Abstract
The synthesis of phenylene-ethynylene rods and their use as rigid spacers is described. Alternation of a Sonogashira reaction and silyl group cleavage was used to obtain rigid spacers with even and odd numbers of phenylene units. Preliminary applications of these rods in divalent systems are shown. Inhibition studies with Pseudomonas Aeruginosa lectin LecA showed that the rigid spacer proved greatly beneficial for the inhibitory potency.
Keywords: multivalent carbohydrates, LecA inhibition, phenylene ethynylene, rigid spacers, Sonogashira reaction
Introduction
Linker or spacer molecules have a wide range of applications in many areas of chemistry as bridging molecules between separate functional units. Spacers are often flexible but depending on the nature of the application, efforts have been made to make rigid linkages between functional units. Examples of these have been reported in areas such as nanoelectronics and nanooptics [1], surfactants [2–4], photoelectrochemical detection [5], catalysis [6], glycosylation reactions [7], and carbohydrate–protein interactions [8]. Many different strategies and molecule types have been used depending on the desired geometry, such as ring formation [9], carbohydrate–triazole conjugation [10], aryl–alkyne linked structures [11–15] and the use of DNA as a rigid bridge between silver nanoparticles and quantum dots [5]. Among the rigid linking units the phenylene-ethynylene unit has seen considerable interest in sensor [16] and molecular-electronics applications [17]. This is due to the specific fluorescent, conducting and electrochemical properties that the conjugated system confers to the molecule [18]. In the study of carbohydrate–protein interactions, it is now well known that making a system multivalent increases the binding or inhibitory potency of ligands, whose monovalent counterparts would otherwise be too weak to have biological relevance [19–22]. The spacer is an important factor in the design of an effective multivalent ligand [23]. While most systems reported thus far contain flexible spacers, there is a major untapped potential for systems based on rigid spacers, even though some flexibility may be needed to overcome design imperfections [10].
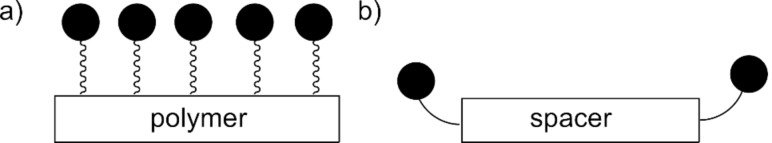
We herein describe the synthesis of rigid spacers of various lengths based on phenylene-ethynylene units and their incorporation into divalent ligands. For design purposes this system has the advantage that due to the high rigidity and linearity it is easy to predict their length, as was recently shown by EPR measurements [24]. The solubility of rigid hydrophobic spacers in an aqueous environment is notoriously poor, and therefore PEG attachments have been employed. Such PEG units were also incorporated in glycopolymers based on the phenylene-ethynylene repeating units by Seeberger and co-workers (see schematically in Figure 1a), who used them for the detection of bacteria [25]. The fluorescent properties of the polymer allowed a fast detection of the E. coli bacteria. Brewer et al. reported a divalent inhibitor with a short rigid spacer containing just a single phenylene-ethynylene [26]. The inhibition shown by this compound was disappointing since it was less potent than its flexible divalent counterpart. There are no other examples in the literature that show inhibition studies of lectins using divalent ligands directly connected by a rigid spacer based on phenylene-ethynylene units (Figure 1b).
Figure 1.
Schematic depiction of (a) a rigid phenylene-ethynylene polymer core with ligands attached via flexible chains, and (b) a divalent ligand using phenylene-ethynylene as a rigid spacer connecting the ligands.
In order to systematically study the effects of spacer lengths on the binding potencies of divalent ligands, having access to a series of spacers of well-defined lengths was imperative. We here report the synthesis of a series of spacers based on phenylene-ethynylene building blocks (Figure 2), with distinct syntheses for the compounds containing an even and an odd number of aromatic rings. One of the spacers was incorporated into the structure of a divalent galactoside ligand and was used to inhibit the virulence-linked lectin LecA of Pseudomonas aeruginosa [27–28].
Figure 2.
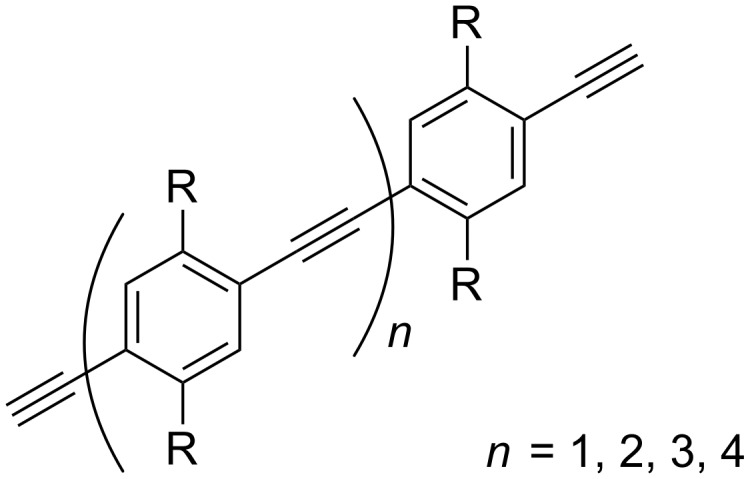
Generic structure of spacers containing an even (n = 1, 3) and an odd (n = 2, 4) number of units.
Results and Discussion
Synthetic strategies
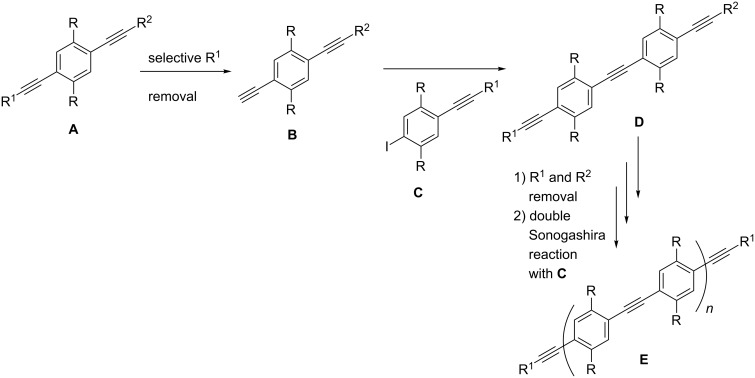
Depending on the number of units in the spacer, two different routes can be applied. The pathway followed to obtain rods containing an even number of units is shown in Figure 3. The R group on the ring is used to increase the solubility of the system. The strategy relies on orthogonal protecting groups R1 and R2 of structure A, to enable the selective deprotection needed to make B. Its free alkyne moiety can undergo a Sonogashira reaction with C to give D. At this point the removal of the protecting group R1 and R2 can be performed, to either couple the ligands or elongate the system by a double Sonogashira reaction.
Figure 3.
Synthetic strategy for rigid spacers with an even number of units.
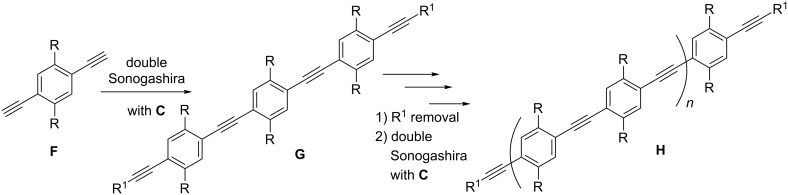
The strategy to prepare spacers containing an odd number of units is shown in Figure 4. The strategy is more straightforward since it does not require any orthogonal deprotection step. Starting with F, a double Sonogashira reaction with C should yield the three-unit system G. Removal of the protecting groups R1 can be performed, to either couple the ligands or elongate the system by a double Sonogashira reaction.
Figure 4.
Synthetic strategy for rigid spacers with an odd number of units.
Synthesis of the building blocks
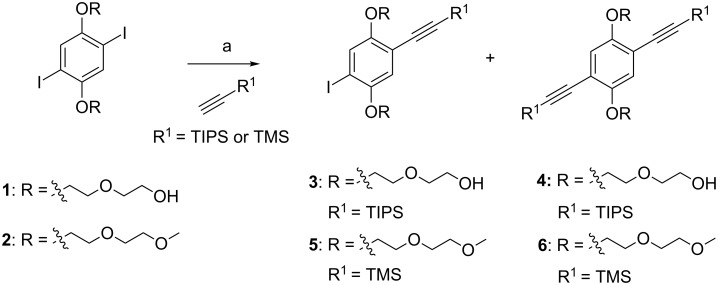
The building blocks were prepared as shown in Scheme 1. In the general structures shown in Figure 3 and Figure 4 the R group is used to increase the solubility. For this purpose diethylene glycol was used as a side chain, which terminated as a free hydroxy group for 1 and a methoxy group for 2. Silyl groups were used as selective protective groups for the alkyne moiety. Monoalkyne 3 and bisalkyne 4 were made from 1 [29] by a Sonogashira reaction with TIPS-acetylene in 31% and 50%, respectively. Similarly, 5 and 6 were obtained from 2, in agreement with a recent literature report [16]. The use of the microwave reactor allowed a shorter reaction time (20 min at 60 °C) than the one reported in the literature (24 h at 40 °C for 6 and 24 h at 10 °C for 5).
Scheme 1.
Synthesis of building blocks; (a) from 1, Pd(PPh3)4 , CuI, PPh3, TEA, toluene, 50 °C, 5 h, 31% for 3 and 50% for 4; from 2, PdCl2(PPh3)2 , CuI, TEA, THF, microwave, 60 °C, 20 min, 35% for 5 and 53% for 6.
Synthesis of a two-unit spacer
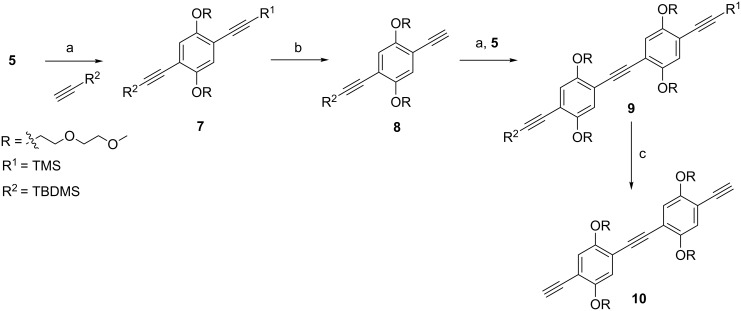
The strategy of Figure 3 was applied to the synthesis of the two-unit spacer. In order to obtain our orthogonally protected intermediate 7, mono-iodo compound 5 was coupled with tert-butyl(ethynyl)dimethylsilane (TBDMS-acetylene, Scheme 2). From 7 the more labile TMS group was removed by using K2CO3 to give 8. This compound was coupled to 5 by a Sonogashira reaction to give the protected two-unit spacer 9. Removal of its silyl protecting groups with TBAF yielded the desired two-unit spacer 10. A slightly different strategy (see Supporting Information, Scheme S1) was used starting from the mono-iodo derivative 3, because partial silyl migration to the free hydroxy groups was observed while attempting mono-desilylation.
Scheme 2.
Synthesis of a two-unit spacer. (a) PdCl2(PPh3)2, CuI, TEA, THF, microwave, 60 °C, 20 min, 81% for 7, 79% for 9; (b) K2CO3, MeOH/CH2Cl2, 45 min, 78%; (c) TBAF, THF, 84%.
Synthesis of three-unit spacers
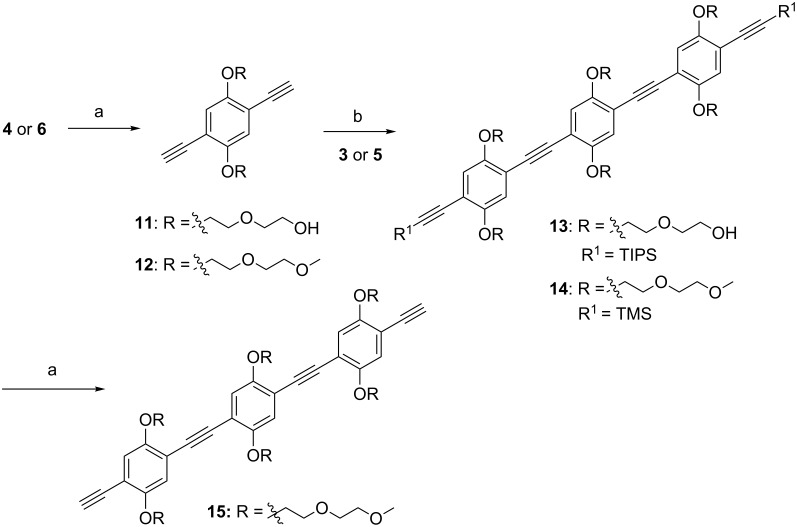
To obtain the three-unit spacer, the odd strategy (Figure 4) was applied starting with compound F. In order to make F, the silyl protecting groups of both 4 and 6 were removed (Scheme 3). For 4 the TIPS groups were removed with an excess of TBAF to give 11. Compound 6, which bears the TMS group, was treated with K2CO3 to afford 12. Both 11 and 12 were elongated in a double Sonogashira reaction with 3 and 5, respectively. Of the products, 14 was deprotected by using K2CO3 to yield the three-unit spacer 15, according to a recent literature report [16].
Scheme 3.
Synthesis of three-unit spacers. (a) from 4: TBAF, THF, 62%; from 6 and 14: K2CO3, MeOH/CH2Cl2, 45 min, 85–88%; (b) from 11: Pd(PPh3)4, CuI, PPh3, TEA, toluene, 50 °C, 14 h, 50%; from 12: PdCl2(PPh3)2, CuI, TEA, THF, microwave, 60 °C, 20 min, 75%.
Synthesis of a four-unit spacer
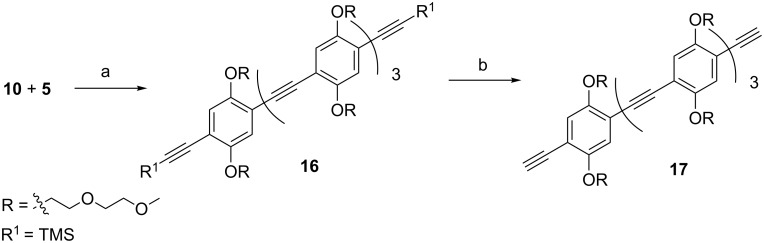
The four-unit spacer was synthesized starting from the two-unit spacer 10 and the iodo compound 5 through a double Sonogashira reaction to give 16 (Scheme 4). Deprotection of the two alkyne moieties with K2CO3 afforded the four-unit spacer 17.
Scheme 4.
Synthesis of a four-unit spacer. (a) PdCl2(PPh3)2, CuI, TEA, THF, microwave, 60 °C, 20 min, 73%; (b) K2CO3, MeOH/CH2Cl2, 45 min, 76%.
Synthesis of a five-unit spacer
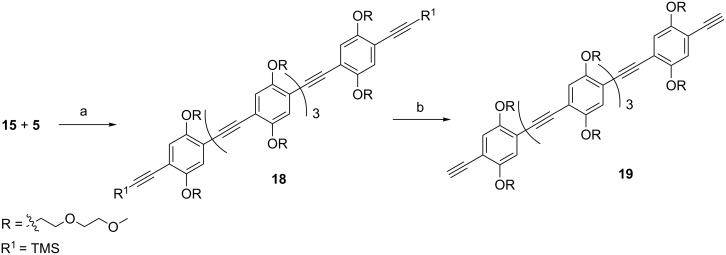
The synthesis of the five-unit spacer started with the elongation of the three-unit spacer 15 through a double Sonogashira reaction with iodo compound 5 to give 18 (Scheme 5). The five-unit spacer 19 was obtained after deprotection of the alkyne moieties with K2CO3.
Scheme 5.
Synthesis of a five-unit spacer. (a) PdCl2(PPh3)2, CuI, TEA, THF, microwave, 60 °C, 20 min, 70%; (b) K2CO3, MeOH/CH2Cl2, 45 min, 75%.
Preliminary application
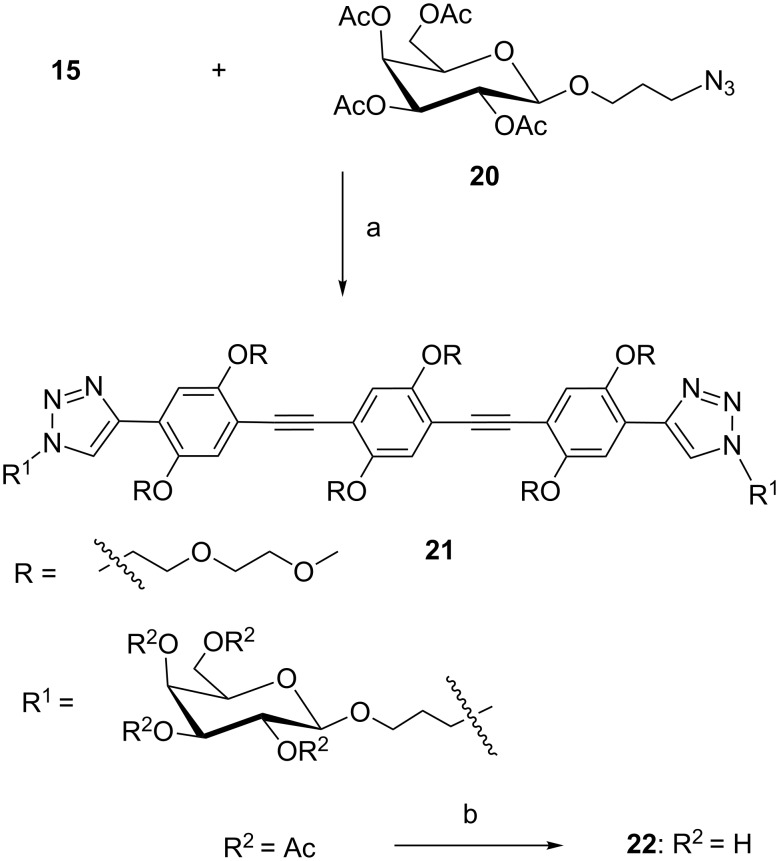
As part of our program on bacterial adhesion inhibition by multivalent carbohydrates, the bacterial lectin LecA, a virulence factor of the problematic pathogen Pseudomonas aeruginosa is a target of interest [30–31]. This tetrameric lectin binds galactosides and the shortest distance between two binding sites is around 26 Å [20,28]. We previously noted that the use of a rigid spacer with some flexibility in the aglycon chain connecting the galactose ligands, led to more potent inhibition [10]. Since the distance to cover is ca. 26 Å, the best match in the phenylene-ethynylene series is the three-unit spacer 15. Just the spacer, without the aglycon linking moiety measures around 22 Å. Coupling of the galactose ligand and the flexible aglycon part should result in a promising compound. CuAAC of 15 with the ligand 20 was performed as shown in Scheme 6 to give 21. The acetyl protecting groups were removed by using NaOMe in MeOH, yielding final product 22.
Scheme 6.
Synthesis of divalent ligand 22. (a) CuSO4·5H2O, Na-ascorbate, DMF/H2O, microwave, 80 °C, 40 min, 85%; (b) NaOMe, MeOH, 41%.
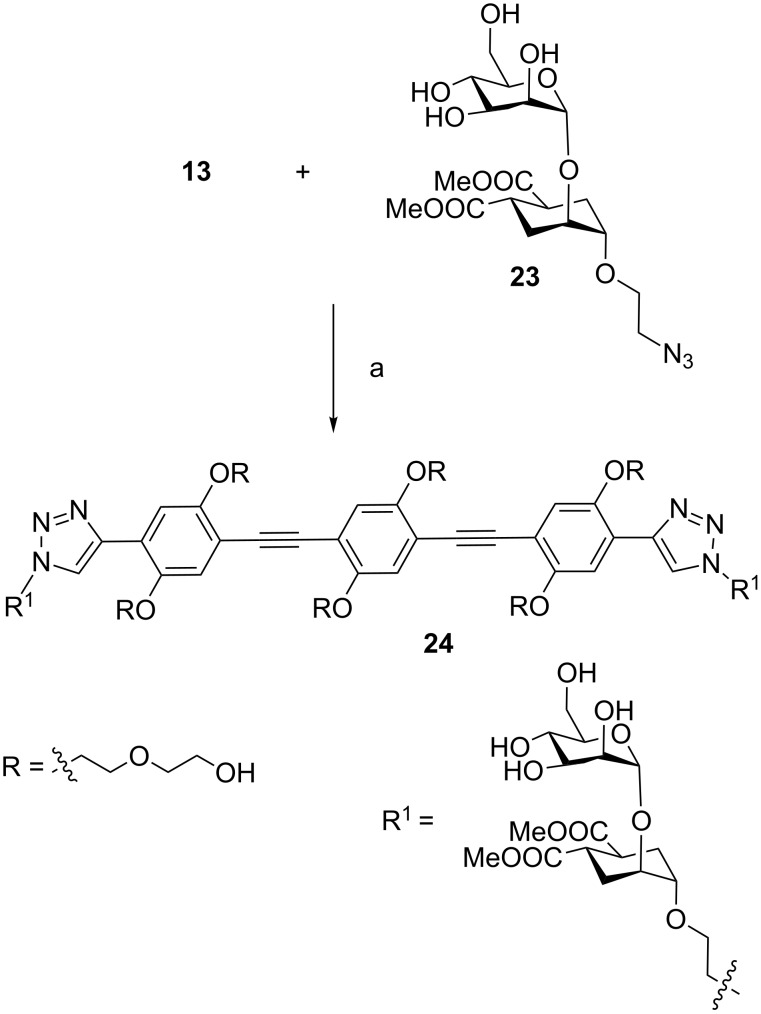
Compound 13, bearing hydroxy-terminated diethylene glycol chains, has the potential of solubilizing in water constructs bearing active spearheads less hydrophilic than simple sugars. We have been developing pseudo-disaccharide molecules such as 23 [32] (Scheme 7) as mimics of mannose disaccharides for the interaction with DC-SIGN and other C-lectins [33–35]. This molecule and its derivatives [36] contain lipophilic moieties that generally increase their affinity for the target proteins, but can create solubility problems. Desilylation of 13 (TBAF, THF, Scheme 7) followed by in situ CuAAC with the pseudo-disaccharide 23 led to the divalent ligand 24, which was found to be fully soluble in water, at least up to millimolar concentrations. Compound 24 is a mimic for DC-SIGN inhibition and its bioactivity will be tested elsewhere.
Scheme 7.
Synthesis of divalent ligand 24. (a) 13, TBAF in THF, rt, 1 h, then H2O, TBTA, CuSO4·5H2O, Na-ascorbate, 23, rt, 18 h, 76%.
Inhibition studies
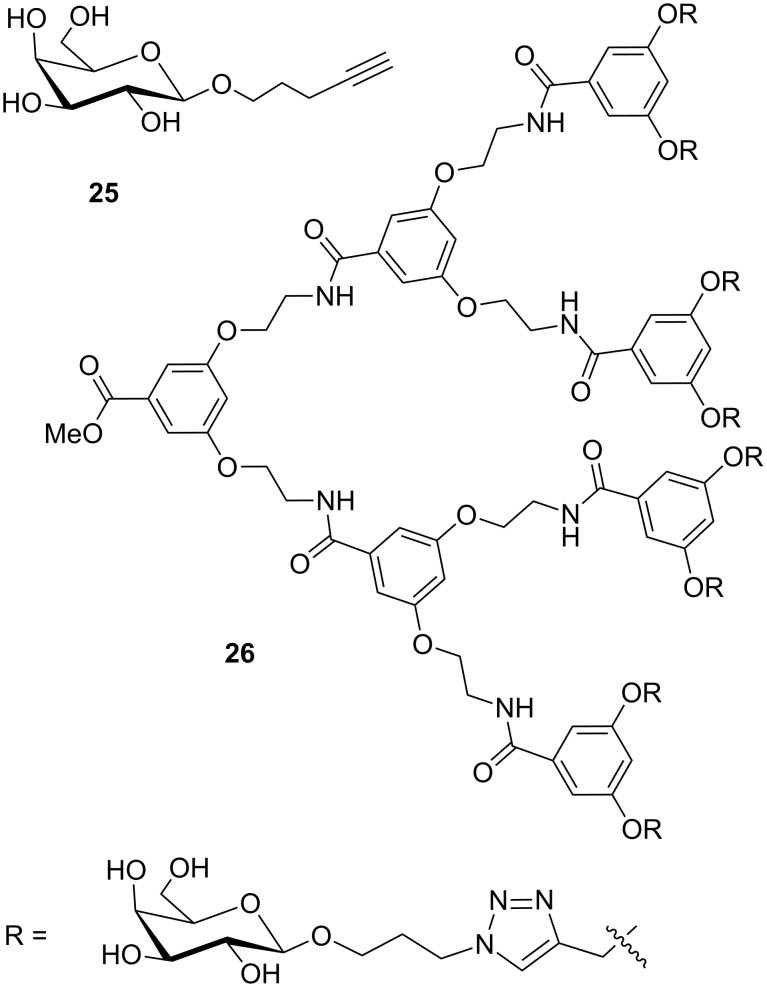
The inhibitory potency of 22 for LecA was studied in an ELISA type assay by using a glycochip as the solid phase [10]. In this assay an IC50 value of 0.9 μM was determined (Table 1). This compared favorably with the monovalent reference compound 25 (Figure 5), which exhibited an IC50 of 120 μM [10]. Similarly, the divalent compound 22 was a more potent inhibitor than the higher valency compound 26, especially when expressed as the relative potency per sugar, which is 11 for the tetravalent 26 [37–38] and 67 for 22.
Table 1.
Inhibitory potency of mono, di- and tetravalent galactosides on LecA bindinga.
| Compound | Valency | IC50/μM | Relative Potency (per sugar) |
| 25 | 1 | 120 | 1 |
| 26 | 4 | 2.7 | 44 (11) |
| 22 | 2 | 0.90 | 133 (67) |
aFITC-labeled LecA, 20 μg mL−1 binding to a galactoside functionalized surface.
Figure 5.
Previously tested compounds.
Conclusion
In this work a strategy for the synthesis of rigid spacers or rods of different length based on phenylene-ethynylene units was developed. On the phenyl ring, two versions of a solubilizing diethylene glycol moiety were used, one terminating in a hydroxy and one terminating in a methoxy group. The hydroxy version led to some migration of the silyl protecting group upon attempted monodeprotection, and alternative strategies had to be devised for the synthesis of the two-unit spacer. Compounds 17 and 19, containing four and five phenylene-ethynylene units, respectively, can still be further elongated, depending on the need of the project, thus enabling the preparation of long spacers with a well-defined number of monomeric units. The CuAAC of the three unit spacer 15 with a galactose ligand gave the divalent ligand 21 in good yield. After deprotection this compound was used to inhibit the lectin LecA from Pseudomonas aeruginosa. A major potency increase was seen with the divalent structure based on the phenylene-ethynylene spacer, with an IC50 of 0.9 μM, i.e., a 133-fold potency increase over a monovalent reference compound, clearly showing the potential for spacers of this nature.
It was also shown that the three-unit spacer 13 could be used in a one-pot desilylation and CuAAC reaction to give 24, which was found to be fully soluble in water, despite the more lipophilic nature of the active ligand. This finding paves the way for the synthesis and evaluation of polyvalent glycomimetics with regularly spaced cores to be used for Man-specific C-lectin inhibition assays. With access to structural data of target lectins, the design of multivalent inhibitors can be performed on a customized level, where the selection of the proper spacer is based on the information about the binding site, such as its density, orientation and position. Furthermore, the rigidity of the rods described above can contribute to overcoming the entropic penalty of flexible multivalent scaffolds, thus improving the overall activity of the ligands.
Supporting Information
Synthetic procedures and spectral data.
Acknowledgments
This research is supported by the Dutch Technology Foundation STW, applied science division of NWO and the Technology Program of the Ministry of Economic Affairs. N.V. was supported by a European fellowship under the ITN project Carmusys (FP7-MC-ITN-213592). We thank the COST Action CM1102 Multiglyconano for support and Stefania Ordanini for optimization of the synthesis of 30 (see Supporting Information File 1, Scheme S1). M.R.C. acknowledges Fundació Crèdit Andorrà for a masters grant.
References
- 1.Rahman A F M M, Wang F, Matsuda K, Kimura T, Komatsu N. Chem Sci. 2011;2:862–867. doi: 10.1039/c0sc00635a. [DOI] [Google Scholar]
- 2.Zhu D-Y, Cheng F, Chen Y, Jiang S-C. Colloids Surf, A. 2012;397:1–7. doi: 10.1016/j.colsurfa.2012.01.006. [DOI] [Google Scholar]
- 3.Zhu S, Liu L, Cheng F. J Surfactants Deterg. 2011;14:221–225. doi: 10.1007/s11743-010-1226-3. [DOI] [Google Scholar]
- 4.Mivehi L, Bordes R, Holmberg K. Langmuir. 2011;27:7549–7557. doi: 10.1021/la200539a. [DOI] [PubMed] [Google Scholar]
- 5.Zhao W-W, Yu P-P, Shan Y, Wang J, Xu J-J, Chen H-Y. Anal Chem. 2012;84:5892–5897. doi: 10.1021/ac300127s. [DOI] [PubMed] [Google Scholar]
- 6.Framery E, Andrioletti B, Lemaire M. Tetrahedron: Asymmetry. 2010;21:1110–1124. doi: 10.1016/j.tetasy.2010.04.028. [DOI] [Google Scholar]
- 7.Tiwari V K, Kumar A, Richard R, Schmidt R R. Eur J Org Chem. 2012:2945–2956. doi: 10.1002/ejoc.201101815. [DOI] [Google Scholar]
- 8.Deniaud D, Julienne K, Gouin S G. Org Biomol Chem. 2011;9:966–979. doi: 10.1039/c0ob00389a. [DOI] [PubMed] [Google Scholar]
- 9.Leyden R, Velasco-Torrijos T, André S, Gouin S, Gabius H-J, Murphy P V. J Org Chem. 2009;74:9010–9026. doi: 10.1021/jo901667r. [DOI] [PubMed] [Google Scholar]
- 10.Pertici F, Pieters R J. Chem Commun. 2012;48:4008–4010. doi: 10.1039/c2cc30234a. [DOI] [PubMed] [Google Scholar]
- 11.Martos-Maldonado M C, Quesada-Soriano I, Casas-Solvas J M, García-Fuentes L, Vargas-Berenguel A. Eur J Org Chem. 2012:2560–2571. doi: 10.1002/ejoc.201101598. [DOI] [Google Scholar]
- 12.Lieffrig J, Yamamoto H M, Kusamoto T, Cui H, Jeannin O, Fourmigué M, Kato R. Cryst Growth Des. 2011;11:4267–4271. doi: 10.1021/cg200843w. [DOI] [Google Scholar]
- 13.Ramachandra S, Schuermann K C, Edafe F, Belser P, Nijhuis C A, Reus W F, Whitesides G M, De Cola L. Inorg Chem. 2011;50:1581–1591. doi: 10.1021/ic1002868. [DOI] [PubMed] [Google Scholar]
- 14.Touaibia M, Roy R. J Org Chem. 2008;73:9292–9302. doi: 10.1021/jo801850f. [DOI] [PubMed] [Google Scholar]
- 15.Bergeron-Brlek M, Giguère D, Shiao T C, Saucier C, Roy R. J Org Chem. 2012;77:2971–2977. doi: 10.1021/jo2025652. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Dong Y, Li J, Huang X, Cheng Y, Zhu C. Chem–Asian J. 2011;6:2725–2729. doi: 10.1002/asia.201100534. [DOI] [PubMed] [Google Scholar]
- 17.Kaliginedi V, Moreno-García P, Valkenier H, Hong W, García-Suárez V M, Buiter P, Otten J L H, Hummelen J C, Lambert C J, Wandlowski T. J Am Chem Soc. 2012;134:5262–5275. doi: 10.1021/ja211555x. [DOI] [PubMed] [Google Scholar]
- 18.Pinto M R, Schanze K S. Synthesis. 2002:1293–1309. doi: 10.1055/s-2002-32541. [DOI] [Google Scholar]
- 19.Fan E, Zhang Z, Minke W E, Hou Z, Verlinde C L M J, Hol W G J. J Am Chem Soc. 2000;122:2663–2664. doi: 10.1021/ja993388a. [DOI] [Google Scholar]
- 20.Kitov P I, Sadowska J M, Mulvey G, Armstrong G D, Ling H, Pannu N S, Read R J, Bundle D R. Nature. 2000;403:669–672. doi: 10.1038/35001095. [DOI] [PubMed] [Google Scholar]
- 21.Branderhorst H M, Liskamp R M J, Visser G M, Pieters R J. Chem Commun. 2007:5043–5045. doi: 10.1039/b711070g. [DOI] [PubMed] [Google Scholar]
- 22.Schwefel D, Maierhofer C, Beck J G, Seeberger S, Diederichs K, Möller H M, Welte W, Wittmann V. J Am Chem Soc. 2010;132:8704–8719. doi: 10.1021/ja101646k. [DOI] [PubMed] [Google Scholar]
- 23.Braun P, Nägele B, Wittmann V, Drescher M. Angew Chem, Int Ed. 2011;50:8428–8431. doi: 10.1002/anie.201101074. [DOI] [PubMed] [Google Scholar]
- 24.Jeschke G, Sajid M, Schulte M, Ramezanian N, Volkov A, Zimmermann H, Godt A. J Am Chem Soc. 2010;132:10107–10117. doi: 10.1021/ja102983b. [DOI] [PubMed] [Google Scholar]
- 25.Disney M D, Zheng J, Swager T M, Seeberger P H. J Am Chem Soc. 2004;126:13343–13346. doi: 10.1021/ja047936i. [DOI] [PubMed] [Google Scholar]
- 26.Dam T K, Oscarson S, Roy R, Das S K, Pagé D, Macaluso F, Brewer C F. J Biol Chem. 2005;280:8640–8646. doi: 10.1074/jbc.M412827200. [DOI] [PubMed] [Google Scholar]
- 27.Imberty A, Wimmerová M, Mitchell E P, Gilboa-Garber N. Microbes Infect. 2004;6:221–228. doi: 10.1016/j.micinf.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Cioci G, Mitchell E P, Gautier C, Wimmerová M, Sudakevitz D, Pérez S, Gilboa-Garber N, Imberty A. FEBS Lett. 2003;555:297–301. doi: 10.1016/S0014-5793(03)01249-3. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Q, Swager T M. J Am Chem Soc. 1995;117:12593–12602. doi: 10.1021/ja00155a023. [DOI] [Google Scholar]
- 30.Pieters R J. Med Res Rev. 2007;27:796–816. doi: 10.1002/med.20089. [DOI] [PubMed] [Google Scholar]
- 31.Pieters R J. Adv Exp Med Biol. 2011;715:227–240. doi: 10.1007/978-94-007-0940-9_14. [DOI] [PubMed] [Google Scholar]
- 32.Mari S, Posteri H, Marcou G, Potenza D, Micheli F, Cañada F J, Jiménez-Barbero J, Bernardi A. Eur J Org Chem. 2004:5119–5125. doi: 10.1002/ejoc.200400520. [DOI] [Google Scholar]
- 33.Reina J J, Sattin S, Invernizzi D, Mari S, Martínez-Prats L, Tabarani G, Fieschi F, Delgado R, Nieto P M, Rojo J, et al. ChemMedChem. 2007;2:1030–1036. doi: 10.1002/cmdc.200700047. [DOI] [PubMed] [Google Scholar]
- 34.Luczkowiak J, Sattin S, Sutkevičiūtė I, Reina J J, Sánchez-Navarro M, Thépaut M, Martínez-Prats L, Daghetti A, Fieschi F, Delgado R, et al. Bioconjugate Chem. 2011;22:1354–1365. doi: 10.1021/bc2000403. [DOI] [PubMed] [Google Scholar]
- 35.Orsini F, Villa P, Parrella S, Zangari R, Zanier E R, Gesuete R, Stravalaci M, Fumagalli S, Ottria R, Reina J J, et al. Circulation. 2012;126:1484–1494. doi: 10.1161/CIRCULATIONAHA.112.103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obermajer N, Sattin S, Colombo C, Bruno M, Švajger U, Anderluh M, Bernardi A. Mol Diversity. 2011;15:347–360. doi: 10.1007/s11030-010-9285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parera Pera N, Branderhost H M, Kooij R, Maierhofer C, van der Kaanden M, Liskamp R M J, Wittman V, Ruijtenbeek R, Pieters R J. ChemBioChem. 2010;11:1896–1904. doi: 10.1002/cbic.201000340. [DOI] [PubMed] [Google Scholar]
- 38.Branderhorst H M, Ruijtenbeek R, Liskamp R M J, Pieters R J. ChemBioChem. 2008;9:1836–1844. doi: 10.1002/cbic.200800195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthetic procedures and spectral data.