 56.7 | 17/30 | 5.9 | 1/17 | 18.8 | 3/16 | 0.0 | 0/3 | 0.0 | 0/2 | 0.0 | 0/0 | 41.7 | 20/48 | 5.0 | 1/20 |
| Noncommercial casual female partner | 5 | 1.0 | 0.0 | 0/3 | 0.0 | 0/0 | 0.0 | 0/2 | 0.0 | 0/0 | 0.0 | 0/0 | 0.0 | 0/0 | 0.0 | 0/5 | 0.0 | 0/0 |
| Noncommercial casual male partner | 180 | 37.3 | 1.3 | 1/77 | 0.0 | 0/1 | 0.0 | 0/96 | 0.0 | 0/0 | 0.0 | 0/7 | 0.0 | 0/0 | 0.6 | 1/180 | 0.0 | 0/1 |
| Commercial male partner | 106 | 22.0 | 6.3 | 3/48 | 66.7 | 2/3 | 1.8 | 1/57 | 100.0 | 1/1 | 0.0 | 0/1 | 0.0 | 0/0 | 3.8 | 4/106 | 75.0 | 3/4 |
| Long-term male partner | 143 | 29.7 | 100.0 | 81/81 | 56.8 | 46/81 | 15.5 | 9/58 | 22.2 | 2/9 | 0.0 | 0/4 | 0.0 | 0/0 | 62.9 | 90/143 | 53.3 | 48/90 |
| Total | 482 | 100.0 | 42.7 | 102/239 | 48.0 | 49/102 | 5.7 | 13/229 | 23.1 | 3/13 | 0.0 | 0/14 | 0.0 | 0/0 | 23.9 | 115/482 | 45.2 | 52/115 |
Abbreviations: HIV, human immunodeficiency virus; MSM, men who have sex with men.
a Number of HIV-infected MSM chosen as index cases.
b Number of reported sexual contacts who had available contact information.
In sum, a total of 100 HIV-infected MSM served as “index cases” in the survey, including 49 cases who were identified through routine surveillance programs and 51 cases who were newly identified using the present contact tracing survey. These 100 MSM reported a total of 1,534 sexual contacts and were able to provide contact information for 482 (31.4%) of those contacts, of whom none appeared in multiple rounds in this study and only 115 (23.9%) actually received HIV testing. Among the 115 contacts who actually received HIV testing, 27 (23.5%)—3 commercial sex partners and 24 long-term male partners—had previously tested negative in the past 12 months. Among those 27 contacts, 8 contacts (29.6%)—2 commercial sex partners and 6 long-term male partners—newly tested HIV-positive in this study. The proportion of contactable sexual contacts who actually received HIV testing was relatively high (62.9%) among long-term male partners and among spouses or long-term female partners (41.7%). A very high proportion (53.3%) of long-term male partners also tested positive for HIV.
Sociodemographic characteristics of HIV-infected MSM
The mean age of the 100 HIV-infected MSM was 30.3 years (standard deviation, 9.3). Among these men, 57.0% were aged 19–29 years, 42.0% were currently married, 67.0% had at least a high school education, and 66.0% were officially registered as local residents or held official permanent residency status (“hukou”) at the study site (Table 2). Sociodemographic characteristics such as age, marital status, and educational level were not significantly different between participants who were local residents of Taizhou City and those who were migrants or nonlocal residents.
Table 2.
Sociodemographic Characteristics, Sexual Behaviors, and HIV Genotyping of HIV-infected MSM in Taizhou Prefecture, Eastern China, 2008–2010
| Characteristic | χ2 | P Value | Local Residents (n1a = 66; 66%) |
Nonlocal Residents (n2a = 34; 34%) |
Total (N = 100; 100%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | % Successfully Genotyped | No. | % | % Successfully Genotyped | No. | % | % Successfully Genotyped | |||
| Age, years | 4.037 | 0.258 | |||||||||
| 19–29 | 37 | 56.1 | 20 | 58.8 | 57 | 57.0 | |||||
| 30–39 | 19 | 28.8 | 10 | 29.4 | 29 | 29.0 | |||||
| 40–49 | 4 | 6.1 | 4 | 11.8 | 8 | 8.0 | |||||
| 50–62 | 6 | 9.1 | 0 | 0.0 | 6 | 6.0 | |||||
| Marital status | 0.492 | 0.782 | |||||||||
| Never married | 35 | 53.0 | 18 | 52.9 | 53 | 53.0 | |||||
| Currently married | 27 | 40.9 | 15 | 44.1 | 42 | 42.0 | |||||
| Divorced/widowed | 4 | 6.1 | 1 | 2.9 | 5 | 5.0 | |||||
| Education | 3.542 | 0.170 | |||||||||
| Elementary school | 1 | 1.5 | 2 | 5.9 | 3 | 3.0 | |||||
| Middle school | 17 | 25.8 | 13 | 38.2 | 30 | 30.0 | |||||
| High school or above | 48 | 72.7 | 19 | 55.9 | 67 | 67.0 | |||||
| No. of sexual partners in the past year | 1.256 | 0.534 | |||||||||
| 2–9 | 39 | 59.1 | 17 | 50.0 | 56 | 56.0 | |||||
| 10–19 | 13 | 19.7 | 10 | 29.4 | 23 | 23.0 | |||||
| ≥20 | 14 | 21.2 | 7 | 20.6 | 21 | 21.0 | |||||
| Condom use for sex with male partners in the past year | 0.19 | 0.663 | |||||||||
| Consistent | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |||||
| Inconsistent | 54 | 81.8 | 29 | 85.3 | 83 | 83.0 | |||||
| Never | 12 | 18.2 | 5 | 14.7 | 17 | 17.0 | |||||
| Condom use for sex with female partners in the past yearb | 0.373 | 0.541 | 35 | 21 | 56 | ||||||
| Consistent | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |||||
| Inconsistent | 9 | 25.7 | 7 | 33.3 | 16 | 28.6 | |||||
| Never | 26 | 74.3 | 14 | 66.7 | 40 | 71.4 | |||||
| HIV genotyping | |||||||||||
| env-based genotyping | 3.747 | 0.154 | |||||||||
| Proportion successfully genotyped | 43 | 65.2 | 21 | 61.8 | 64 | 64.0 | |||||
| CRF01_AE | 33 | 76.7 | 12 | 57.1 | 45 | 70.3 | |||||
| CRF07_BC | 7 | 16.3 | 8 | 38.1 | 15 | 23.4 | |||||
| B | 3 | 7.0 | 1 | 4.8 | 4 | 6.3 | |||||
| gag-based genotyping | 1.730 | 0.630 | |||||||||
| Proportion successfully genotyped | 41 | 62.1 | 25 | 73.5 | 66 | 66.0 | |||||
| CRF01_AE | 28 | 68.3 | 17 | 68.0 | 45 | 68.2 | |||||
| CRF07_BC | 7 | 17.1 | 4 | 16.0 | 11 | 16.7 | |||||
| B | 6 | 14.6 | 3 | 12.0 | 9 | 13.6 | |||||
| CRF08_BC | 0 | 0.0 | 1 | 4.0 | 1 | 1.5 | |||||
Abbreviations: CRF, circulating recombinant form; HIV, human immunodeficiency virus; MSM, men who have sex with men.
a Number of HIV-infected MSM.
b Fifty-six HIV-infected MSM had had female partners in the past year; for 48 (85.7%) of them, female partners were spouses only.
Sexual behaviors and sexual networks of HIV-infected MSM
More than half (56.0%) of the HIV-infected MSM participants had had both male and female sexual partners in the past year; 23.0% had had 10–19 sexual partners and 21.0% had had 20 or more. The median number of sexual partners in the past year was 8, and the range was 2–79. During sex with male partners in the past year, none of the HIV-infected MSM participants had consistently used condoms; 83.0% reported inconsistent condom use, and 17.0% reported never using condoms. During sex with female partners, none of the HIV-infected MSM participants had consistently used condoms; 28.6% reported inconsistent condom use, and 71.4% reported never using condoms (Table 2).
The 100 HIV-infected MSM participants reported a total of 482 sexual contacts with full tracing information. The majority (89.0%) of these sexual contacts were male sexual partners (Table 1).
By following the flow of the contact tracing survey, we constructed 49 independent sexual networks for the HIV-infected MSM and their sexual contacts (see Web Figure 1, available at http://aje.oxfordjournals.org/). These include 35 networks with 2 or more HIV-infected persons within each network and 14 networks with only 1 HIV-infected MSM within each network.
HIV genotyping
All 100 HIV-infected MSM received HIV genotyping. Among them, 28 (28.0%) could not be genotyped because of an undetectable viral load. The other 72 MSM (72.0%) were successfully genotyped by means of either the env or the gag gene sequence, 58 (58.0%) were genotyped by both env and gag genes, 6 (6.0%) were genotyped by env only, and 8 (8.0%) were genotyped by gag only. The proportion of HIV-infected MSM who were successfully genotyped was 77.6% (38/49) of the initial index cases and 66.7% (34/51) of the persons with newly identified infections in the contact tracing survey, including 32 (66.7% of 48) long-term male partners and 2 (66.7% of 3) commercial male partners. The results of HIV genotyping are presented in detail in Table 2 and Web Figures 1 and 2.
The HIV-positive wife of an HIV-infected MSM was genotyped as CRF01_AE by means of both the env and gag genes. CRF01_AE was the dominant HIV subtype among HIV-infected MSM, accounting for 71.9% (46/64) of those genotyped by the env gene and 69.7% (46/66) of those genotyped by the gag gene. The distribution of HIV subtypes did not differ significantly by residency (Table 2) and did not differ significantly according to whether or not the participant had had female sex partners in the past year (data not shown). On the other hand, 6 (10.3%) of the 58 HIV-infected MSM who were genotyped by means of both the env and gag genes were genotyped with discordant HIV subtypes based on env and gag gene sequences—1 with B(env)/CRF01_AE(gag), 1 with CRF01_AE(env)/B(gag), and 4 with CRF07_BC(env)/B(gag). The HIV genotypes of genotyped HIV-infected MSM are shown in Web Figure 1.
Identification of potential HIV transmission pairs and clusters
As Web Figure 1 shows, 7 of the 49 independent sexual networks were determined to be potential HIV transmission pairs or clusters according to the behavioral and molecular criteria (Web Figure 1a). There were also 16 networks with behaviorally linked (i.e., having unprotected sex) HIV-infected MSM pairs or clusters who either had discordant HIV subtypes (3 clusters) or had the same HIV subtype but large HIV genetic distances (13 pairs or clusters) (Web Figure 1b). Phylogenetic trees of these HIV subtypes are presented in Web Figure 2. As expected, phylogenetic analysis indicated that the nucleotide sequences from the 7 potential HIV transmission pairs were clustered together for env and gag genes.
DISCUSSION
To the best of our knowledge, this was the first study in China to identify HIV infections through risky behavioral networks of HIV-infected MSM, coupled with traced HIV transmission pairs or clusters based on molecular evidence. The study showed that contact tracing is a useful strategy for identifying new HIV infections in MSM and their spouses or long-term sexual partners whose HIV infection status might have otherwise remained unrecognized; such a strategy, however, was unsuccessful in bringing commercial or noncommercial casual sex partners of HIV-infected MSM in for HIV testing because of the unavailability of their contact information. Nonetheless, our findings retain public health significance, since knowing one's HIV status is the first step toward accessing care and preventing further infection (29).
Population system epidemiology illustrates that systems of interacting individuals rather than independent individuals determine the infection transmission models (15). The structure of sexual contact networks plays a key role in the epidemiology of sexually transmitted infections (including HIV) (30). In the present study, HIV-infected MSM had multiple sexual partners and complex sexual networks in which condoms were rarely used, and thus were at high risk of HIV transmission. Moreover, more than 40% of the HIV-infected MSM had ever been married or maintained long-term heterosexual relationships in which the rate of condom use was very low. These men, therefore, might have played an important bridging role in transmitting HIV to the general female population. In fact, 1 (5%) of 20 female spouses or long-term partners of HIV-infected MSM in the study tested positive for HIV.
On the other hand, since HIV is characterized by high genetic variability and has a number of subtypes or mosaic strains known as circulating recombinant forms (CRF) which show specific geographical distributions and transmission patterns, HIV genotyping and phylogenetic analysis have been used extensively in tracing the source of the epidemic. In China, although the HIV epidemic started with subtype B′ among injection drug users in Yunnan Province in the early 1990s (31), it has now spread to all populations, with a variety of HIV subtypes and CRFs. Among them, subtypes B′, CRF07_BC, and CRF01_AE are postulated to be responsible for most of the recent cases in China (32, 33). Nevertheless, little is known about the molecular epidemiology and transmission patterns of HIV among MSM in China. Contrary to this observation, our findings revealed that CRF01_AE was found to be the dominant subtype of HIV type 1 among MSM. Moreover, CRF01_AE is also a highly prevalent HIV subtype for heterosexual transmission in China (13), suggesting a mixture of HIV transmission between homosexual and heterosexual people.
Perhaps the most significant contribution of this study is its combination of both behavioral and molecular evidence to identify HIV transmission pairs or clusters in the MSM population and beyond. Previous studies have been based primarily on either behavioral connections or molecular connections but not both, in order to determine patterns or locate sources of HIV transmission (34, 35). However, the lengthy infectious period and low risk of HIV infection have made the interpretation of sexual networks difficult. Consequently, the most common network data in HIV epidemiology indicate only the class of persons epidemiologically contacted rather than the particular individuals involved in HIV transmission. In this study, the integration of behavioral network analysis and phylogenetic analysis of genotyped HIV strains provides enhanced evidence for studying HIV transmission between HIV-infected MSM and their partners. Although 7 networks were determined to be potential HIV transmission pairs or clusters in this study, phylogenetic analysis of 3 of the networks revealed that 2 or more HIV-infected MSM were actually infected with different subtypes of HIV and thus had different sources of HIV transmission. Nevertheless, unprotected sex between persons infected with different HIV subtypes opens the possibility for mixture of different HIV subtypes and development of new recombinants (36). In fact, 6 HIV-infected participants in this study were genotyped with discordant HIV subtypes based on env or gag gene sequences, respectively, indicating the existence of mixed HIV infections in the study sample. This molecular epidemiologic information is essential not only for the design of prevention strategies but also for guiding the design of vaccine approaches in the targeted region.
We were unable to contact a substantial proportion of reported sexual contacts, and among those who were contactable, the proportion willing to receive voluntary HIV counseling and testing was very low. There could be a variety of reasons for this unwillingness, particularly stigma and discrimination regarding both homosexual behavior and HIV infection status and low awareness of HIV risks (37, 38). This is a serious challenge not only for the success of contact tracing as a strategy for identifying new HIV infections but more importantly for successful control of the HIV/AIDS epidemic (e.g., partner service) (37–40). In this regard, tremendous efforts such as extensive health education and intensive counseling are needed to eliminate stigma or discrimination associated with homosexual behavior and HIV infection and thus promote risk awareness and partner notification among MSM. Alternative strategies for HIV testing targeting MSM should also be explored. Another limitation of this study was the relatively low rate of HIV genotyping, which was very likely due to undetectable plasma HIV viral load among some participants who might be at the set-point stage of the disease with well-controlled viral replication. Behavioral networks and molecular epidemiologic studies involving large samples of HIV-infected persons with different transmission modes and different dominances of HIV subtypes in various regions are needed to gain a thorough understanding of HIV transmission and successfully control the epidemic in China.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, School of Public Health, Fudan University, Shanghai, China (Haijiang Lin, Na He, Sujuan Zhou, Yingying Ding, Tiejun Zhang); Key Laboratory of Public Health Safety (Fudan University), Ministry of Education, Shanghai, China (Haijiang Lin, Na He, Sujuan Zhou, Yingying Ding, Tiejun Zhang); Taizhou City Center for Disease Control and Prevention, Taizhou City, Zhejiang Province, China (Haijiang Lin, Danhong Qiu); Department of Behavioral Sciences and Health Education, Rollins School of Public Health, Emory University, Atlanta, Georgia (Na He, Frank Y. Wong); and The Hubert Department of Global Health, Rollins School of Public Health, Emory University, Atlanta, Georgia (Frank Y. Wong).
This work was supported by the Shanghai Municipal Health Bureau (grant XBR2011043), the Shanghai Leading Disciplinary Project (grant B118), the Chinese National Major Science and Technology Project of Infectious Diseases (grant 2008ZX10001-003), the US National Institutes of Health (grant R01HD056956), and the Emory Center for AIDS Research (grant P30 AI050409).
The authors thank Brian Smith for his editorial assistance.
Conflict of interest: none declared.
REFERENCES
- 1.Winfield J, Latif AS. Tracing contacts of persons with sexually transmitted diseases in a developing country. Sex Transm Dis. 1985;12(1):5–7. doi: 10.1097/00007435-198501000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kimbrough LW, Fisher HE, Jones KT, et al. Accessing social networks with high rates of undiagnosed HIV infection: the Social Networks Demonstration Project. Am J Public Health. 2009;99(6):1093–1099. doi: 10.2105/AJPH.2008.139329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klovdahl AS. Social networks and the spread of infectious diseases: the AIDS example. Soc Sci Med. 1985;21(11):1203–1216. doi: 10.1016/0277-9536(85)90269-2. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Use of social networks to identify persons with undiagnosed HIV infection—seven U.S. cities, October 2003–September 2004. MMWR Morb Mortal Wkly Rep. 2005;54(24):601–605. [PubMed] [Google Scholar]
- 5.Heffelfinger JD, Sullivan PS, Branson BM, et al. Advancing HIV prevention demonstration projects: new strategies for a changing epidemic. Public Health Rep. 2008;123(3):5–15. doi: 10.1177/00333549081230S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wykoff RF, Heath CW, Hollis SL, et al. Contact tracing to identify human immunodeficiency virus infection in a rural community. JAMA. 1988;259(24):3563–3566. [PubMed] [Google Scholar]
- 7.Ye R, Xiang L, Yang Y, et al. Tracing the sources of newly reported HIV infections in Dehong prefecture of Yunnan province. Zhonghua Liu Xing Bing Xue Za Zhi (Chin J Epidemiol) 2010;31(1):39–42. [PubMed] [Google Scholar]
- 8.Lin H, Feng J, Wu Q, et al. Risk behavioral networks of newly reported HIV infections in Taizhou prefecture, Zhejiang province. Zhonghua Liu Xing Bing Xue Za Zhi (Chin J Epidemiol) 2010;31(11):1227–1230. [PubMed] [Google Scholar]
- 9.Wang L, Wang L, Ding ZW, et al. HIV prevalence among populations at risk, using sentinel surveillance data from 1995 to 2009 in China. Zhonghua Liu Xing Bing Xue Za Zhi (Chin J Epidemiol) 2011;32(1):20–24. [PubMed] [Google Scholar]
- 10.Li H, Peng R, Li J, et al. HIV incidence among men who have sex with men in China: a meta-analysis of published studies. PLoS ONE. 2011;6(8):e23431. doi: 10.1371/journal.pone.0023431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, Wang Y. Introduction: China meets new AIDS challenges. J Acquir Immune Defic Syndr. 2010;53(1):S1–S3. doi: 10.1097/QAI.0b013e3181c7d379. [DOI] [PubMed] [Google Scholar]
- 12.Ministry of Health, People's Republic of China; Joint United Nations Programme on HIV/AIDS; World Health Organization. 2011 Estimates for the HIV/AIDS Epidemic in China. Beijing, China: Ministry of Health, People's Republic of China; 2012. http://www.chinaids.org.cn/n16/n1193/n4073/n745902.files/n745901.pdf. (Accessed January 29, 2012) [Google Scholar]
- 13.Wang N. Some new trends of HIV/AIDS epidemic in China. Zhonghua Liu Xing Bing Xue Za Zhi (Chin J Epidemiol) 2010;31(11):1205–1209. [PubMed] [Google Scholar]
- 14.Rothenberg RB, Potterat JJ, Woodhouse DE, et al. Social network dynamics and HIV transmission. AIDS. 1998;12(12):1529–1536. doi: 10.1097/00002030-199812000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Koopman JS, Lynch JW. Individual causal models and population system models in epidemiology. Am J Public Health. 1999;89(8):1170–1174. doi: 10.2105/ajph.89.8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eames KT, Keeling MJ. Monogamous networks and the spread of sexually transmitted diseases. Math Biosci. 2004;189(2):115–130. doi: 10.1016/j.mbs.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Eames KT, Keeling MJ. Modeling dynamic and network heterogeneities in the spread of sexually transmitted diseases. Proc Natl Acad Sci USA. 2002;99(20):13330–13335. doi: 10.1073/pnas.202244299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghina M, Nahla H, Francisca AA, et al. HIV-1 molecular epidemiology evidence and transmission patterns in the Middle East and North Africa. Sex Transm Infect. 2011;87(2):101–106. doi: 10.1136/sti.2010.043711. [DOI] [PubMed] [Google Scholar]
- 19.Lewis F, Hughes GJ, Rambaut A, et al. Episodic sexual transmission of HIV revealed by molecular phylodynamics. PLoS Med. 2008;5(3):e50. doi: 10.1371/journal.pmed.0050050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilcher CD, Wong JK, Pillai SK. Inferring HIV transmission dynamics from phylogenetic sequence relationships. PLoS Med. 2008;5(3):e69. doi: 10.1371/journal.pmed.0050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Li S, Li X, et al. Characterization of HIV-1 subtypes and viral antiretroviral drug resistance in men who have sex with men in Beijing, China. AIDS. 2007;21(8):S59–S65. doi: 10.1097/01.aids.0000304698.47261.b1. [DOI] [PubMed] [Google Scholar]
- 22.Delwart EL, Herring B, Rodrigo AG, et al. Genetic subtyping of human immunodeficiency virus using a heteroduplex mobility assay. PCR Methods Appl. 1995;4(5):S202–S216. doi: 10.1101/gr.4.5.s202. [DOI] [PubMed] [Google Scholar]
- 23.Fischetti L, Opare-Sem O, Candotti D, et al. Molecular epidemiology of HIV in Ghana: dominance of CRF02_AG. J Med Virol. 2004;73(2):158–166. doi: 10.1002/jmv.20070. [DOI] [PubMed] [Google Scholar]
- 24.Xin R, Feng Y, Chen C, et al. Primers of gag gene for HIV-1 subtyping in China and application thereof in practice. Natl Med J China. 2009;89(13):876–880. [PubMed] [Google Scholar]
- 25.Zhao F, Wang Z, Li W. Human immunodeficiency virus type 1 subtypes prevalence in central China. Yonsei Med J. 2009;50(5):644–649. doi: 10.3349/ymj.2009.50.5.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bezemer D, van Sighem A, de Wolf F, et al. Combination antiretroviral therapy failure and HIV super-infection. AIDS. 2008;22(2):309–311. doi: 10.1097/QAD.0b013e3282f37489. [DOI] [PubMed] [Google Scholar]
- 27.Hue S, Clewley JP, Cane PA, et al. HIV-1 pol gene variation is sufficient for reconstruction of transmissions in the era of antiretroviral therapy. AIDS. 2004;18(5):719–728. doi: 10.1097/00002030-200403260-00002. [DOI] [PubMed] [Google Scholar]
- 28.Bezemer D, van Sighem A, Lukashov VV, et al. Transmission networks of HIV-1 among men having sex with men in the Netherlands. AIDS. 2010;24(2):271–282. doi: 10.1097/QAD.0b013e328333ddee. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Increasing Access to HIV Testing and Counseling. Report of a WHO Consultation. Geneva, Switzerland:: 2002. World Health Organization http://www.who.int/hiv/pub/vct/pub36/en/index.html. (Accessed November 19–21, 2002) [Google Scholar]
- 30.Kretzschmar M. Sexual network structure and sexually transmitted disease prevention: a modeling perspective. Sex Transm Dis. 2000;27(10):627–635. doi: 10.1097/00007435-200011000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Graf M, Shao Y, Zhao Q, et al. Cloning and characterization of a virtually full-length HIV type 1 genome from a subtype B′-Thai strain representing the most prevalent B-clade isolate in China. AIDS Res Hum Retroviruses. 1998;14(3):285–288. doi: 10.1089/aid.1998.14.285. [DOI] [PubMed] [Google Scholar]
- 32.Lu L, Jia M, Ma Y, et al. The changing face of HIV in China. Nature. 2008;455(7213):609–611. doi: 10.1038/455609a. [DOI] [PubMed] [Google Scholar]
- 33.Yu X, Yuan L, Huang Y, et al. Susceptibility of HIV-1 subtypes B′, CRF07_BC and CRF01_AE that are predominantly circulating in China to HIV-1 entry inhibitors. PLoS One. 2011;6(3):e17605. doi: 10.1371/journal.pone.0017605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng W, Fu P, Bao L, et al. Molecular epidemiological tracing of HIV-1 outbreaks in Hainan Island of southern China. AIDS. 2009;23(8):977–985. doi: 10.1097/QAD.0b013e328329217d. [DOI] [PubMed] [Google Scholar]
- 35.Bao L, Vidal N, Fang H, et al. Molecular tracing of sexual HIV type 1 transmission in the southwest border of China. AIDS Res Hum Retroviruses. 2008;24(5):733–742. doi: 10.1089/aid.2007.0269. [DOI] [PubMed] [Google Scholar]
- 36.Kijak GH, McCutchan FE. HIV diversity, molecular epidemiology, and the role of recombination. Curr Infect Dis Rep. 2005;7(6):480–488. doi: 10.1007/s11908-005-0051-8. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan SG, Wu Z, Detels R. Missed opportunities for HIV testing and counselling in Asia. AIDS. 2010;24(3):S49–S53. doi: 10.1097/01.aids.0000390089.60682.26. [DOI] [PubMed] [Google Scholar]
- 38.Wu Z, Sun X, Sullivan SG, et al. HIV testing in China. Science. 2006;312(9):1475–1476. doi: 10.1126/science.1120682. [DOI] [PubMed] [Google Scholar]
- 39.Ma W, Detels R, Feng Y, et al. Acceptance of and barriers to voluntary HIV counselling and testing among adults in Guizhou province, China. AIDS. 2007;21(8):S129–S135. doi: 10.1097/01.aids.0000304708.64294.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou L, Guo J, Fan L, et al. Survey of motivation for use of voluntary counseling and testing services for HIV in a high risk area of Shenyang, China. BMC Health Serv Res. 2009;9:e23. doi: 10.1186/1472-6963-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.