Abstract
Objectives
Identifying the likelihood of a patient having coronary artery disease (CAD) at the time of emergency department (ED) presentation with chest pain could reduce the need for stress testing or coronary imaging after myocardial infarction (MI) has been excluded. The authors aimed to determine if a novel cardiac biomarker consisting of plasma cholesteryl ester (CE) levels typically derived from the activity of the enzyme acyl-CoA:cholesterol acyltransferase (ACAT2) are predictive of CAD in a clinical model.
Methods
A single-center prospective cohort design enrolled participants with symptoms of acute coronary syndrome undergoing coronary computed tomography angiography (CCTA) or invasive angiography. Plasma samples were analyzed for CE composition with mass spectrometry. The primary endpoint was any CAD determined at angiography. Multivariable logistic regression analyses were used to estimate the relationship between the sum of the plasma concentrations from cholesteryl palmitoleate (16:1) and cholesteryl oleate (18:1) (defined as ACAT2-CE) and the presence of CAD. The added value of ACAT2-CE to the model was analyzed comparing the C statistics and integrated discrimination improvement (IDI).
Results
The study cohort was comprised of 113 participants with a mean (± standard deviation [SD]) age 49 (SD ± 11.7) years, 59% had CAD at angiography, and 23% had an MI within 30 days. The median (interquartile range [IQR]) plasma concentration of ACAT2-CE was 938 μM (IQR, 758 to 1,099 μM) in patients with CAD and 824 μM (IQR 683 to 998 μM) in patients without CAD (p = 0.03). When considered with age, sex, and the number of conventional CAD risk factors, ACAT2-CE levels were associated with a 6.5% increased odds of having CAD per 10 μM increase in concentration. The addition of ACAT2-CE significantly improved the C statistic (0.89 vs 0.95, p = 0.0035), and IDI (0.15, p < 0.001) compared to the reduced model. In the subgroup of low-risk observation unit patients, the CE model had superior discrimination compared to the Diamond Forrester classification (IDI 0.403, p < 0.001).
Conclusions
Plasma levels of ACAT2-CE have strong potential to predict a patient’s likelihood of having CAD when considered in a clinical model but not when used alone. In turn, a clinical model containing ACAT2-CE could reduce the need for cardiac imaging after the exclusion of myocardial infarction.
INTRODUCTION
During the evaluation of patients with acute chest pain, appropriateness criteria for cardiac stress testing place emphasis on the likelihood of a patient having obstructive coronary artery disease (CAD).1,2 However, it has been previously shown that plaque rupture typically occurs in plaques without previous obstructive CAD.3 Further, appropriateness criteria and guidelines do not suggest a pretest probability threshold below which patients should not receive testing. Therefore, following current guidelines,4 even patients at very low risk for having CAD receive an emergent evaluation with serial cardiac markers and stress testing, or coronary CT angiography (CCTA). The result is that patients being evaluated in chest pain units are receiving lengthy and costly evaluations, yet these chest pain units are reporting ischemic cardiac event rates as low as 1.1% to 1.7%,5,6 with 70% or greater stenosis found in only 1.5% to 2.2%.7,8
In patients with low-risk chest pain, most events are due to myocardial infarction (MI), with fewer due to unstable angina. In these low-risk patients without established CAD, after exclusion of MI with serial troponins, unstable angina rates range between 0.9% to 2%.9,10 Stress testing to detect this low event rate is unlikely to be cost-effective; yet missing these patients nearly doubles their risk-adjusted mortality.11 After exclusion of MI, a biomarker able to detect CAD, the necessary precursor for most causes of unstable angina, could eliminate the need for stress testing in the vast majority of patients, and identify patients most likely to benefit from further testing.
Recently, acyl-CoA:cholesterol acyltransferase-2 (ACAT2) activity has been shown in monkey and murine models to correlate with atherosclerosis.12-14 Hepatic ACAT2 is the primary source of cholesteryl esters (CE) produced from the mono-unsaturated fatty acids palmitoleic acid (16:1) and oleic acid (18:1), and has also been associated with CE produced from palmitic acid (16:0). Higher plasma concentrations of these CEs have been linked to increased risk for MI in a longitudinal cohort of men followed over 19 years.15 Whether measurement of these CEs in plasma may be informative to care providers when assessing patients with acute chest pain remains to be determined.
The objective of this study was to explore whether there is diagnostic value in a clinical model utilizing plasma levels of ACAT2-associated CEs, in association with other commonly available risk stratification elements, when evaluating patients with possible acute coronary syndrome (ACS). The premise is that when evaluating patients with acute chest pain, identifying those unlikely to have CAD could eliminate the need for objective cardiac testing after exclusion of MI. This investigation examines first whether there is any association between measured CE levels and any definable CAD measured at angiography. Second, we describe the potential clinical effect of implementing this biomarker in a low-risk chest pain population in terms of missed ACS events and reducing the need for objective cardiac testing.
METHODS
Study Design
The study, a single-site observational cohort study, was approved by the Institutional Review Board of the Wake Forest School of Medicine, and all participants provided written informed consent.
Study Setting and Population
Participants were recruited from Wake Forest Baptist Health, a tertiary care medical center serving an urban, suburban, and rural population. The emergency department (ED) has an annual volume of approximately 97,000 visits, including approximately 600 with low-risk chest pain managed in the observation unit; about 2,700 cardiac catheterizations are performed annually at this facility. The study population consisted of patients who were at least 18 years old, who were either referred for invasive coronary angiography or were undergoing CCTA in the observation unit of the ED. The overall goal of the study was to assemble a repository for the testing of novel cardiac biomarkers; therefore, only patients with anemia (hemoglobin < 8.0) or inability to follow up were excluded. Patients with MI or ACS were not excluded. The analysis of plasma CE composition to use as a biomarker was the a priori primary analysis from this cohort.
This analysis includes patients enrolled from either the ED or the hospital setting with chest pain or suspected angina equivalent. The ED population consists of patients who received a primary assessment by the care providers consistent with low risk for ACS based on initial cardiac biomarkers and the initial electrocardiogram (ECG). After the primary assessment, the patients were placed in the ED observation unit, had a CCTA ordered by the primary clinical provider, and were then approached for enrollment. The basis for the clinical assessment was an overall impression of low risk based on the framework set forth in the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines4 and a Thrombolysis in Myocardial Infarction (TIMI) risk score16 of 0 or 1, correlating to a short-term risk for ACS of 2% to 5%.17 Patients recruited from the hospital setting were patients with and without known ACS being referred to angiography for either definition of coronary anatomy or coronary intervention.
Study Protocol
After obtaining informed consent, participants underwent a single blood draw, provided background information about their history and demographics, and completed a food frequency questionnaire. The food frequency questionnaire administered was the Block Brief 2000 (NutritionQuest, Berkeley, CA), chosen for both its brevity and the ability to quantify monounsaturated, polyunsaturated, and saturated fat intake. The questionnaire is based on a previous validated abbreviated questionnaire and contains a reduced food list consisting of about 70 food items developed from the National Health and Nutrition Examination Survey dietary recall data.18 The questionnaire asks individual portion size, displays pictures of food items, takes 15 to 20 minutes to complete, and was administered in the observation unit or inpatient setting. Clinical outcomes at 30 days were assessed using a scripted telephone interview and a structured medical record review. The primary investigator reviewed all case report forms to make a final determination of ACS during the index hospital visit, and at 30 days based on an objective study definition and blinded to CE measurements. Clinical data collection was consistent with standardized guidelines for ED patients with possible ACS.19
Coronary imaging
Participants undergoing coronary angiography had findings abstracted from the clinical reports. Invasive angiography was performed using conventional techniques, and the most severe diameter stenosis was recorded on the case report form for each reported vessel. Participants with prior bypass grafts had stenoses recorded for both native and graft vessels. Methods used at the study institution to assess the coronary vasculature with CCTA have been previously described.20 In brief, all participants underwent coronary imaging with a 64-slice LightSpeed VCT (GE Healthcare, Milwaukee, WI). Most patients first received oral and/or IV beta-blockade. Initial scout images were obtained followed by a low-dose, noncontrast, ECG-gated acquisition for calcium scoring. The subsequent contrast injection was a triple-phase intravenous (IV) injection consisting of 100 mL of nonionic iodinated contrast (Optiray 350; Mallinckrodt Medical, Hazelwood, MO) followed by an ECG-gated acquisition. Raw image data were then used to create multiphase images that were post-processed and analyzed on an independent 3D workstation (Advantage Workstation 4.2, GE Healthcare). Images were interpreted by radiologists and cardiologists meeting level 2 or 3 training guidelines from the ACC/AHA for cardiac CT.21 Coronary stenosis was measured using an electronic caliper and comparing the average luminal diameter in the most stenotic region with the average luminal diameter of a normal proximal or distal reference segment located within 1 cm of the stenosis without intervening branch vessels. As a measure of disease burden, CAD severity scores22 were calculated among participants with significant coronary stenosis. Image interpretation occurred as part of clinical care, but was abstracted and recorded for the purposes of the study.
Data handling and follow-up
Sources of data included the participants, care providers, and the medical record. Data templates were used to collect data directly from the patient and care providers for data fields that were anticipated to be unreliable in the medical record. Abstraction of medical record data was guided by a “sources of data” document describing the expected location and definition of each data field. Data from paper case report forms were then entered into a web-based electronic database.
Sample acquisition and processing
Whole blood was collected via venipuncture into Vacutainer tubes (BD Vacutainer, Franklin Lakes, NJ) containing ethylenediaminetetraacetic acid (EDTA). Cells were separated from plasma by centrifugation at a minimum of 1,700 RPM for 15 minutes in a tabletop centrifuge at 4° C. Plasma was subsequently aspirated from the cell layer. Plasma was stored at −70° C until analysis was done. Lipoprotein cholesterol distributions were determined on whole plasma using size separations via gel filtration chromatography, similar to the method of Garber et al.23 Plasma (containing about 15 μg of cholesterol) diluted 1:1 with cold phosphate buffered saline was applied to a Superose 6 column (GE Healthcare, Little Chalfont, Buckinghamshire, UK). The column was eluted with 0.9% saline containing 0.01% EDTA and 0.01% sodium azide at a flow rate of 0.4 ml/min using a LaChromElite HPLC system (Hitachi High Technologies, Tokyo, Japan) and the column eluate was continuously mixed online with 0.125 ml/min cholesterol reagent (Cholesterol Liquid Reagent Set, Pointe Scientific, Inc., Canton, MI) that was then passed through a 5 mL knitted reaction coil maintained at 37°C. Data readout is proportional to cholesterol concentration in the eluate, and fractions containing very low density lipoprotein (VLDL), low density lipoprotein (LDL), and high density lipoprotein (HDL) were identified so that the percentage of cholesterol in each could be determined. The concentrations in each lipoprotein class were then calculated from a direct measure of cholesterol concentration in an aliquot of the starting plasma. The measurement of cholesterol and triglyceride concentrations in whole plasma was done using enzymatic methods for cholesterol24 and triglycerides.25
Mass spectrometry was used for measurement of CEs. Samples were stored at −70°C before analysis. After thawing, 5 μL of plasma was added to 1 mL methanol solution containing 5 ng/μL 17:0 CE (internal standard) and 10 ng/μL sodium formate. The solution was vortexed for 10 seconds and then allowed to stand at room temperature for 30 minutes. One hundred μL of the first solution was diluted 1:10 with high purity methanol. Individual CEs were measured using a Quattro II mass spectrometer equipped with a Z-spray interface. Analysis parameters were as follows: capillary voltage = 3.2 kV, cone voltage = 50V, source temperature = 80° C, and desolvation temperature = 200° C. Samples were maintained at 15°C in a temperature-controlled Spark Holland Reliance autosampler/stacker (Spark Holland, The Netherlands) while awaiting analysis. 25μL of each sample was infused into the mass spectrometer at 10μL/min. CE were quantified in the positive ion mode by monitoring the common neutral loss of 368.4 Da. The CE profile and quantitation were calculated from these data and presented in an electronic spreadsheet. Cholesteryl esters measured included those with the following fatty acids: palmitate,16:0; palmitoleate, 16:1; stearate, 18:0; oleate, 18:1; linoleate, 18:2; linolenate,18:3; arachidonate, 20:4; eicosapentenoate, 20:5; and docosahexanoate, 22:6. Measurements were reported both in percentages of total CE by mass as well as plasma concentrations (10−3 mol/m3) of each, and both measures were considered in the data analysis. Analyses of CE measurements conducted to assess reproducibility in plasma or blood demonstrated a mean error for repeated analysis on the same day was 3.4% for 18:2 and 5.4% for 18:1 after isotope correction. Reproducibility for repeated analysis of the same sample on different days was 5.4% and 9.6% for 18:2 and 18:1, respectively.
Primary outcome
The primary outcome was the presence of CAD, measured either by CCTA or invasive angiography. Severity of the maximal diameter of stenosis was recorded and the patients were dichotomized as 1) positive or negative for CAD, and 2) positive or negative for significant CAD (≥50%). Ranges of stenosis were recorded as the highest value of the range. Stenosis caused by myocardial bridging alone was not included in this analysis.
Data Analysis
Sample size
Variance and effect size data for this novel biomarker were not available in humans. At the outset it was empirically estimated that approximately 100 participants with varying degrees of CAD would provide at least preliminary evidence of a clinically important relationship, if one existed. It was estimated that this would provide sufficient event rates of coronary stenosis to satisfactorily fit exploratory multiple variable logistic regression models, considering the standard “rule of ten” recommending ten events for every degree of freedom considered in the multivariate model.26
The first objective was to determine if there was an association between measured CE levels and coronary stenosis. Two definitions of coronary stenosis were examined: any measurable CAD and significant (>50%) stenosis. Means and standard deviations (SD), medians with interquartile ranges (IQR), and proportions were used to describe normally distributed continuous variables, skewed continuous variables, and categorical variables, respectively. Cholesterol esters related to ACAT2 (16:0, 16:1, and 18:1) and their sums were examined among participants with and without coronary stenosis and compared with Kruskal-Wallis tests. Cholesteryl palmitate concentrations contributed in a different direction than cholesteryl palmitoleate and cholesteryl oleate. Mechanistically, ACAT2 is directly involved in the synthesis of the latter two cholesteryl esters after the activity of steroyl CoA desaturase 1 to introduce the double bond, whereas the synthesis of cholesteryl palmitate does not require prior steroyl CoA desaturase 1 activity. Therefore this CE was removed and the sum of 16:1 and 18:1 (ACAT2-CE) were further examined. Exploratory analyses with other CE composites are displayed in the Data Supplement (S1).
Logistic regression was used to assess the relationship between the sum of cholesteryl palmitoleate plus cholesteryl oleate and coronary stenosis. Separate models using absolute CE concentrations, mass percentages, and both CAD endpoints (any stenosis, >50% stenosis) were constructed. Univariate logistic regression was performed between potential covariates and the endpoint under consideration, and those covariates with a Wald p < 0.20 were then considered for inclusion in the multivariate model. Covariates included age, dietary intake of monounsaturated and polyunsaturated fat, sex, total plasma cholesterol, LDL, HDL, body mass index, and a summary measure of traditional coronary risk factors (smoking, diabetes, hypertension, dyslipidemia) ranging from zero to four. Graphic examination and fractional polynomial analysis27 indicated that continuous variables were best treated as linear within the models. Model construction was performed manually beginning with the full model with backward removal of covariates in order to build a parsimonious model. The CE term was retained at all steps; a Wald p < 0.05 was required for other covariates to be retained within the model. Interaction terms were generated via the product method and assessed for inclusion, as were covariates with the potential to be effect modifiers of the relationship between the CE and the endpoint. Given the exploratory nature of this analysis, we made no adjustment to any p values for the construction of multiple models. The Hosmer-Lemeshow goodness of fit test was used to assess model fit, and receiver area operating curves were generated to produce a C statistic. Contributions of CE were assessed between the final full model and a reduced model without the CE by comparison of the receiver operator characteristic (ROC) curve C statistic,28 and integrated discrimination improvement (IDI).29 The IDI is a measure of the discriminatory ability of the models with and without the marker of interest.29,30 Each model was assessed for outliers and overly influential points using Pregibon’s dbeta statistic.
The secondary objective was to examine the potential effect of the resulting final model in the subgroup of ED patients with low-risk chest pain. This was first accomplished by comparing the final model with the Diamond and Forrester classification31 (S1), using the IDI. The final model was also then applied to the low-risk subgroup to determine the potential reduction in imaging and the resulting missed ACS rate at 30 days. Data analysis was conducted with SAS Enterprise Guide, v4.2 (SAS Institute Inc., Cary, NC) and Stata/IC 11.2 (College Station, TX).
RESULTS
The observational cohort consisted of 150 participants enrolled over 24 months at the time of this analysis. Of these 150, 37 participants were excluded for meeting one or more of the following exclusions: no available blood samples (n = 10), no coronary imaging (n = 7), no food frequency questionnaire data (n = 21), and no acute chest pain (n = 3). The final dataset for this analysis consisted of 113 participants with complete data; 65 (58%) were enrolled from the observation unit and 48 (42%) were enrolled from the angiography suite. Demographics of included subjects are in Table 1. During the index visit, 23% experienced MI and 26% received revascularization. At 30 days, 32% had at least one ACS event (Table 2).
Table 1.
Participant Demographics and Past Medical History
| Patient Characteristics | No CAD n / N ( %) |
CAD n / N ( %) |
|---|---|---|
| Age (yrs), mean (±SD) | 40.9 (±8.6) | 55.0 (±10.0) |
| Female sex | 23/46 (50.0) | 20/67 (29.9) |
| White race | 28/46 (60.9) | 53/67 (79.1) |
| Hypertension | 12/46 (26.1) | 36/67 (53.7) |
| Diabetes mellitus | 4/46 (8.7) | 17/67 (25.4) |
| Current smoking | 17 (37.0) | 25/67 (37.3) |
| History of cocaine use | 8/46 (17.4) | 10/67 (14.9) |
| Hyperlipidemia (by history) | 9/46 (19.6) | 38/67 (56.7) |
| Body mass index, mean (±SD) | 29.7 (±5.8) | 29.8 (±5.8) |
| Prior heart failure | 0/46 (0) | 3/67 (4.5) |
| Prior myocardial infarction | 1/46 (2.2) | 17/67 (25.4) |
CABG = Coronary artery bypass graft
Table 2.
Presenting Characteristics and Physical Exam Findings
| No CAD n / N ( %) |
CAD n / N ( %) |
|
|---|---|---|
| Presenting Characteristics | ||
| Chest pain chief complaint | 39/44 (88.6) | 61/66 (92.4) |
| Chest pain at rest | 36/43 (83.7) | 45/65 (69.2) |
| Multiple episodes of symptoms within 24 hours of presentation |
15/43 (34.9) | 29/64 (45.3) |
| Chest pain pleuritic | 8/43 (18.6) | 12/63 (19.0) |
| Physical Exam | ||
| Heart rate (beats/minute)* | 78.9 (±14.4) | 68.9 (±23.1) |
| Systolic blood pressure (mmHg)* | 136.6 (±35.4) | 131.3 (±31.6) |
| Murmur | 1/42 (2.4) | 6/65 (9.2) |
| Rales | 0/44 (0) | 6/65 (9.2) |
| Jugular venous distention | 0/43 (0) | 0/65 (0) |
| Overall electrocardiogram classification | ||
| Normal | 30/46 (65.2) | 30/67 (44.8) |
| Nonspecific changes | 13/46 (28.3) | 19/67 (28.4) |
| Early repolarization only | 0/46 (0) | 2/67 (3.0) |
| Abnormal but not diagnostic of ischemia | 1/46 (2.2) | 2/67 (3.0) |
| Infarction or ischemia known to be old | 0 (0) | 4/67 (6.0) |
| Infarction or ischemia not known to be old | 1/46 (2.2) | 7/67 (10.5) |
| Suggestive of myocardial infarction | 1/46 (2.2) | 3/67 (4.5) |
| Risk Stratification | ||
| TIMI risk score | ||
| 0 | 25/46 (54.4) | 12/67 (17.9) |
| 1 | 17/46 (37.0) | 16/67 (23.9) |
| 2 | 3/46 (6.5) | 11/67 (16.4) |
| 3 | 1/46 (2.2) | 15/67 (22.4) |
| 4 | 0/46 (0) | 10/67 (14.9) |
| 5 | 0/46 (0) | 2/67 (3.0) |
| 6 | 0/46 (0) | 1/67 (1.5) |
| 30-day acute coronary syndrome | 1/46 (2.2) | 35/67 (52.2) |
| Cardiovascular death | 0/46 (0) | 1/67 (1.5) |
| Myocardial infarction | 1/46 (2.2) | 25/67 (37.3) |
| Revascularization | 0/46 (0) | 29/67 (43.3) |
data presented as mean (±SD); TIMI = thrombolysis in myocardial infarction
Participants were a mean age of 49 (SD ± 11.7) years, and 38% were women (Table 1). Conventional coronary risk factors among study participants included hypertension (43%), current smoking (37%), diabetes (19%), hyperlipidemia (42%), history of cocaine use (16%), and 16% of the study population had experienced a prior MI. Most participants had a chief complaint of chest pain (91%), and a normal (53%) or nonspecific (28%) ECG nearest to enrollment.
Computed tomography coronary angiography was the diagnostic modality for coronary imaging in 56% of participants, with the remainder undergoing invasive angiography. At angiography, 59% had CAD and 43% had significant coronary stenosis in at least one coronary artery. Two participants undergoing CCTA had major coronary segments that could not be quantified due to artifact and were classified based on the available information. CAD severity scores (excluding the two participants with incomplete data for this calculation), averaged across all four main coronary vessels (including left main), was 4.3 (SD ± 6.1). At least 50% stenosis was seen in 1, 2, 3, and 4 coronary distributions in 17, 11, 9, and 1 participant, respectively.
Stratifying the cohort into participants with and without CAD, dietary fat intake and plasma lipid concentrations were similar between groups (Table 3). Cholesteryl ester concentrations were significantly higher for 16:1, 18:1, 18:3, 20:4, 20:5, and 22:6 among participants with coronary stenosis (Table 4, Figure 1). Based on the study hypothesis, the sum CE (16:1 and 18:1) (ACAT2-CE) was of primary interest and was significantly higher in both mass percent and concentration in those with CAD. Figure 1 suggests that ACAT2-CE may be more useful in patients 40 years old or younger. This subgroup consisted of 29 participants, 6 with any CAD.
Table 3.
Dietary intake, serum and plasma results
| No CAD Mean (±SD) |
CAD Mean (±SD) |
p value | |
|---|---|---|---|
| Dietary intake (daily estimated intake) | |||
| Total fat (g) | 79.8 (39.2) | 81.3 (51.2) | 0.86 |
| Saturated fat (g) | 26.5 (13.2) | 26.4 (17.2) | 0.96 |
| Monounsaturated fat (g) | 31.0 (16.1) | 31.6 (19.6) | 0.86 |
| Polyunsaturated fat (g) | 16.0 (7.8) | 17.1 (11.7) | 0.54 |
| Dietary cholesterol (g) | 235.7 (137.2) | 257.0 (203.3) | 0.51 |
| Olive oil use for cooking, n/N (%) | 23/46 (50.0) | 30/67 (44.8) | 0.70 |
| Plasma lipids (mg/dl) | |||
| Total cholesterol | 166.6 (27.5) | 178.7 (44.6) | 0.0780 |
| VLDL | 19.7 (12.1) | 23.8 (14.5) | 0.1237 |
| LDL | 105.0 (21.0) | 113.7 (36.0) | 0.1094 |
| HDL | 41.9 (13.1) | 41.3 (16.2) | 0.83 |
Comparisons were conducted using t-tests.
VLDL = very low density lipoprotein; LDL = low density lipoprotein; HDL = high density lipoprotein
For all analyses, p ≤ 0.05 denotes statistical significance, with no adjustment for the multiple comparisons.
Table 4.
Plasma cholesteryl ester results
| Median concentration in μmol/l (Q1, Q3) | Median percentage of CE (Q1, Q3) | |||||
|---|---|---|---|---|---|---|
| No CAD | CAD | p value | No CAD | CAD | p value | |
| 16:0 | 449.4 (375.3-489.0) | 440.4 (379.0-547.9) | 0.52 | 10.2 (9.4-11.1) | 9.6 (8.6-10.4) | 0.0066 |
| 16:1 | 112.1 (85.1-156.0) | 150.5 (118.8-214.8) | 0.0007 | 2.8 (2.2-3.5) | 3.3 (2.6-4.2) | 0.0128 |
| 18:0 | 58.2 (45.2-64.8) | 66.9 (45.8-85.0) | 0.0641 | 1.3 (1.1-1.5) | 1.4 (1.0-1.7) | 0.41 |
| 18:1 | 645.8 (568.4-767.4) | 791.4 (648.7-939.8) | 0.0016 | 15.8 (14.1-17.0) | 16.2 (15.0-17.7) | 0.0879 |
| 18:2 | 2421.3 (2108.2-2733.6) | 2657.5 (2195.6-3049.0) | 0.0674 | 56.3 (51.4-59.2) | 54.6 (51.3-58.0) | 0.0766 |
| 18:3 | 81.6 (64.8-106.4) | 111.2 (90.3-146.4) | 0.0002 | 1.9 (1.6-2.3) | 2.3 (2.1-2.6) | 0.0014 |
| 20:4 | 425.6 (359.5-515.0) | 474.8 (404.7-615.8) | 0.0166 | 10.3 (8.9-12.0) | 10.8 (9.3-12.8) | 0.2264 |
| 20:5 | 34.1 (27.9-47.4) | 41.2 (33.1-62.5) | 0.0256 | 0.9 (0.7-1.1) | 0.9 (0.7-1.2) | 0.50 |
| 22:6 | 19.8 (15.7-26.1) | 22.6 (17.5-31.1) | 0.0402 | 0.5 (0.4-0.6) | 0.5 (0.4-0.6) | 0.32 |
|
| ||||||
| Sum (16:1, 18:1) | 775.5 (669.3-937.4) | 945.5 (757.5-1,106.6) | 0.0012 | 18.4 (16.0-21.0) | 19.3 (17.8-22.1) | 0.0354 |
Figure 1.
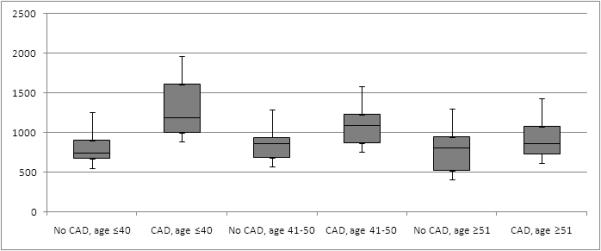
Cholesteryl ester values by the presence of coronary disease and age
The boxplots represent the values of the sum of cholesteryl esters 16:1 and 18:1 measured in participants. The box margins represent the 25th and 75th percentiles, the bar within the box represents the median, and the whiskers represent the range of values.
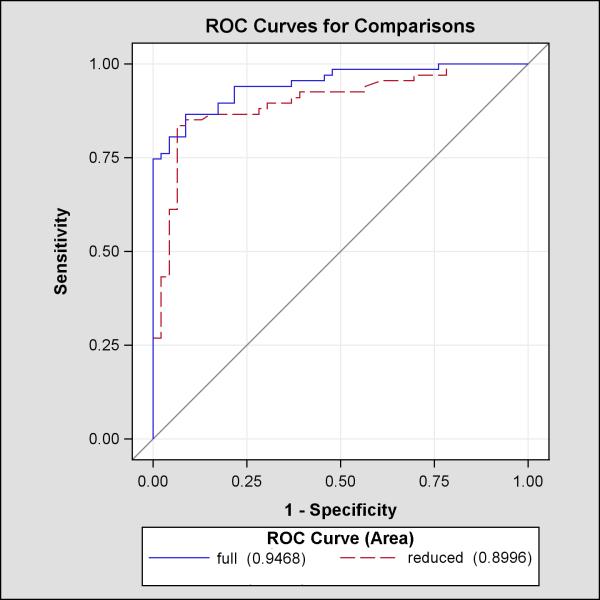
In logistic regression modeling using participants with complete data (n = 113), the absolute concentration of ACAT2-CE was predictive of any coronary stenosis in the final model also containing age, sex, and number of conventional coronary risk factors (Table 5) (AUC 0.95, 95% CI = 0.91 to 0.98). In this model, each 10 micromolar increase in concentration was associated with a 6.5% increase in the odds of having coronary stenosis (p < 0.001). No interaction terms were found to be additive to the explanatory value of the model. The Hosmer-Lemeshow goodness of fit test (χ2(8) = 13.9, p = 0.08) provided no reason to reject the primary model on the basis of fit. Further models, including those using coronary stenosis >50% as the endpoint and ACAT2-CE mass percentage as the primary predictor, were found to be less informative. Additional models are included in the Data Supplement (S1). To determine the contribution of ACAT2-CE to the model, ROC curves were created and are shown in Figure 2 for the model with and without ACAT2-CE. The model without ACAT2-CE had a significant reduction in the C statistic to 0.89 (95% CI = 0.81 to 0.94, p for comparison 0.004). The estimated integrated discrimination improvement from adding ACAT2-CE was 0.15 (p < 0.001), suggesting the sum CE adds to the ability of the model to discriminate between those with and without CAD.
Table 5.
Multiple variable logistic regression model of the relationship between plasma ACAT2-CE and the odds of having any coronary artery disease at angiography (n=113)
| Adjusted odds ratio |
95% CI | p-value | |
|---|---|---|---|
| ACAT2-CE* | 1.0065 | 1.0029-1.0101 | <0.001 |
| Age | 1.2412 | 1.1263-1.3678 | <0.001 |
| Male | 9.4364 | 1.9159-46.4762 | 0.006 |
| Number of risk factors† | 1.9978 | 1.0698-3.7307 | 0.030 |
odds ratio expressed per each 10−3 mol/m3 increase in plasma concentrations
risk factors = smoking, diabetes, hypertension, dyslipidemia;
ACAT2-CE = acyl-CoA:cholesterol acyltransferase-2 cholesteryl esters, and represents the sum of palmitoleic acid (16:1) and oleic acid (18:1)
For all analyses, p ≤ 0.05 denotes statistical significance, with no adjustment for the multiple comparisons.
Figure 2.
Receiver operator characteristic curves for full (CE, age, sex, number of conventional risk factors) and reduced (full model minus CE) models to predict any CAD among the entire study cohort.
The full model has a significantly higher C statistic, p = 0.0219.
CE = cholesteryl esters; CAD = coronary artery disease
The performance of the final model for CAD was then compared to the Diamond Forrester risk stratification framework in the subgroup of low-risk observation unit patients with complete data for this calculation (n = 58). When compared, the clinical model had superior performance based on an estimated IDI (0.403, p < 0.001), indicating that the final model for CAD had better discriminatory ability. In low-risk patients with serial negative troponin results (n = 64), implementing a 50% predicted probability threshold to determine the need for further testing after serial cardiac markers would have led to a calculated maximum post-test probability of 1% based on the prevalence of ACS after excluding MI of 2%,10 (2%*50% = 1.0%). At this threshold, 42 of 64 (66%) patients would have been considered negative; one had maximal stenosis of 50%, none with 30-day ACS. A 50% predicted probability threshold would have resulted in 22 of 64 (34%) patients with positive results; eight had maximal stenosis ≥ 50% and none had 30-day ACS.
DISCUSSION
The results of this analysis suggest that plasma concentrations of cholesteryl esters typically synthesized by ACAT2 are associated with CAD in patients with acute chest pain. ACAT2-CE had predictive value beyond that obtained from clinical variables alone. However, the greatest potential utility appears to be incorporating ACAT2-CE results in a clinical model rather than considering the results in isolation. Together, these findings suggest that ACAT2-CE levels integrated into a clinical decision model may predict the presence of CAD and thereby serve as a test for unstable angina in patients presenting with acute chest pain.
The background information suggesting that cholesteryl ester composition might matter in coronary artery atherosclerosis comes from numerous studies done in animal models and in humans. Perhaps the first observations to directly establish a link came in 1997 when it was shown that the cholesteryl ester secretion rate from primate livers in isolated perfusion was predictive of the extent of coronary artery atherosclerosis in the liver donor monkeys.14 The acyl composition for cholesteryl esters made by the tissue esterifying enzyme ACAT was predominantly oleate, and the percentage of this CE was enhanced when the diet was enriched in oleate. The plasma enzyme LCAT contributes mostly cholesteryl linoleate to the plasma CE pool. Subsequent studies in ARIC32 and in Sweden33 have confirmed that in humans, plasma cholesteryl oleate is positively associated with carotid intimal medial thickness, or mortality from cardiovascular disease, respectively. Numerous studies in genetically engineered mice lacking the enzyme ACAT2 have also showed that marked reductions in cholesteryl oleate in plasma LDL occur together with reduced aortic atherosclerosis when ACAT2 is absent.34 Thus, a convincing picture becomes available showing that a higher percentage of cholesteryl oleate in plasma LDL CE is consistently associated with increased atherosclerosis.
In order to be useful as a biomarker, CE measurements have to be obtainable. The measurements in this analysis were conducted in a research lab using a mass spectrometer. These instruments are relatively expensive, but multiplexing an instrument use for more than one type of analyses would substantially reduce sample cost. Technical expertise would require an individual who can dilute 5 to 10 μL of plasma or blood into a fixed volume of solvent. Mass spectrometer upkeep would require a service contract and an individual who could tune and check the instrument daily. Since most academic institutions have one or more mass spectrometer facilities, establishing this sort of facility would be feasible. A typical cost for sample analysis in an academic mass spectrometer facility is on the order of $30. If a modern electrospray mass spectrometer is installed, the turn-around time would be minimal, 0.5 to 1 hour.
There exists a substantial need for more refined risk stratification schemes to guide the evaluation of patients with acute chest pain. Existing clinical decision rules comprised of combinations of traditional risk factors, biomarkers of necrosis, and clinical variables have proven insufficiently sensitive to exclude ACS without cardiac imaging.17,35,36 Further, conventional risk factors for atherosclerosis have not proven useful when evaluating patients with acute chest pain.37,38 In distinction to prior efforts that aspired to predict major adverse cardiac events,5,39-41 we chose to predict the presence of coronary stenosis. ACS consists of MI and unstable angina, both of which are most commonly the result of coronary atherosclerotic disease. MI, by definition, is excluded with serial cardiac markers of necrosis. In contrast, unstable angina is defined by non-elevated markers of necrosis and currently requires cardiac imaging for proper diagnosis. However, if we knew with considerable accuracy that there was no CAD present, pursuing cardiac imaging would not be expected to yield useful diagnostic findings. Reducing the need for cardiac imaging could improve health care by reducing exposure to ionizing radiation, avoiding false-positive results, and could improve health care efficiency by reducing the number of unnecessary imaging tests. Therefore, predicting a precursor for unstable angina, coronary atherosclerotic disease, could improve health and increase health care efficiency. It should be noted that this approach could miss the relatively rare secondary causes of ACS that are not associated with myocardial necrosis, such as de-novo coronary thrombosis, coronary embolism, or coronary vasospasm.
The most clinically useful model in our analysis appears to be the sum of the plasma concentrations of CE 16:1 and CE 18:1, combined with age, sex, and the number of traditional coronary risk factors to predict the presence of any coronary atherosclerosis in low-risk patients. If validation of this model is successful, this measure could be used to determine the need for coronary imaging or stress testing after the exclusion of MI. Our first examination in our low-risk subgroup suggests that this approach could result in a meaningful reduction in imaging. Further prospective investigations should validate these findings, evaluate the potential clinical utility, refine appropriate cut-points, and examine model calibration.
LIMITATIONS
This analysis is subject to limitations. First, the study cohort contains a broad spectrum of risk for CAD ranging from very low risk, to some participants known to have CAD. This range of risk allowed us to assess the potential use of this biomarker with a smaller study size and across the risk spectrum. Second, the outcome was measured using two methods, angiography with either CT or by traditional invasive catheterization. This is unlikely to affect our results because cardiac catheterization and CCTA have a high degree of correlation,42,43 and our measures of stenosis were divided into broad categories of stenosis. Therefore, small deviations in measurement of stenosis are unlikely to have a meaningful effect on our findings. Third, the low-risk subgroup is small and has a predominance of participants without coronary stenosis. As a result, modeling in this subgroup could lead to an overfit model. However, we feel it is less likely that this affected our results, because our findings are similar and largely unchanged in the entire cohort compared to the low-risk subgroup. Fourth, the performance of the Block Brief 2000 dietary questionnaire in patients with acute chest pain is not described, and could have provided inaccurate dietary estimates. Ultimately, dietary intake did not contribute to our models. Fifth, very little human data were available to guide this preliminary analysis. Consistent with the exploratory intent of this analysis, we analyzed and reported two definitions of CAD (any CAD and significant CAD), both with similar results. This could over-estimate the effect due to multiple comparisons. In addition, we used adjusted odds ratios instead of risk ratios for our analysis. There is varied opinion regarding the most appropriate metric to describe differentiated probabilities of outcomes. Given that risk ratios are less able to be applied homogeneously across subgroups when confounding exists compared to odds ratios,44 and given a reasonable a priori assumption that confounding due to covariates would be present, we elected to proceed with standard logistic regression and odds ratios. Similarly, the effect of MI and plaque rupture on ACAT2-CE is unknown; however, for the most part, CEs come from the liver or intestine as a product of cholesterol metabolism, and are a part of cholesterol homeostatic mechanisms. The amount of CEs in plaques is very small compared to that in plasma, and therefore we feel the occurrence of MI in our patients is unlikely to have confounded our results. Due to these limitations, our findings are an initial step in determining whether these CEs have potential to be a useful biomarker, and leave unanswered questions for future research.
CONCLUSIONS
Plasma levels of ACAT2-CE have strong potential to predict a patient’s likelihood of having coronary artery disease when considered in a clinical model, but not when used alone. In turn, a clinical model containing ACAT2-CE could reduce the need for cardiac imaging after the exclusion of myocardial infarction. Further study of monounsaturated fatty acid-containing cholesteryl esters as biomarkers in patients with suspected acute coronary syndrome is needed to validate these findings, evaluate the clinical utility, refine cut-points, and examine model calibration.
Supplementary Material
Acknowledgments
Funding Sources: Grant numbers: HL-49373 (Rudel); NCRR 5 M01 RR07122-17 (Applegate); Translational Science Institute, Wake Forest School of Medicine (Miller and Rudel)
Footnotes
Reprints not available from the authors
Disclosures: Dr. Miller (past 24 months): Research support from Siemens, Pennsylvania Department of Health, NIH / NHLBI, EKR Therapeutics, 3M. Dr. Rudel has consulted with Glaxo-Smith Kline and with Merck. Drs. Miller, Rudel, and Thomas have filed a patent related to this work.
The rest of the authors have no relevant financial disclosures or potential conflicts of interest to report.
Prior Presentations: none
References
- 1.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Circulation. 2002;106:1883–92. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 2.Douglas PS, Khandheria B, Stainback RF, et al. ACCF/ASE/ACEP/AHA/ASNC/SCAI/ SCCT/SCMR 2008 appropriateness criteria for stress echocardiography: a report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, American Society of Echocardiography, American College of Emergency Physicians, American Heart Association, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance: endorsed by the Heart Rhythm Society and the Society of Critical Care Medicine. Circulation. 2008;117:1478–97. doi: 10.1161/CIRCULATIONAHA.107.189097. [DOI] [PubMed] [Google Scholar]
- 3.Little WC, Downes TR, Applegate RJ. The underlying coronary lesion in myocardial infarction: implications for coronary angiography. Clin Cardiol. 1991;14:868–74. doi: 10.1002/clc.4960141103. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction) Circulation. 2007;116:e148–304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 5.Mahler SA, Hiestand BC, Goff DCJ, Hoekstra JW, Miller CD. Can the HEART score safely reduce stress testing and cardiac imaging in patients at low risk for major adverse cardiac events? Crit Pathw Cardiol. 2011;10:128–33. doi: 10.1097/HPC.0b013e3182315a85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell AM, Garvey JL, Kline JA. Multimarker panel to rule out acute coronary syndromes in low-risk patients. Acad Emerg Med. 2006;13:803–6. doi: 10.1197/j.aem.2006.03.553. [DOI] [PubMed] [Google Scholar]
- 7.Hollander JE, Chang AM, Shofer FS, McCusker CM, Baxt WG, Litt HI. Coronary computed tomographic angiography for rapid discharge of low-risk patients with potential acute coronary syndromes. Ann Emerg Med. 2009;53:295–304. doi: 10.1016/j.annemergmed.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Khare RK, Powell ES, Venkatesh AK, Courtney DM. Diagnostic uncertainty and costs associated with current emergency department evaluation of low risk chest pain. Crit Pathw Cardiol. 2008;7:191–6. doi: 10.1097/HPC.0b013e318176faa1. [DOI] [PubMed] [Google Scholar]
- 9.Hermann LK, Weingart SD, Duvall WL, Henzlova MJ. The limited utility of routine cardiac stress testing in emergency department chest pain patients younger than 40 years. Ann Emerg Med. 2009;54:12–6. doi: 10.1016/j.annemergmed.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Nabi F, Chang SM, Pratt CM, et al. Coronary artery calcium scoring in the emergency department: identifying which patients with chest pain can be safely discharged home. Ann Emerg Med. 2010;56:220–9. doi: 10.1016/j.annemergmed.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163–70. doi: 10.1056/NEJM200004203421603. [DOI] [PubMed] [Google Scholar]
- 12.Lee RG, Kelley KL, Sawyer JK, Farese RV, Jr, Parks JS, Rudel LL. Plasma cholesteryl esters provided by lecithin:cholesterol acyltransferase and acyl-coenzyme a:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circ Res. 2004;95:998–1004. doi: 10.1161/01.RES.0000147558.15554.67. [DOI] [PubMed] [Google Scholar]
- 13.Willner EL, Tow B, Buhman KK, et al. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA. 2003;100:1262–7. doi: 10.1073/pnas.0336398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudel LL, Haines J, Sawyer JK, Shah R, Wilson MS, Carr TP. Hepatic origin of cholesteryl oleate in coronary artery atherosclerosis in African green monkeys. Enrichment by dietary monounsaturated fat. J Clin Invest. 1997;100:74–83. doi: 10.1172/JCI119524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Öhrvall M, Berglund L, Salminen I, Lithell H, Aro A, Vessby B. The serum cholesterol ester fatty acid composition but not the serum concentration of alpha tocopherol predicts the development of myocardial infarction in 50-year-old men: 19 years follow-up. Atherosclerosis. 1996;127:65–71. doi: 10.1016/s0021-9150(96)05936-9. [DOI] [PubMed] [Google Scholar]
- 16.Antman EM, Cohen M, Bernink PJLM, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–42. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 17.Pollack CV, Jr, Sites FD, Shofer FS, Sease KL, Hollander JE. Application of the TIMI risk score for unstable angina and non-ST elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med. 2006;13:13–8. doi: 10.1197/j.aem.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 18.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Hollander JE, Blomkalns AL, Brogan GX, et al. Standardized reporting guidelines for studies evaluating risk stratification of ED patients with potential acute coronary syndromes. Acad Emerg Med. 2004;11:1331–40. doi: 10.1197/j.aem.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Miller CD, Litt HI, Askew K, et al. Implications of 25% to 50% coronary stenosis with cardiac computed tomographic angiography in ED patients. Am J Emerg Med. 2011 doi: 10.1016/j.ajem.2011.02.015. in press. [DOI] [PubMed] [Google Scholar]
- 21.Thomas JD, Zoghbi WA, Beller GA, et al. ACCF 2008 Training Statement on Multimodality Noninvasive Cardiovascular Imaging A Report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training Developed in Collaboration With the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society for Vascular Medicine. J Am Coll Cardiol. 2009;53:125–46. doi: 10.1016/j.jacc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 23.Garber DW, Kulkarni KR, Anantharamaiah GM. A sensitive and convenient method for lipoprotein profile analysis of individual mouse plasma samples. J Lipid Res. 2000;41:1020–6. [PubMed] [Google Scholar]
- 24.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 25.Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077–80. [PubMed] [Google Scholar]
- 26.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 27.Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimonious parametric modeling. Appl Statist. 1994;43(3):429–67. [Google Scholar]
- 28.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 29.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 30.Cook NR, et al. Comments on ’Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond’ by M. J. Pencina. Stat Med. 2008;27:191–5. doi: 10.1002/sim.2987. [DOI] [PubMed] [Google Scholar]
- 31.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–8. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 32.Ma J, Folsom A, Lewis L, Eckfeldt J. Relation of plasma phospholipid and cholesterol ester fatty acid composition to carotid artery intima-media thickness: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 1997;65:551–9. doi: 10.1093/ajcn/65.2.551. [DOI] [PubMed] [Google Scholar]
- 33.Warensjo E, Sundstrom J, Vessby B, Cederholm T, Riserus U. Markers of dietary fat quality and fatty acid desaturation as predictors of total and cardiovascular mortality: a populationbased prospective study. Am J Clin Nutr. 2008;88:203–9. doi: 10.1093/ajcn/88.1.203. [DOI] [PubMed] [Google Scholar]
- 34.Bell TA, 3rd, Kelley K, Wilson MD, Sawyer JK, Rudel LL. Dietary fat-induced alterations in atherosclerosis are abolished by ACAT2-deficiency in ApoB100 only, LDLr-/- mice. Arterioscler Thromb Vasc Biol. 2007;27:1396–402. doi: 10.1161/ATVBAHA.107.142802. [DOI] [PubMed] [Google Scholar]
- 35.Limkakeng A, Jr, Gibler WB, Pollack C, et al. Combination of Goldman risk and initial cardiac troponin I for emergency department chest pain patient risk stratification. Acad Emerg Med. 2001;8:696–702. doi: 10.1111/j.1553-2712.2001.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 36.Marsan RJ, Jr, Shaver KJ, Sease KL, Shofer FS, Sites FD, Hollander JE. Evaluation of a clinical decision rule for young adult patients with chest pain. Acad Emerg Med. 2005;12:26–31. doi: 10.1197/j.aem.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 37.Han JH, Lindsell CJ, Storrow AB, et al. The role of cardiac risk factor burden in diagnosing acute coronary syndromes in the emergency department setting. Ann Emerg Med. 2007;49:145–52. doi: 10.1016/j.annemergmed.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 38.Anderson JLAC, Antman EM, Bridges CR, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non–ST-Elevation Myocardial Infarction) Circulation. 2007;116:e148–304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 39.Than M, Cullen L, Reid CM, et al. A 2-h diagnostic protocol to assess patients with chest pain symptoms in the Asia-Pacific region (ASPECT): a prospective observational validation study. Lancet. 2011;377:1077–84. doi: 10.1016/S0140-6736(11)60310-3. [DOI] [PubMed] [Google Scholar]
- 40.Backus BE, Six AJ, Kelder JC, et al. Chest pain in the emergency room: a multicenter validation of the HEART score. Crit Pathw Cardiol. 2010;9:164–9. doi: 10.1097/HPC.0b013e3181ec36d8. [DOI] [PubMed] [Google Scholar]
- 41.Hess EP, Brison RJ, Perry JJ, et al. Development of a clinical prediction rule for 30-Day cardiac events in emergency department patients with chest pain and possible acute coronary syndrome. Ann Emerg Med. 2012;59(2):115–25. doi: 10.1016/j.annemergmed.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 42.Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–44. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 43.Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–32. doi: 10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 44.Cook TD. Advanced statistics: up with odds ratios! A case for odds ratios when outcomes are common. Acad Emerg Med. 2002;9:1430–4. doi: 10.1111/j.1553-2712.2002.tb01616.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.