Abstract
Background/Objectives
Diabetes mellitus is a strong risk factor for functional decline in older patients. It is unclear whether Hemoglobin A1c (HbA1c) levels predict functional decline.
Design
Longitudinal cohort study
Participants
Community-dwelling, nursing-home (NH) eligible patients with diabetes enrolled at On Lok between 10/2002 and 12/2008 (367 patients, 1579 HbA1c measurements).
Setting
On Lok Lifeways, the original model for Programs of All-Inclusive Care for the Elderly (PACE).
Measurements
The outcomes were 1) functional decline and 2) functional decline or death at 2 years. Our primary predictor was HbA1c. We adjusted for age, gender, race/ethnicity, baseline function, comorbid conditions, length of time enrolled at On Lok, insulin use, as well as the clustering of HbA1c within patients with mixed-effects Poisson regression.
Results
Mean age was 80 years and 185 patients (50%) were taking insulin. Sixty-three percent of our participants experienced functional decline and 75% experienced death or functional decline during the study period. At 2 years, higher HbA1c was associated with less functional decline or death (p for trend=0.006). Accounting for clustering and confounding factors, HbA1c 8–8.9% was associated with a decreased relative risk of functional decline or death compared to HbA1c 7–7.9% (0.88, 95% CI: 0.79–0.99).
Conclusion
Among community-dwelling, NH-eligible patients with diabetes, HbA1c of 8–8.9% is associated with better functional outcomes at 2 years compared to HbA1c of 7–7.9%. Our results suggest that the current AGS guideline recommending a HbA1c target of ≤8% for older patients with limited life expectancy may be lower than necessary to maintain function.
Keywords: Glycemic control, functional decline, Hemoglobin A1c
INTRODUCTION
Diabetes mellitus is common in older Americans and is strongly associated with disability and functional decline. In 2004, an estimated 324,000 Americans with diabetes were living in nursing homes1 and a similar number of nursing-home (NH) eligible elders with diabetes lived in the community with formal and informal caregiver support.2 Further, this growing population of older patients with diabetes has a two-fold increased risk of disability and functional decline compared to older patients without diabetes.3–5 The increased risk of functional decline is especially important in older adults since function has been shown to be strongly associated with outcomes such as health-related quality of life,6,7 nursing home placement, mortality and cost.8,9
Although the presence of diabetes appears to be a strong risk factor for functional decline, it is unclear if the level of glycemic control affects functional outcomes. Poor glycemic control may lead to worsening neuropathy, malaise, urinary incontinence and malnutrition leading to functional decline.10 Conversely, tight glycemic control may lead to more frequent hypoglycemia or falls, leading to functional decline.11–15 Thus, both tight glycemic control and poor glycemic control may lead to functional decline; however, it is currently unknown whether any association exists in NH-eligible elders who are at highest risk for functional decline. Determining the level of glycemic control that is associated with best functional outcomes would help providers determine the most appropriate glycemic target for older patients with diabetes.
Thus, we examined the relationship between Hemoglobin A1c (HbA1c) levels and 2 year functional decline in community-dwelling, NH-eligible older adults with diabetes enrolled at On Lok Lifeways, the original model for Programs of All-Inclusive Care for the Elderly (PACE). We hypothesized that PACE enrollees with diabetes who were able to achieve the American Geriatrics Society (AGS) recommended HbA1c target of ≤8%16 would have less functional decline than those whose HbA1c is >8%.
METHODS
Participants
We conducted a longitudinal cohort study using repeated measures of glycemic control and functional decline in On Lok enrollees with diabetes mellitus between October 2002 (when an electronic medical record system was implemented) and December 2008. On Lok, the original model for the PACE program, requires enrollees to be certified as “NH-eligible,” indicating that the participant requires ongoing skilled help and is unable to live independently.17,18 On Lok helps NH-eligible enrollees remain in the community by providing and coordinating all healthcare services including primary and specialist physician services, adult day health care, home care, acute and post-acute hospitalization and custodial nursing home care for those who require it. Patients receive health services in PACE centers which comprise of co-located adult day health centers and medical clinics. The program provides participants transportation to PACE centers where the following services are provided: meals, medication management, help with bathing/showering, recreational activities, physical and occupational therapy, social work and nursing and physician services. Each participant receives a comprehensive health assessment upon enrollment and every six months thereafter. These assessments include medical evaluations and functional assessments performed by nurses, occupational therapists and physical therapists.
Participants were included in the study if they 1) were enrolled at On Lok at the start of the study period or entered On Lok between October 2002 and December 2006 (allowing for 24 months follow-up), 2) had a diagnosis of diabetes mellitus identified by ICD-9 code (250.xx), and 3) had at least one HbA1c value between October 2002 and December 2006 (464 participants with 2144 HbA1c measurements). Since enrollment at On Lok is often associated with medication and functional changes, HbA1c measurements were excluded if a) they were collected less than 30 days after the initial enrollment (159 measurements excluded), or b) corresponding baseline functional assessments were collected less than 30 days after initial enrollment (116 measurements excluded). HbA1c measurements were also excluded if functional data was unavailable due to no baseline functional assessment within 6 months before the HbA1c measurement (64 measurements excluded), disenrollment before 24 month follow-up (100 measurements), or follow-up functional data were missing (75 measurements). Finally, measurements were also excluded if participants were enrolled in end-of-life care (ICD9: V66.7) (51 measurements excluded), leading to our final analytic cohort of 1579 HbA1c measurements from 367 enrollees.
Measures: Outcomes
The co-primary outcomes of our study were 1) functional decline and 2) functional decline or death within 2 years. Functional decline was defined as a decline in Activity of Daily Living (ADL) score at follow-up compared to baseline. The ADL score (range 0–10) was determined by combining each enrollee's level of functioning in 5 basic ADLs (bathing, dressing, toileting, transferring and eating) as independent (2 points), partially dependent (1 point) or completely dependent (0 points). Baseline ADL score was defined as the closest ADL score within 6 months prior to the HbA1c measurement and the follow-up ADL score was defined as the ADL measurement 24 ± 3 months after the HbA1c measurement. For patients who died within the 24 month follow-up period, functional decline was determined by comparing their baseline and last ADL score before death.
Because our ADL assessments occurred every 6 months, participants who suffer less than 6 months of functional decline before death may be missed using our definition of functional decline. Thus, we also examined the outcome of functional decline or death within 24 months. As intermediate outcomes, we also examined functional decline or death at 6 months and 12 months.
Measures: Predictors and Confounding Variables
Our primary predictor was the HbA1c level divided into 4 categories: <7%, 7–7.9%, 8–8.9%, and ≥9%. HbA1c was categorized since previous studies suggest that the relationship between HbA1c and outcomes may be non-linear.19 To characterize how HbA1c levels changed during follow-up, we compared the baseline HbA1c levels with the last HbA1c measurement before 2 year follow-up or death.
We accounted for factors which may confound the relationship between HbA1c and functional status including gender and race/ethnicity. Age, length of time at On Lok and the use of oral antihyperglycemic medications or insulin was determined at each HbA1c measurement. Baseline function was defined as the baseline ADL score. Since patients requiring insulin may differ in complex ways from patients on oral medications and prior studies suggest insulin use was associated with higher risk of physical disability,3,20 we performed analyses stratified by treatment (any insulin versus oral antihyperglycemic medications only) to determine whether the HbA1c-functional decline relationship differed by treatment. For our non-stratified analyses, we included treatment as a confounding factor. Since our previous study showed that an educational intervention in 2005 was successful in changing glycemic control practices at On Lok, we accounted for year in our analysis.21 Comorbid conditions were captured through ICD9 codes associated with hospitalizations, Emergency Room (ER) visits and outpatient physician visits.
Statistical Analysis
Our unit of analysis was the HbA1c measure (predictor) and 2 year death or functional decline (outcome). We used a population averaged mixed-effects Poisson regression to account for clustering of HbA1c values by patients. We chose Poisson regression over logistic regression because our outcome was common, making odds ratios more difficult to interpret than risk ratios. We chose the HbA1c level of 7.0–7.9% as the reference since the AGS guideline recommends HbA1c ≤ 8% as the appropriate glycemic target for “frail elders with limited life expectancy”16 and was consistent with On Lok practices during our study period. The statistical significance of trends was tested by examining whether the slope of the outcome-HbA1c regression line from the unadjusted mixed-effects model differed from zero. As a sensitivity analysis, we categorized all participants who disenrolled as having functional decline and found that our results were unchanged.
All statistics were performed using Stata MP (version 10.1; StataCorp 2007, College Station, TX) and SAS (Version 9.2, SAS System for Windows, 2008, SAS Institute Inc, Cary, NC). The Committee on Human Research of the University of California, San Francisco and the San Francisco Veterans Affairs Research and Development committee approved this study.
RESULTS
Characteristics of the Participants
Table 1 shows baseline characteristics for the 367 participants and their 1579 HbA1c measurements. Participants were elderly, with mean age of 80 years and the majority of participants were female (67%) and Asian (65%). Almost one-third of participants were on only oral antihyperglycemics at baseline and half were on insulin. Most patients had functional limitations with 66% of enrollees having a baseline ADL score of 8 or less. Most patients also showed evidence of cognitive impairment, with 79% of enrollees having an SPMSQ score of 6 or less. The most common oral antihyperglycemic medications were sulfonylureas (e.g. glipizide) in 51% of participants and biguanides (e.g. metformin) in 40% participants. Out of our 367 participants, 231 (63%) had a decline in functional status and 275 (75%) died or had a decline in functional status during the study period.
Table 1.
Characteristics of Subjects (n=367)
| Characteristic | |
|---|---|
| # of HbA1c measurements | 1579 |
| Age (years ± SD) | 80 ± 9 |
| Female | 246 (67%) |
| Race/Ethnicity | |
| White | 54 (15%) |
| African-American | 32 (9%) |
| Hispanic | 42 (12%) |
| Asian | 239 (65%) |
| Months enrolled in On-Lok (range) | 18 (6–53) |
| Medications | |
| No antihyperglycemic medications | 66 (18%) |
| Oral antihyperglycemic medications (no insulin) | 116 (32%) |
| Insulin | 185 (50%) |
| Baseline ADL score | |
| 10 - 9 | 124 (34%) |
| 8 – 7 | 83 (23%) |
| 6 – 5 | 92 (25%) |
| 4 or less | 68 (19%) |
| Hospitalizations or ER visits | |
| No hospitalizations or ER visits | 176 (48%) |
| Only ER visits | 38 (10%) |
| Hospitalizations | 153 (42%) |
| Cognitive Score | |
| SPMSQ 7–10 | 78 (21%) |
| SPMSQ ≤ 6 | 289 (79%) |
| Comorbidities | |
| Chronic Obstructive Pulmonary Disease | 70 (20%) |
| Congestive Heart Failure | 125 (34%) |
| Cancer | 27 (7%) |
| Kidney Disease | 42 (11%) |
| End Stage Renal Disease | 17 (5%) |
SD is standard deviation
SPMSQ is Short Portable Mental Status Questionnaire
Percentages are rounded to nearest whole number, therefore may not add to 100
Relationship between HbA1c, Functional Decline and Death
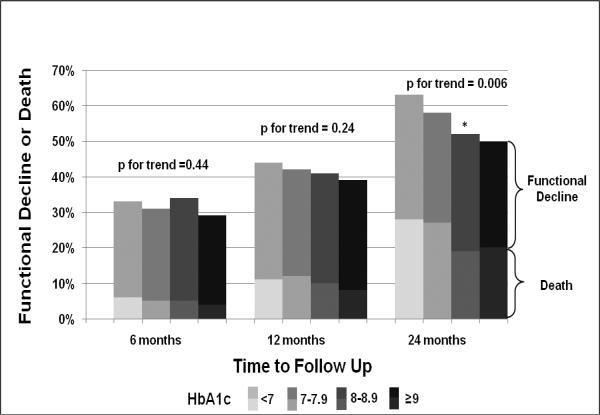
Figure 1 shows our unadjusted results at 6, 12 and 24 months. Twenty to 30% of our cohort died by 2 years, confirming that our study population was at high risk for adverse outcomes. At 6 months, no clear association was seen between HbA1c level and our composite outcome of functional decline or death, with a p-value for trend of 0.44 (see Figure 1). At 12 months, each higher level of HbA1c appeared to be associated with a lower rate of functional decline or death; however, this trend was not statistically significant (p-value for trend = 0.24). By 2 years, higher HbA1c was associated with less functional decline or death, with a significant test for trend at p = 0.006.
Figure 1.
Functional Decline or Death over Time by HbA1c Levels
* p-value = 0.03 for difference between our reference group (HbA1c 7–7.9) and HbA1c 8–8.9. All other comparisons with reference group (HbA1c 7–7.9), p > 0.05
After 2 years of follow-up, functional decline was identified after 49% of HbA1c measurements, death occurred after 25% of HbA1c measurements and the combined outcome of functional decline or death occurred after 58% of HbA1c measurements (see Table 2). Patients who experienced functional decline before death were considered to have experienced both the functional decline outcome and the death outcome. Because these patients are only counted once for the combined outcome of functional decline or death, the combined outcome is less than the sum of the individual outcomes.
Table 2.
Risk of Functional Decline or Death at 2 years by Baseline levels of Hemoglobin A1c (All Enrollees with Diabetes)
| HbA1c Category | Number of HbA1c measurements | Mean Baseline HbA1c (± SD) | Mean HbA1c at 24 months (± SD) | % Death | Adjusted RR for Death (95% CI) | % Functional Decline | Adjusted RR for Functional Decline (95% CI) | % Functional Decline or Death* | Adjusted RR for Functional Decline or Death (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| <7 | 698 | 6.3 ± 0.03 | 6.5 ± 0.1 | 29% | 1.06 (0.92, 1.21) | 53% | 1.07 (0.95, 1.21) | 63% | 1.07 (0.98, 1.17) |
| 7–7.9 | 412 | 7.4 ± 0.02 | 7.4 ± 0.1 | 29% | Ref | 46% | Ref | 58% | Ref |
| 8–8.9 | 223 | 8.5 ± 0.02 | 8.1 ± 0.1 | 22% | 0.95 (0.80, 1.13) | 46% | 0.90 (0.78, 1.05) | 52% | 0.88 (0.79–0.99) |
| >9 | 246 | 10.2 ± 0.2 | 8.9 ± 0.2 | 22% | 1.09 (0.91, 1.29) | 45% | 0.98 (0.82, 1.16) | 50% | 0.97 (0.84, 1.12) |
Adjusted models accounted for: age, gender, race/ethnicity, time enrolled in On Lok, baseline ADL, comorbidities (cancer, congestive heart failure, chronic obstructive lung disease, renal disease, dialysis) and medications (none, orals or insulin)
Because many patients experienced functional decline before death, the combined category of % Functional Decline or Death is not the sum of the individual % Death and % Functional Decline categories
There was evidence of treatment intensification in response to poor glycemic control, with decreases in HbA1c when initial HbA1c levels were 8–8.9% and ≥9%. Conversely, there was evidence of appropriate less aggressive treatment in patients with baseline HbA1c <7. There was no change in the level of glycemic control in our reference group with mean HbA1c staying at 7.4%.
In our fully-adjusted analysis, HbA1c levels of 8–8.9% was associated with best outcomes, with the lowest relative risk (RR) of death, functional decline and the combined outcome of functional decline or death. (see Table 2) For our combined outcome, HbA1c of 8–8.9% was associated with a statistically significant decreased RR (0.88, 95% CI: 0.79, 0.99) compared to our reference group of HbA1c 7–7.9%. Across all outcomes (death, functional decline and our combined outcome), patients with a HbA1c level <7% had the highest risk of poor outcomes.
Results Stratified by Use of Oral Antihyperglycemic Medications and Insulin
The analysis stratified by medications showed qualitatively similar results for the oral antihyperglycemic medications and insulin subgroups, with both patients taking insulin and oral antihyperglycemic medications showing a U-shaped relationship between HbA1c and our outcomes. In both the oral antihyperglycemic and insulin groups, the HbA1c level of 8–8.9% conferred the lowest adjusted RR of our combined outcome of functional decline or death (see Tables 3 and 4). This result reached statistical significance in the insulin group (RR 0.82, 95% CI: 0.71, 0.96) but not the oral antihyperglycemic group (RR 0.89, 95% CI: 0.72, 1.10). Although the results were qualitatively similar between insulin and oral antihyperglycemic medication subgroups, there was evidence of statistical interaction across medication strata (p=0.04).
Table 3.
Risk of Functional Decline or Death at 2 years by Baseline levels of Hemoglobin A1c (Oral Antihyperglycemics Only)
| HbA1c Category | Number of HbA1c measurements | Mean Baseline HbA1c (± SD) | Mean HbA1c at 24 months (± SD) | % Death | Adjusted RR for Death (95% CI) | % Functional Decline | Adjusted RR for Functional Decline (95% CI) | % Functional Decline or Death* | Adjusted RR for Functional Decline or Death (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| <7 | 241 | 6.3 ± 0.1 | 6.5 ± 0.1 | 25% | 0.97 (0.71, 1.33) | 61% | 1.17 (0.95, 1.44) | 67% | 1.18 (0.99, 1.40) |
| 7–7.9 | 159 | 7.4 ± 0.02 | 7.3 ± 0.1 | 19% | Ref | 42% | Ref | 48% | Ref |
| 8–8.9 | 54 | 8.5 ± 0.04 | 7.9 ± 0.2 | 20% | 1.16 (0.76, 1.77) | 42% | 0.88 (0.70, 1.09) | 48% | 0.89 (0.72, 1.10) |
| >9 | 45 | 10.0 ± 0.2 | 8.3 ± 0.3 | 20% | 1.16 (0.74, 1.82) | 36% | 1.10 (0.81, 1.49) | 42% | 1.09 (0.82, 1.44) |
Adjusted models accounted for: age, gender, race/ethnicity, time enrolled in On Lok, baseline ADL, comorbidities (cancer, congestive heart failure, chronic obstructive lung disease, renal disease, dialysis) and medications (none, orals or insulin)
Because many patients experienced functional decline before death, the combined category of % Functional Decline or Death is not the sum of the individual % Death and % Functional Decline categories
Table 4.
Risk of Functional Decline or Death at 2 years by Baseline levels of Hemoglobin A1c (Insulin)
| HbA1c Category | Number of HbA1c measurements | Mean Baseline HbA1c (± SD) | Mean HbA1c at 24 months (± SD) | % Death | Adjusted RR for Death (95% CI) | % Functional Decline | Adjusted RR for Functional Decline (95% CI) | % Functional Decline or Death* | Adjusted RR for Functional Decline or Death (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| <7 | 265 | 6.4 ± 0.03 | 6.7 ± 0.1 | 36% | 1.11 (0.94, 1.30) | 53% | 1.04 (0.90, 1.21) | 66% | 1.04 (0.92, 1.17) |
| 7–7.9 | 225 | 7.4 ± 0.02 | 7.5 ± 0.1 | 35% | Ref | 51% | Ref | 64% | Ref |
| 8–8.9 | 164 | 8.5 ± 0.03 | 8.2 ± 0.1 | 21% | 0.90 (0.73, 1.11) | 46% | 0.84 (0.70, 1.02) | 51% | 0.82 (0.71, 0.96) |
| >9 | 201 | 10.2 ± 0.2 | 9.0 ± 0.2 | 22% | 1.04 (0.84, 1.28) | 47% | 0.92 (0.74, 1.14) | 51% | 0.91 (0.76, 1.08) |
Adjusted models accounted for: age, gender, race/ethnicity, time enrolled in On Lok, baseline ADL, comorbidities (cancer, congestive heart failure, chronic obstructive lung disease, renal disease, dialysis) and medications (none, orals or insulin)
Because many patients experienced functional decline before death, the combined category of % Functional Decline or Death is not the sum of the individual % Death and % Functional Decline categories
DISCUSSION
Contrary to our original hypothesis, we found that HbA1c above the AGS guideline-recommended target of 8% is not associated with a higher risk of functional decline or mortality for community-dwelling, NH-eligible elders with diabetes. In fact, HbA1c of 8–8.9% was associated with a lower risk of 2 year functional decline or death than HbA1c 7–7.9%. Our results suggest that the 2003 AGS guideline recommending a HbA1c target of ≤8% may need to be revisited and possibly updated.16
Glycemic control is a core element of diabetes care and HbA1c has become the cornerstone measure of the quality of diabetes care.22,23 Much of the research on optimal levels of glycemic control has focused on younger patients and there are few studies on the appropriate levels of glycemic control in NH-eligible elders who make up a large and growing segment of the diabetes population. Although guidelines agree that glycemic control should be less aggressive for elders with advanced illness and limited life expectancy, there is substantial disagreement over what the specific target should be. Besides the AGS guidelines which recommend HbA1c ≤8%, the Veterans Affairs / Department of Defense (VA/DoD) recommends a target HbA1c of 8 – 9%,24 and the American Diabetes Association recommends a “less stringent” target than HbA1c <7% for frail elderly patients.25 Our study suggests that the VA/DoD guidelines may be the most appropriate HbA1c target for community-dwelling, NH-eligible elders with diabetes.
Since the HbA1c 8–8.9% group's glycemic control improved during our study, one possible interpretation of our results is that treatment to improve glycemic control may have contributed to the better outcomes in this group. Specifically, we found that our HbA1c of 8–8.9% group had a baseline mean HbA1c of 8.5% which dropped to 8.1% at 2-year follow-up, suggesting that treatments were intensified to improve glycemic control. However, since the mean HbA1c level remained above the target HbA1c of 8%, our results suggest that the current recommended HbA1c target of ≤8% is lower than necessary to maintain function.
A second interpretation of our findings is that our HbA1c 7–7.9% reference group may have had many declining patients with marginal nutritional status, leading to our HbA1c 8–8.9% group to have better outcomes by comparison. This interpretation is unlikely since the HbA1c level of our reference group does not change from baseline to 24 months (mean HbA1c 7.4 at baseline and at 24 months). If this group was comprised of declining patients with marginal nutritional status, a decrease in HbA1c levels would be expected over 2 years of follow up. Further, we chose the HbA1c of 7–7.9% rather than the <7% as our reference group to minimize the chances of including declining patients in our reference group. Thus, although our reference group may have some participants whose glycemic control improved due to advancing illness, it is unlikely that this fully explains our findings.
We performed a stratified analysis to determine whether the relationship between HbA1c and function differed between patients taking insulin or relying on oral medications. Overall, our stratified analysis showed that our results were similar in patients taking insulin or oral medications. In both the insulin and oral medication groups, the best outcomes occurred in patients with a HbA1c between 8 and 8.9%. In both groups, HbA1c >9% appeared to be associated with worse outcomes than HbA1c 8–8.9%, suggesting that HbA1c 9% or higher should be avoided. Finally, HbA1c <7% was associated with worst outcomes, suggesting this is a high-risk group for functional decline who may require additional support to maintain function.
The results of our unadjusted interval analysis show that there was no significant trend between HbA1c and functional decline or death at 6 months. However, a trend appears to start to develop at 12 months with higher HbA1c being associated with lower rates of functional decline or death (p=0.24). At 2 years, this result becomes statistically significant (p=0.006), suggesting that the functional benefits of HbA1c 8–8.9% took 1–2 years to realize. In contrast, previous studies in younger patients suggest that ~8 years of tight glycemic control (HbA1c 7%) is needed to see decreased rates of microvascular complications.16 Thus, our study suggests that for maintaining function in community-dwelling, NH-eligible elders, the HbA1c target can be between 8–8.9%, and the benefits are seen within 2 years.
Strengths, Limitations and Next Steps
Our study has both strengths and limitations. The major strength of our study is that, to our knowledge, it is the first study examining the longitudinal relationship between glycemic control and functional outcomes in PACE enrollees with diabetes. Much of what is known about the frail elderly is based on NH populations, even though more elders who are eligible for NH care live in the community.2 Thus, this is one of the first studies examining the relationship between glycemic control and functional outcomes in community-dwelling, NH-eligible elders.
Our study may also inform the care of healthier elders who are not NH-eligible. Previous intervention trials of glycemic control have focused on younger, healthier patients while the current study focuses on NH-eligible older patients in poorer health. Thus, the current study and previous trials represent “bookends,” highlighting the levels of glycemic control associated with best outcomes across the spectrum of health in older adults. By focusing on NH-eligible elders in poorer health, this study provides important additional data to clinicians, allowing them to rationally individualize glycemic targets for patients who are healthy, in poor health or somewhere in between.
Our study needs to be interpreted in light of its limitations. First, our study is observational and On Lok clinicians may have treated individual study participants more or less aggressively because of a variety of unmeasured factors. This confounding by indication suggests that study participants who had HbA1c levels 7–7.9% are likely different from study participants who had lower or higher HbA1c levels. We attempted to account for these differences by adjusting for demographic factors, comorbidities and baseline function as well as stratifying by glycemic treatment (oral antihyperglycemic medications versus insulin). However, we were unable to account for other possibly important factors such as social support, which may affect both glycemic control and functional decline.26 Thus, some residual confounding is likely, suggesting our results are a basis for future studies rather than a definitive statement of the optimal levels of glycemic control for community-dwelling, NH-eligible elders with diabetes.
Second, although HbA1c has been shown to correlate tightly with glucose levels in most patients, other factors including race and red blood cell (RBC) turnover can lead to HbA1c levels that are less indicative of glycemic control. For example, patients treated with iron or erythropoietin therapy may have increased RBC turnover leading to lower HbA1c levels, which may have contributed to the worse outcomes we observed for patients with low HbA1c.27 However, regardless of the shortcomings of the HbA1c test, it is the most widely used test of glycemic control and our study suggests that HbA1c levels <7% are predictive of worse outcomes.
Third, patients and families must choose to enroll in PACE programs and elders who choose to enroll are likely different from elders who do not enroll. Next, our subjects were predominantly Asian due to the history and location of On Lok in San Francisco and there may be ethnic differences in the relationship between glycemic control and outcomes.28–30 Finally, this study design cannot address the mechanisms underlying the observed associations between levels of glycemic control and outcomes.
Further research is needed. First, our observational results suggest that a HbA1c goal of 8–8.9% may be reasonable in the community-dwelling, NH-eligible patients. Due to concerns about residual confounding, a randomized trial is required to confirm or refute this observational result. Second, our study highlights the importance of non-vascular outcomes in geriatric diabetes research.31 Future research should focus on outcomes such as incontinence, falls and quality of life along with mortality, stroke and myocardial infarctions.
In summary, among community-dwelling, NH-eligible patients with diabetes, HbA1c levels between 8–8.9% appears to be associated with less functional decline or death at 2 years compared to HbA1c levels between 7–7.9%. This result suggests that the current AGS HbA1c target of 8% or less for frail elders may be lower than is necessary to maintain function and delay death for this vulnerable population.
ACKNOWLEDGMENTS
Presentations: Presented as an oral plenary abstract at the Society of General Internal Medicine Annual National Meeting in Phoenix, AZ (May 7, 2011) and as an oral abstract at the Bay Area Clinical Research Symposium in San Francisco, CA (November 4, 2011)
Sponsor's Role: The funding sources had no role in the design or conduct of the study, data management or analysis, or manuscript preparation
Funding Sources: Dr. Lee's effort was supported by NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130 and the Hellman Family Award for Early Career Faculty at UCSF.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Dr. Yau designed the study, interpreted the data and wrote the manuscript.
Dr. Eng supported data collection and provided critical revisions of the manuscript.
Ms. Cenzer provided statistical support and provided critical revisions of the manuscript.
Dr. Boscardin supervised the statistical analysis and provided critical revisions of the manuscript.
Ms. Rice-Trumble supported data collection and provided critical revisions of the manuscript.
Dr. Lee provided supervision in all phases of the study.
No other parties contributed substantially to this research or to preparation of this manuscript.
REFERENCES
- 1.Resnick HE, Heineman J, Stone R, et al. Diabetes in U.S. nursing homes, 2004. Diabetes Care. 2008;31(2):287–8. doi: 10.2337/dc07-1425. [DOI] [PubMed] [Google Scholar]
- 2.Manton KG, Corder LS, Stallard E. Estimates of change in chronic disability and institutional incidence and prevalence rates in the U.S. elderly population from the 1982, 1984, and 1989 National Long Term Care Survey. J Gerontol. 1993;48:S153–166. doi: 10.1093/geronj/48.4.s153. [DOI] [PubMed] [Google Scholar]
- 3.Gregg EW, Beckles GL, Williamson DF, et al. Diabetes and physical disability among older U.S. adults. Diabetes Care. 2000;23:1272–1277. doi: 10.2337/diacare.23.9.1272. [DOI] [PubMed] [Google Scholar]
- 4.Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care. 2002;25:61–67. doi: 10.2337/diacare.25.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Sinclair AJ, Allard I, Bayer A. Observations of diabetes care in long-term institutional settings with measures of cognitive function and dependency. Diabetes Care. 1997;20:778–784. doi: 10.2337/diacare.20.5.778. [DOI] [PubMed] [Google Scholar]
- 6.Giles LC, Hawthorne G, Crotty M. Health-related Quality of Life among hospitalized older people awaiting residential aged care. Health Qual Life Outcomes. 2009;7:71. doi: 10.1186/1477-7525-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroll M, Schlettwein D, van Staveren W, et al. Health related quality of life and physical performance. SENECA 1999. J Nutr Health Aging. 2002;6:15–19. [PubMed] [Google Scholar]
- 8.Guralnik JM, Fried LP, Salive ME. Disability as a public health outcome in the aging population. Annu Rev Public Health. 1996;17:25–46. doi: 10.1146/annurev.pu.17.050196.000325. [DOI] [PubMed] [Google Scholar]
- 9.Keeler E, Guralnik JM, Tian H, et al. The impact of functional status on life expectancy in older persons. J Gerontol A Biol Sci Med Sci. 2010;65:727–733. doi: 10.1093/gerona/glq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araki A, Ito H. Diabetes mellitus and geriatric syndromes. Geriatr Gerontol Int. 2009;9:105–114. doi: 10.1111/j.1447-0594.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen LK, Chen YM, Lin MH, et al. Care of elderly patients with diabetes mellitus: A focus on frailty. Ageing Res Rev. 2010;9(Suppl 1):S18–22. doi: 10.1016/j.arr.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Chen LK, Lin MH, Lai HY, et al. Care of patients with diabetes mellitus in long-term care facilities in Taiwan: diagnosis, glycemic control, hypoglycemia, and functional status. J Am Geriatr Soc. 2008;56:1975–1976. doi: 10.1111/j.1532-5415.2008.01904.x. [DOI] [PubMed] [Google Scholar]
- 13.Nelson JM, Dufraux K, Cook PF. The relationship between glycemic control and falls in older adults. J Am Geriatr Soc. 2007;55:2041–2044. doi: 10.1111/j.1532-5415.2007.01430.x. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz AV, Vittinghoff E, Sellmeyer DE, et al. Diabetes-related complications, glycemic control, and falls in older adults. Diabetes Care. 2008;31:391–396. doi: 10.2337/dc07-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shorr RI, Ray WA, Daugherty JR, et al. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med. 1997;157:1681–1686. [PubMed] [Google Scholar]
- 16.Brown AF, Mangione CM, Saliba D, et al. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51(5 Suppl Guidelines):S265–280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 17.Bodenheimer T. Long-term care for frail elderly people--the On Lok model. N Engl J Med. 1999;341:1324–1328. doi: 10.1056/NEJM199910213411722. [DOI] [PubMed] [Google Scholar]
- 18.Eng C, Pedulla J, Eleazer GP, et al. Program of All-inclusive Care for the Elderly (PACE): An innovative model of integrated geriatric care and financing. J Am Geriatr Soc. 1997;45:223–232. doi: 10.1111/j.1532-5415.1997.tb04513.x. [DOI] [PubMed] [Google Scholar]
- 19.Vijan S, Hofer TP, Hayward RA. Estimated benefits of glycemic control in microvascular complications in type 2 diabetes. Ann Intern Med. 1997;127:788–795. doi: 10.7326/0003-4819-127-9-199711010-00003. [DOI] [PubMed] [Google Scholar]
- 20.Caruso LB, Silliman RA, Demissie S, et al. What can we do to improve physical function in older persons with type 2 diabetes? J Gerontol A Biol Sci Med Sci. 2000;55:M372–377. doi: 10.1093/gerona/55.7.m372. [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Boscardin WJ, Stijacic Cenzer I, et al. The risks and benefits of implementing glycemic control guidelines in frail older adults with diabetes mellitus. J Am Geriatr Soc. 2011;59:666–672. doi: 10.1111/j.1532-5415.2011.03362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2002;25:213–229. doi: 10.2337/diacare.25.1.213. [DOI] [PubMed] [Google Scholar]
- 23.Ahmann AJ. Guidelines and performance measures for diabetes. Am J Manag Care. 2007;13(Suppl 2):S41–46. [PubMed] [Google Scholar]
- 24.Pogach LM, Brietzke SA, Cowan CL, Jr., et al. Development of evidence-based clinical practice guidelines for diabetes: The Department of Veterans Affairs/Department of Defense guidelines initiative. Diabetes Care. 2004;27(Suppl 2):B82–89. doi: 10.2337/diacare.27.suppl_2.b82. [DOI] [PubMed] [Google Scholar]
- 25.Standards of medical care in diabetes--2007. Diabetes Care. 2007;30(Suppl 1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 26.Okura T, Heisler M, Langa KM. Association between cognitive function and social support with glycemic control in adults with diabetes mellitus. J Am Geriatr Soc. 2009;57:1816–1824. doi: 10.1111/j.1532-5415.2009.02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng JM, Cooke M, Bhandari S, et al. The effect of iron and erythropoietin treatment on the A1C of patients with diabetes and chronic kidney disease. Diabetes Care. 2010;33:2310–2313. doi: 10.2337/dc10-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryson CL, Ross HJ, Boyko EJ, et al. Racial and ethnic variations in albuminuria in the US Third National Health and Nutrition Examination Survey (NHANES III) population: associations with diabetes and level of CKD. Am J Kidney Dis. 2006;48:720–726. doi: 10.1053/j.ajkd.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Kehl KG, Findeisen HM, Fardo DW, et al. Race-ethnicity as an effect modifier of the association between HbAlc and mortality in U.S. adults without diagnosed diabetes. Eur J Endocrinol. 165:275–281. doi: 10.1530/EJE-11-0171. [DOI] [PubMed] [Google Scholar]
- 30.McWilliams JM, Meara E, Zaslavsky AM, et al. Differences in control of cardiovascular disease and diabetes by race, ethnicity, and education: U.S. trends from 1999 to 2006 and effects of medicare coverage. Ann Intern Med. 2009;150:505–515. doi: 10.7326/0003-4819-150-8-200904210-00005. [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ, Eng C. Goals of glycemic control in frail older patients with diabetes. JAMA. 2011;305:1350–1351. doi: 10.1001/jama.2011.404. [DOI] [PMC free article] [PubMed] [Google Scholar]