Abstract
Background:
The aim of this study was to compare clinical and pathological outcomes after neoadjuvant chemotherapy between oestrogen receptor (ER)-positive invasive pure lobular carcinoma (ILC) and invasive ductal carcinoma (IDC).
Methods:
This analysis included 1895 patients (n=177 ILC; n=1718 IDC), with stage I–III breast cancer, who received neoadjuvant chemotherapy. Clinical and pathological response rates, the frequency of positive surgical margins and rate of breast-conserving surgery were compared.
Results:
There was a trend for fewer good clinical responses in ILC compared with IDC. Tumour downstaging was significantly less frequent in ILC. Positive or close surgical resection margins were more frequent in ILC, and breast-conserving surgery was less common (P<0.001). These outcome differences remained significant in multivariate analysis, including tumour size, nodal status, age, grade and type of chemotherapy. Invasive pure lobular carcinoma was also associated with a significantly lower pathological complete response (pCR) rate in univariate analysis, but this was no longer significant after adjusting for tumour size and grade.
Conclusion:
Neoadjuvant chemotherapy results in lower rates of clinical benefit, including less downstaging, more positive margins and fewer breast-conserving surgeries in ER-positive ILC compared with ER-positive IDC. Pathological complete responses are rare in both groups, but do not significantly differ after adjusting for other variables.
Keywords: breast cancer, lobular carcinoma, neoadjuvant chemotherapy, pathological complete response, clinical response, margins
Pure invasive lobular carcinomas (ILCs) account for 10–15% of all breast cancers and are almost invariably oestrogen receptor (ER)-positive and tend to have low histological grades (grades I and II) (Fisher et al, 1975; Wellings et al, 1975; World Health Organisation, 1982). Invasive pure lobular carcinoma is characterised by small, round cells with scant cytoplasm that infiltrate the stroma in single files, which makes it more difficult to palpate or detect this type of cancer with mammogram (Katz et al, 2007). This histological feature may also lead to higher rates of positive surgical margins after breast-conserving surgery (Porter et al, 1999; Molland et al, 2004; Waljee et al, 2008; Boughey et al, 2009). The rates of pathological complete response (pCR) to neoadjuvant chemotherapy are also significantly lower in ILC compared with invasive ductal carcinomas (IDCs) (Cristofanilli et al, 2005). Several investigators have suggested that ILC histology is a relative contraindication for preoperative chemotherapy because the expected benefit is modest because of less frequent clinical responses, low pCR rates and more frequent positive surgical margins (Katz et al, 2007; Boughey et al, 2009; Purushotham et al, 2010).
Comparing outcomes between ILC and IDC in general has substantial limitations because of the uneven distribution of confounders, including histological grade and ER status between these two distinct histological subtypes. The question whether histology itself, after adjusting for differences in grade and ER, remains a predictor of lower response rates and higher rates of positive margins remain controversial. Previous studies performed multivariate analysis to address this question and most results suggested that histology remains an important independent factor. However, Boughey et al (2009) and Wagner et al (2009) reported frequent clinical responses in ILCs to preoperative chemotherapy and margin positivity rates after breast-conserving surgery were similar between patients who received neoadjuvant chemotherapy and those who did not. In this study, we compare rates of pathological response, surgical margin status and rates of breast-conserving surgery between ER-positive ILC and IDC that received neoadjuvant chemotherapy. We excluded ER-negative tumours from the analysis to eliminate an important confounder.
Patients and methods
Patient population
Patients were identified for this study from a prospectively maintained clinical database of the University of Texas MD Anderson Cancer Centre. Patients were selected for inclusion if they had ER-positive stage I–III breast cancer diagnosed between 1990 and 2010 and received neoadjuvant chemotherapy. This search initially identified 2592 patients. After reviewing the medical records, the following patients were excluded: male breast cancer (n=12), patients with axillary metastasis without an identifiable primary breast tumour (n=13), metastatic disease at diagnosis (n=13), patients who received preoperative radiation therapy alone (n=10) or underwent partial excisional biopsy before neoadjuvant chemotherapy (n=214). We also excluded patients with rare or mixed histological subtypes (n=224) to focus on the comparison of pure lobular vs pure ductal carcinomas. Patients with overexpression of human epidermal growth factor receptor-2 (HER2) were also included. Review of medical records also revealed miscoding of ER and progesterone receptor (PR) results in 21 patients who had hormone receptor-negative breast cancer and in 189 patients who received neoadjuvant endocrine therapy alone. A total of 1895 patients were included in the final analysis (Supplementary Figure 1).
The patients received neoadjuvant chemotherapy with anthracycline-based regimen (n=236), with a taxane-based regimen (n=137) or with a combination of an anthracycline and taxane (n=1515); 263 patients also received trastuzumab in combination with neoadjuvant chemotherapy. Postoperatively, 451 patients (24%) received adjuvant chemotherapy and 1522 (81%) received adjuvant hormonal therapy. The Institutional Review Board of the University of Texas MD Anderson Cancer Centre (MDACC) in Houston approved this study.
Assessment of clinical and pathological outcomes
The pre-treatment tumour size was determined by physical examination and mammography. If the two methods yielded discordant results, the radiological measurement was used as the tumour's size. Pre-treatment lymph node status was evaluated with a combination of clinical and ultrasonographic examination. If ultrasonogram showed suspicious lymph nodes, a diagnostic fine-needle aspiration was performed. Post-treatment, residual cancer size was determined by pathological examination. All outside pathology reports and slides were reviewed by a dedicated breast pathologist at MDACC to confirm diagnosis and to assess the adequacy of ER, PR and HER2 measurements (World Health Organisation, 1982). Oestrogen receptor and PR positivity were defined as nuclear staining ⩾10% and HER2 positivity was defined as 3+ staining on immunohistochemistry or gene amplification by FISH. Histological grade was assessed following the modified Black's nuclear grading system.
After completion of neoadjuvant chemotherapy, 1827 (96.4%) patients underwent primary breast surgery and 1826 (96.5%) had axillary lymph node staging (level I and II dissection or sentinel lymph node biopsy). If invasive or in situ carcinomas were seen within 2 mm of the surgical margin on microscopic examination (i.e. positive or close tumour margins), a second operation was performed to achieve clear margins. Pathological complete response was defined as no evidence of invasive carcinoma in the breast and axillary lymph nodes.
Statistical analysis
The χ2 test (or Fisher's exact test when the sample size was small) was used to evaluate associations between categorical variables and histological subtype. The Student's t-test was used for continuous variables. We also performed stratified analysis by histological grade (grade I/II vs III). Univariate logistic regressions were performed, including histological subtype, nuclear grade, nodal status tumour size, multifocality, age, race, menopausal status, HER2 status, Ki-67 score and the type of neoadjuvant chemotherapy as variables to identify predictors of breast-conserving therapy, positive margins and pCR. From this model, an odds ratio (OR) for each variable was determined with a 95% confidence interval (CI). All significant variables from the univariate analysis were included in a subsequent multivariate analysis. Median overall survival and distant disease-free survival were determined using the Kaplan–Meier methods. All analyses were performed using R package with Survival, Design, Hmisc, Rpart and Lexis libraries (http://lib.stat.cmu.edu//R/CRAN/).
Results
Patient characteristics are summarised in Table 1: 177 patients had ILC (9%), and 1718 patients had IDC (91%). Patients with ILC were older, had larger and lower grade (grades I/II) tumours and had fewer HER2-positve cancers compared with IDC. Anthracycline-based or taxane and anthracycline combination regimens were used equally frequently in both histological groups, but trastuzumab use was more common in IDC (Table 1).
Table 1. Patient demographic and treatment clinical characteristics.
|
ILC (n=177) |
IDC (n=1718) |
||||
|---|---|---|---|---|---|
| Demographic or clinical characteristics | No. of patients | % | No. of patients | % | χ2 P-value |
|
Age (years) | |||||
| Median | 54 | 50 | <0.001 | ||
| Range |
35–62 |
|
21–83 |
|
|
|
Race | |||||
| Asian | 6 | 3 | 96 | 6 | 0.51 |
| Black | 19 | 11 | 223 | 13 | |
| White | 150 | 85 | 1375 | 80 | |
| Other |
2 |
1 |
24 |
1 |
|
|
Menopausal status | |||||
| Yes |
114 |
64 |
899 |
52 |
0.003 |
|
Concurrent bilateral breast cancer | |||||
| Yes |
16 |
9 |
74 |
4 |
0.008 |
|
Tumour size (cm) | |||||
| Median | 4.5 | 3.4 | <0.001 | ||
| Range |
0.4–12 |
|
0.4–20 |
|
|
|
Multifocal tumour | |||||
| Yes |
42 |
24 |
326 |
19 |
0.1 |
|
T stage | |||||
| T1–2 | 99 | 56 | 1187 | 69 | <0.001 |
| T3–4 |
78 |
44 |
529 |
31 |
|
|
N stage | |||||
| N1 or sup |
102 |
58 |
1089 |
63 |
0.15 |
|
AJCC stage | |||||
| I/II | 111 | 63 | 1076 | 63 | 0.94 |
| III/IV |
66 |
37 |
641 |
37 |
|
|
HER2 status | |||||
| Negative | 84 | 47 | 793 | 46 | 0.006 |
| Positive |
13 |
7 |
288 |
17 |
|
|
Nuclear grade | |||||
| I–II | 145 | 82 | 722 | 42 | <0.001 |
| III |
24 |
14 |
980 |
57 |
|
|
Ki-67 scorea | |||||
| <20 | 48 | 27 | 224 | 13 | <0.001 |
| ⩾20 |
28 |
16 |
518 |
30 |
|
|
Neoadjuvant chemotherapy regimenb | |||||
| Anthracycline and taxane based | 137 | 77 | 1378 | 80 | 0.4 |
| Anthracycline-based only | 27 | 15 | 209 | 12 | 0.5 |
| Taxane-based only | 13 | 7 | 124 | 7 | 1 |
| Trastuzumab | 9 | 5 | 254 | 15 | <0.001 |
| Neoadjuvant hormonotherapy in combination with chemotherapy | 3 | 2 | 54 | 3 | 0.4 |
| Adjuvant chemotherapy | 32 | 18 | 419 | 24 | 0.07 |
| Adjuvant hormonotherapy | 146 | 82 | 1382 | 80 | 0.6 |
| Adjuvant radiotherapy | 146 | 82 | 1311 | 76 | 0.08 |
Abbreviations: A=adriamycin; AJCC=American Joint Committee on Cancer; C=cyclophosphamide; E=epirubicin; F=5-fluorouracil; H=herceptin; HER2=human epidermal growth factor receptor 2; IDC=invasive ductal carcinoma; ILC=invasive lobular carcinoma; T=taxane.
Ki-67 score were available in 76 patients in ILC group and in 742 IDC group.
The most common regimen consisted of T, F, A or E and C (T/FAC or FEC), n=1106; FAC or FEC, n=230; FAC or FEC±H and TH (TH/FAC or FEC±H), n=218; ET or AT, n=74; T alone, n=91.
Significant downstaging was observed in both histological types (P<0.0001) (Supplementary Figure 2A), but it was more common among IDC. Forty-one per cent of ILCs had lower tumour T stage after neoadjuvant chemotherapy compared with baseline, whereas similar downstaging occurred in 64% of IDCs (P<0.0001) (Supplementary Figure 2B).
Positive or close surgical resection margins were significantly more frequent in ILC patients (19 vs 11% P=0.001) and this remained significant even after multivariate analysis, including tumour size and grade (OR=1.82; 95% CI, 1.13–2.93; P=0.01). At the end, breast-conserving surgery was less frequent in ILC patients than in IDC patients (19 vs 34% P<0.001) (Table 2) and histology remained an independent predictor of mastectomy (OR=1.86; 95% CI, 1.15–2.99; P=0.01) even after adjusting for age, tumour grade, initial tumour size, multifocality, nodal status and clinical stage (Table 3).
Table 2. Surgical and pathological outcomes.
|
ILC (n=177) |
IDC (n=1718) |
||||
|---|---|---|---|---|---|
| No. of patients | % | No. of patients | % | χ2 P-value | |
|
Final surgical outcome | |||||
| Conservative | 33 | 19 | 576 | 34 | <0.001 |
| Mastectomy | 139 | 79 | 1078 | 63 | |
| No surgery |
5 |
3 |
64 |
4 |
|
|
Pathological response | |||||
| No pCR | 165 | 93 | 1404 | 82 | <0.001 |
| pCR | 6 | 3 | 246 | 14 | |
Abbreviations: IDC=invasive ductal carcinoma; ILC=invasive lobular carcinoma; pCR=pathological complete response.
Table 3. Predictors of mastectomy after neoadjuvant chemotherapy.
|
Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Factor | ORa | 95% CI | P-value | ORa | 95% CI | P-value |
| Age (years) |
0.99 |
0.98–1.00 |
0.02 |
0.99 |
0.98–1.00 |
0.02 |
| Multifocal tumour |
|
|
<0.001 |
|
|
|
| No | 1.00 | — | — | 1.00 | — | — |
| Yes |
4.6 |
3.28–6.46 |
|
3.85 |
2.70–5.48 |
<0.001 |
| Tumour size (cm) |
1.42 |
1.33–1.51 |
<0.001 |
1.30 |
1.21–1.40 |
<0.001 |
|
N stage | ||||||
| N0 | 1.00 | — | — | 1.00 | — | — |
| N1 or sup |
2.03 |
1.66–2.48 |
<0.001 |
1.39 |
1.09–1.77 |
0.007 |
|
AJCC stage | ||||||
| I/II | 1.00 | — | — | 1.00 | — | — |
| III/IV |
3.51 |
2.78–4.41 |
<0.001 |
1.89 |
1.41–2.54 |
<0.001 |
|
Nuclear grade | ||||||
| I | 1.00 | — | — | 1.00 | — | — |
| II | 0.81 | 0.47–1.37 | 0.43 | 1.43 | 0.75–2.69 | 0.27 |
| III |
0.51 |
0.35–1.00 |
0.05 |
0.85 |
0.44–1.62 |
0.61 |
|
Histological subtype | ||||||
| IDC | 1.00 | — | — | 1.00 | — | — |
| ILC | 2.25 | 1.52–3.33 | <0.001 | 1.86 | 1.15–2.99 | 0.01 |
Abbreviations: AJCC=American Joint Committee on Cancer; CI=confidence interval; IDC=invasive ductal carcinoma; ILC=invasive lobular carcinoma; OR=odds ratio.
OR=1 is the reference; OR<1, factor associated with lower mastectomy rate; OR>1, factor associated with higher mastectomy rate.
Invasive lobular histology was also associated with significantly lower pCR rates (3.5 vs 14% P<0.001) (Table 2). In univariate analysis, multifocal tumour, higher tumour size, node-positive status and lower nuclear grade were also significantly associated with lower pCR rates. In multivariate analysis, including the above variables, histology was no longer significant (Table 4). Similarly, in an analysis stratified by grade, pCR rates were no longer significantly different between ILC and IDC (Supplementary Table 1).
Table 4. Predictors of pathological complete response.
|
Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Factor | ORa | 95% CI | P-value | ORa | 95% CI | P-value |
| Age (years) |
0.99 |
0.98–1 |
0.2 |
1 |
0.99–1.01 |
0.8 |
|
Multifocal tumoura | ||||||
| No | 1.00 | — | — | 1.00 | — | — |
| Yes |
0.69 |
0.48–1 |
0.05 |
0.75 |
0.5–1.12 |
0.15 |
|
Nuclear grade | ||||||
| I | 1.00 | — | — | 1.00 | — | — |
| II | 4.91 | 0.67–36.14 | 0.1 | 2.91 | 0.38–22.56 | 0.31 |
| III |
20.33 |
2.81–147.17 |
0.002 |
11.26 |
1.47–86.39 |
0.01 |
|
Baseline T stage | ||||||
| T1–T2 | 1.00 | — | — | 1.00 | — | — |
| T3–T4 |
0.69 |
0.51–0.93 |
0.01 |
0.65 |
0.46–0.92 |
0.01 |
|
Baseline N stage | ||||||
| N0 | 1.00 | — | — | 1.00 | — | — |
| N1 or sup |
0.73 |
0.56–0.96 |
0.02 |
0.66 |
0.49–0.9 |
0.008 |
|
Histological subtype | ||||||
| IDC | 1.00 | — | — | 1.00 | — | — |
| ILC |
0.21 |
0.09–0.47 |
<0.001 |
0.5 |
0.19–1.3 |
0.1 |
|
Neoadjuvant chemotherapy regimen | ||||||
| Taxane basedb | 2.67 | 1.56–4.59 | <0.001 | 2.14 | 1.2–3.84 | 0.01 |
| Traztuzumabc | 6 | 4.44–8.12 | <0.001 | 5.03 | 3.64–6.95 | <0.001 |
Abbreviations: CI=confidence interval; IDC=invasive ductal carcinoma; ILC=invasive lobular carcinoma; OR=odds ratio; pCR=pathological complete response.
OR=1 is the reference; OR<1, factor associated with lower pCR rate; OR>1, factor associated with higher pCR rate.
Taxane-based regimen was compared with no taxane-based regimen.
Trastuzumab regimen was compared with no trastuzumab regimen.
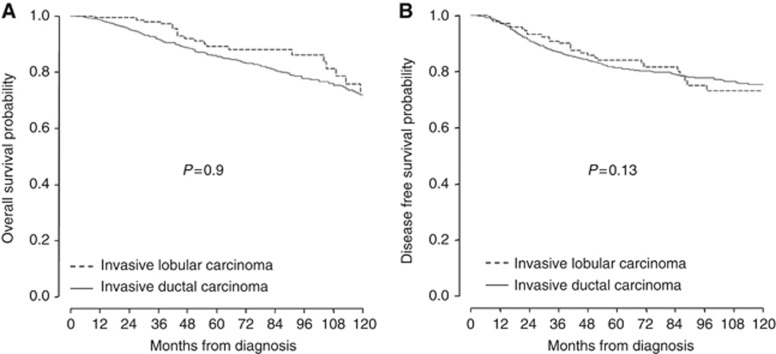
Disease-free survival and overall survival were evaluated with a median follow-up time of 44 months (range, 1–221 months). In all, 290 patients had developed a recurrence (222 distant recurrences only, 19 local recurrences only, 49 distant and local recurrences), and 262 had died. Histological type was not associated with significant difference in overall survival (hazard ratio=1.01; 95% CI, 0.7–1.47; P=0.9), disease-free survival (hazard ratio=0.92; 95% CI, 0.66–1.28; P=0.13) (Figure 1) or local recurrence-free survival (hazard ratio=0.8; 95% CI, 0.36–1.90; P=0.65) (Supplementary Figure 3).
Figure 1.
(A) Overall survival probability and (B) disease-free survival probability by histological subtype.
Discussion
We examined if patients with pure ILC benefit differently from neoadjuvant chemotherapy than patients with IDC. Approximately, 30–40% of IDCs are ER-negative and these cancers have different chemotherapy sensitivity and clinical behaviour compared with ER-positive IDCs (Arpino et al, 2004; Rouzier et al, 2005). Previous studies tried to adjust for the variable distribution of ER status by performing multivariate analysis. However, multivariate analysis has limitations particularly when confounders are only partially independent (Katz, 2003). In this study, we excluded ER-negative IDCs from the current analysis to address directly whether histology confers significant differences in sensitivity to neoadjuvant chemotherapy. Chemotherapy sensitivity was measured by pathological tumour response rates and the rate of breast-conserving surgery.
Similar to previous reports, we observed low pCR rates in both histological subtypes, IDC (14%) and ILC (3.5%) (Cocquyt et al, 2003; Mathieu et al, 2004; Cristofanilli et al, 2005; Tubiana-Hulin et al, 2006; Katz et al, 2007; Sullivan and Apple, 2009; Huober et al, 2010; Straver et al, 2010). However, pCR rates were not significantly different by histological type after adjusting for differences in tumour grade. This is different from earlier reports that suggested significantly lower pCR rates in ILC. Our results show that low- and intermediate-grade ILC and IDC both have similar, very low pCR rates. This result supports the idea that response to neoadjuvant chemotherapy in terms of pCR is more related to intrinsic tumour characteristics, reflected to some extent in grade than histology itself (Lips et al, 2012). Pathological complete response is a powerful early surrogate of good survival in ER-negative and HER2-positive cancers, but its prognostic value is less important in ER-positive cancers because many patients with extensive residual cancer continue to do well probably because of the benefit from adjuvant endocrine therapy (von Minckwitz et al, 2012). Our findings confirm that survivals were similar for both ER-positive IDC and ILC. We recognise that a median follow-up of 44 months is short for ER-positive breast cancers, which is a limitation of the current analysis. However, it is unlikely that late recurrence rates would differ significantly by histology among ER-positive cancers.
An important clinical benefit from neoadjuvant chemotherapy is clinical tumour response that leads to downstaging and smaller surgical resection volume (Fisher et al, 1998; Boughey et al, 2006). Tumour resection margins were more commonly positive or close (⩽2 mm) in ILC (19 vs 11%) and the rate of breast-conserving surgery was also lower (19 vs 34%). These differences in clinical benefit remained significant after adjusting for other clinical variables, including grade and tumour size. These observations are consistent with the majority of the literature that reports low rates of breast conservation therapy for patients with ILC following neoadjuvant chemotherapy (Soucy et al, 2008; Boughey et al, 2009). These results collectively indicate that clinical benefit from neoadjuvant chemotherapy in operable ER-positive ILC is less compared with ER-positive IDCs due to the inherently lower chemotherapy sensitivity of these cancers and their unique anatomical features, which make determination of cancer margins more difficult intraoperatively.
Our study is the largest report of outcomes of ILC subtype after neoadjuvant chemotherapy, but there were some limitations. As a retrospective survey there was heterogeneity in our population, especially regarding chemotherapy regimens. Moreover, important variables, such as proliferation (i.e. Ki-67 staining), were not available. However, nuclear grade may be considered as a crude surrogate for proliferation activity.
However, a simple conclusion that ILC does not respond to neoadjuvant chemotherapy would represent an oversimplification. Forty-one per cent of ILCs had lower tumour T stage compared with baseline after neoadjuvant chemotherapy. What further complicates clinical decision-making for patients with ILC is that clinical response rates over 50% have also been reported with the use of 3–6 months of neoadjuvant endocrine therapy alone (Eiermann et al, 2001; Cataliotti et al, 2006; Semiglazov et al, 2007; Mlineritsch et al, 2008; Mustacchi et al, 2009; Ellis et al, 2011). In addition, the majority of patients with ILC have a low or intermediate recurrence score (Oncotype DX, Redwood, CA, USA), which is associated with no or very modest benefit from adjuvant chemotherapy in terms of improved survival (Kelly et al, 2010; Mook et al, 2010; Allison et al, 2012). Taking all this information together, it is reasonable to conclude that most patients with ILC are unlikely to derive substantial short-term clinical benefit (substantial tumour reduction with clear margins or pathological CR) from neoadjuvant chemotherapy. However, ILC may derive similar long-term survival benefit from neoadjuvant chemotherapy as ER-positive IDCs, but this benefit is likely to be modest.
Acknowledgments
Funding Support, Grants from ‘La Fondation de France' and ‘La Fondation pour la Recherche Médicale' was provided to Yann Delpech, and Lajos Pusztai was supported by the Breast Cancer Research Foundation.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Allison KH, Kandalaft PL, Sitlani CM, Dintzis SM, Gown AM. Routine pathologic parameters can predict cncotype DX recurrence scores in subsets of ER positive patients: who does not always need testing. Breast Cancer Res Treat. 2012;131 (2:413–424. doi: 10.1007/s10549-011-1416-3. [DOI] [PubMed] [Google Scholar]
- Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6 (3:R149–R156. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughey JC, Peintinger F, Meric-Bernstam F, Perry AC, Hunt KK, Babiera GV, Singletary SE, Bedrosian I, Lucci A, Buzdar AU, Pusztai L, Kuerer HM. Impact of preoperative versus postoperative chemotherapy on the extent and number of surgical procedures in patients treated in randomized clinical trials for breast cancer. Ann Surg. 2006;244 (3:464–470. doi: 10.1097/01.sla.0000234897.38950.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughey JC, Wagner J, Garrett BJ, Harker L, Middleton LP, Babiera GV, Meric-Bernstam F, Lucci A, Hunt KK, Bedrosian I. Neoadjuvant chemotherapy in invasive lobular carcinoma may not improve rates of breast conservation. Ann Surg Oncol. 2009;16 (6:1606–1611. doi: 10.1245/s10434-009-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataliotti L, Buzdar AU, Noguchi S, Bines J, Takatsuka Y, Petrakova K, Dube P, de Oliveira CT. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative ‘Arimidex' Compared to Tamoxifen (PROACT) trial. Cancer. 2006;106 (10:2095–2103. doi: 10.1002/cncr.21872. [DOI] [PubMed] [Google Scholar]
- Cocquyt VF, Blondeel PN, Depypere HT, Praet MM, Schelfhout VR, Silva OE, Hurley J, Serreyn RF, Daems KK, Van Belle SJ. Different responses to preoperative chemotherapy for invasive lobular and invasive ductal breast carcinoma. Eur J Surg Oncol. 2003;29 (4:361–367. doi: 10.1053/ejso.2002.1404. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Gonzalez-Angulo A, Sneige N, Kau SW, Broglio K, Theriault RL, Valero V, Buzdar AU, Kuerer H, Buccholz TA, Hortobagyi GN. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol. 2005;23 (1:41–48. doi: 10.1200/JCO.2005.03.111. [DOI] [PubMed] [Google Scholar]
- Eiermann W, Paepke S, Appfelstaedt J, Llombart-Cussac A, Eremin J, Vinholes J, Mauriac L, Ellis M, Lassus M, Chaudri-Ross HA, Dugan M, Borgs M. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol. 2001;12 (11:1527–1532. doi: 10.1023/a:1013128213451. [DOI] [PubMed] [Google Scholar]
- Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, Parker JS, Luo J, DeSchryver K, Allred DC, Esserman LJ, Unzeitig GW, Margenthaler J, Babiera GV, Marcom PK, Guenther JM, Watson MA, Leitch M, Hunt K, Olson JA. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype – ACOSOG Z1031. J Clin Oncol. 2011;29 (17:2342–2349. doi: 10.1200/JCO.2010.31.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz AB, Hoehn JL, Lees AW, Dimitrov NV, Bear HD. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16 (8:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- Fisher ER, Gregorio RM, Fisher B, Redmond C, Vellios F, Sommers SC. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4) Cancer. 1975;36 (1:1–85. doi: 10.1002/1097-0142(197507)36:1<1::aid-cncr2820360102>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Huober J, von Minckwitz G, Denkert C, Tesch H, Weiss E, Zahm DM, Belau A, Khandan F, Hauschild M, Thomssen C, Hogel B, Darb-Esfahani S, Mehta K, Loibl S. Effect of neoadjuvant anthracycline-taxane-based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat. 2010;124 (1:133–140. doi: 10.1007/s10549-010-1103-9. [DOI] [PubMed] [Google Scholar]
- Katz A, Saad ED, Porter P, Pusztai L. Primary systemic chemotherapy of invasive lobular carcinoma of the breast. Lancet Oncol. 2007;8 (1:55–62. doi: 10.1016/S1470-2045(06)71011-7. [DOI] [PubMed] [Google Scholar]
- Katz MH. Multivariable analysis: a primer for readers of medical research. Ann Intern Med. 2003;138 (8:644–650. doi: 10.7326/0003-4819-138-8-200304150-00012. [DOI] [PubMed] [Google Scholar]
- Kelly CM, Krishnamurthy S, Bianchini G, Litton JK, Gonzalez-Angulo AM, Hortobagyi GN, Pusztai L. Utility of oncotype DX risk estimates in clinically intermediate risk hormone receptor-positive, HER2-normal, grade II, lymph node-negative breast cancers. Cancer. 2010;116 (22:5161–5167. doi: 10.1002/cncr.25269. [DOI] [PubMed] [Google Scholar]
- Lips EH, Mukhtar RA, Yau C, de Ronde JJ, Livasy C, Carey LA, Loo CE, Vrancken-Peeters MJ, Sonke GS, Berry DA, Van't Veer LJ, Esserman LJ, Wesseling J, Rodenhuis S, Shelley Hwang E. Lobular histology and response to neoadjuvant chemotherapy in invasive breast cancer. Breast Cancer Res Treat. 2012;136 (1:35–43. doi: 10.1007/s10549-012-2233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu MC, Rouzier R, Llombart-Cussac A, Sideris L, Koscielny S, Travagli JP, Contesso G, Delaloge S, Spielmann M. The poor responsiveness of infiltrating lobular breast carcinomas to neoadjuvant chemotherapy can be explained by their biological profile. Eur J Cancer. 2004;40 (3:342–351. doi: 10.1016/j.ejca.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Mlineritsch B, Tausch C, Singer C, Luschin-Ebengreuth G, Jakesz R, Ploner F, Stierer M, Melbinger E, Menzel C, Urbania A, Fridrik M, Steger G, Wohlmuth P, Gnant M, Greil R. Exemestane as primary systemic treatment for hormone receptor positive post-menopausal breast cancer patients: a phase II trial of the Austrian Breast and Colorectal Cancer Study Group (ABCSG-17) Breast Cancer Res Treat. 2008;112 (1:203–213. doi: 10.1007/s10549-007-9843-x. [DOI] [PubMed] [Google Scholar]
- Molland JG, Donnellan M, Janu NC, Carmalt HL, Kennedy CW, Gillett DJ. Infiltrating lobular carcinoma – a comparison of diagnosis, management and outcome with infiltrating duct carcinoma. Breast. 2004;13 (5:389–396. doi: 10.1016/j.breast.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Mook S, Schmidt MK, Weigelt B, Kreike B, Eekhout I, van de Vijver MJ, Glas AM, Floore A, Rutgers EJ, van 't Veer LJ. The 70-gene prognosis signature predicts early metastasis in breast cancer patients between 55 and 70 years of age. Ann Oncol. 2010;21 (4:717–722. doi: 10.1093/annonc/mdp388. [DOI] [PubMed] [Google Scholar]
- Mustacchi G, Mansutti M, Sacco C, Barni S, Farris A, Cazzaniga M, Cozzi M, Dellach C. Neo-adjuvant exemestane in elderly patients with breast cancer: a phase II, multicentre, open-label, Italian study. Ann Oncol. 2009;20 (4:655–659. doi: 10.1093/annonc/mdn687. [DOI] [PubMed] [Google Scholar]
- Porter PL, El-Bastawissi AY, Mandelson MT, Lin MG, Khalid N, Watney EA, Cousens L, White D, Taplin S, White E. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 1999;91 (23:2020–2028. doi: 10.1093/jnci/91.23.2020. [DOI] [PubMed] [Google Scholar]
- Purushotham A, Pinder S, Cariati M, Harries M, Goldhirsch A. Neoadjuvant chemotherapy: not the best option in estrogen receptor-positive, HER2-negative, invasive classical lobular carcinoma of the breast. J Clin Oncol. 2010;28 (22:3552–3554. doi: 10.1200/JCO.2009.27.8184. [DOI] [PubMed] [Google Scholar]
- Rouzier R, Pusztai L, Delaloge S, Gonzalez-Angulo AM, Andre F, Hess KR, Buzdar AU, Garbay JR, Spielmann M, Mathieu MC, Symmans WF, Wagner P, Atallah D, Valero V, Berry DA, Hortobagyi GN. Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol. 2005;23 (33:8331–8339. doi: 10.1200/JCO.2005.01.2898. [DOI] [PubMed] [Google Scholar]
- Semiglazov VF, Semiglazov VV, Dashyan GA, Ziltsova EK, Ivanov VG, Bozhok AA, Melnikova OA, Paltuev RM, Kletzel A, Berstein LM. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110 (2:244–254. doi: 10.1002/cncr.22789. [DOI] [PubMed] [Google Scholar]
- Soucy G, Belanger J, Leblanc G, Sideris L, Drolet P, Mitchell A, Leclerc YE, Dufresne MP, Beaudet J, Dube P. Surgical margins in breast-conservation operations for invasive carcinoma: does neoadjuvant chemotherapy have an impact. J Am Coll Surg. 2008;206 (3:1116–1121. doi: 10.1016/j.jamcollsurg.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Straver ME, Rutgers EJ, Rodenhuis S, Linn SC, Loo CE, Wesseling J, Russell NS, Oldenburg HS, Antonini N, Vrancken Peeters MT. The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Ann Surg Oncol. 2010;17 (9:2411–2418. doi: 10.1245/s10434-010-1008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PS, Apple SK. Should histologic type be taken into account when considering neoadjuvant chemotherapy in breast carcinoma. Breast J. 2009;15 (2:146–154. doi: 10.1111/j.1524-4741.2009.00689.x. [DOI] [PubMed] [Google Scholar]
- Tubiana-Hulin M, Stevens D, Lasry S, Guinebretiere JM, Bouita L, Cohen-Solal C, Cherel P, Rouesse J. Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol. 2006;17 (8:1228–1233. doi: 10.1093/annonc/mdl114. [DOI] [PubMed] [Google Scholar]
- von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30 (15:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- Wagner J, Boughey JC, Garrett B, Babiera G, Kuerer H, Meric-Bernstam F, Singletary E, Hunt KK, Middleton LP, Bedrosian I. Margin assessment after neoadjuvant chemotherapy in invasive lobular cancer. Am J Surg. 2009;198 (3:387–391. doi: 10.1016/j.amjsurg.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waljee JF, Hu ES, Newman LA, Alderman AK. Predictors of re-excision among women undergoing breast-conserving surgery for cancer. Ann Surg Oncol. 2008;15 (5:1297–1303. doi: 10.1245/s10434-007-9777-x. [DOI] [PubMed] [Google Scholar]
- Wellings SR, Jensen HM, Marcum RG. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975;55 (2:231–273. [PubMed] [Google Scholar]
- World Health Organisation The World Health Organization Histological Typing of Breast Tumours – Second Edition. The World Organization. Am J Clin Pathol. 1982;78 (6:806–816. doi: 10.1093/ajcp/78.6.806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.