Abstract
Background:
There are no reliable markers of malignancy in phaeochromocytomas (PCC) and paragangliomas (PGL). We investigated the relevance of the mammalian target of rapamycin (mTOR)/AKT and hypoxic pathways as novel immunohistochemical markers of malignancy.
Methods:
Tissue microarray blocks were constructed with a total of 100 tumours (10 metastatic) and 20 normal adrenomedullary samples. Sections were immunostained for hypoxia-inducible factor 1α (Hif-1α), vascular endothelial growth factor A (VEGF-A), mTOR, carbonic anhydrase IX (CaIX) and AKT. The predictive performance of these markers was studied using univariate, multivariate and receiver operating characteristic analyses.
Results:
In all, 100 consecutive patients, 64% PCC, 29% familial with a median tumour size of 4.7 cm (range 1–14) were included. Univariate analyses showed Hif-1α overexpression, tumour necrosis, size >5 cm, capsular and vascular invasion to be predictors of metastasis. In multivariate analysis, Hif-1α, necrosis and vascular invasion remained as independent predictors of metastasis. Hif-1α was the most discriminatory biomarker for the presence of metastatic diffusion. Strong membranous CaIX expression was seen in von Hippel–Lindau (VHL) PCC as opposed to other subtypes.
Conclusion:
Lack of vascular invasion, tumour necrosis and low Hif-1α expression identify tumours with lower risk of malignancy. We propose membranous CaIX expression as a potential marker for VHL disease in patients presenting with PCC.
Keywords: Phaeochromocytoma, paraganglioma, carbonic anhydrase IX, Hif-1α, VEGF, mTOR
Phaeochromocytomas (PCC) and paragangliomas (PGL) are rare neuroendocrine tumours of chromaffin origin arising, respectively, from the adrenal medulla (80% of cases) or sympathetic nervous system ganglia (20%), with an estimated incidence of 1 case per 300 000 inhabitants per year (Manger and Eisenhofer, 2004; Gimm et al, 2012). Symptoms and signs of catecholamine excess such as hypertension, tachycardia, headache and anxiety are the key presenting features. In some cases, PCC/PGL can lead to potentially life-threatening consequences such as heart failure, myocardial infarction, arrhythmias or stroke (Reisch et al, 2006).
At least 25% of PCC and PGL arise in the context of well-defined hereditary tumour syndromes due to germline mutations in specific susceptibility loci, such as succinate dehydrogenase (SDH) subunits B, C and D; von Hippel–Lindau (VHL), neurofibromatosis type 1 (NF-1) and RET (Gimenez-Roqueplo et al, 2008). However, the proportion of hereditary tumour syndromes is likely to expand as a result of novel pathogenic genes and mutations being discovered, such as TMEM127, MAX, SDHAF2 and SDH-A (DeLellis et al, 2004).
Although PCC/PGLs are generally regarded as benign in at least 80% of cases, a significant proportion are malignant, and there are no proven effective markers or methods to assess the risk of recurrence or to identify malignancy a priori (Harari and Inabnet, 2011). Recurrence is unpredictable, potentially occurring months to years after surgery (Allibhai et al, 2004). The presence of lymphatic, visceral or bone spread at diagnosis or at disease recurrence represents the only reliable feature of true malignant behaviour (Gao et al, 2008). As a result, all patients with PCC/PGL require intensive and expensive follow up after surgical excision to identify signs of recurrence. This is by means of periodic clinical assessment, biochemical evaluation for excessive catecholamine or metanephrine secretion and serial cross-sectional imaging, preferably with modalities that do not utilise radiation (Petri et al, 2009). Unlike other tumours, the diagnosis of malignant PCC/PGL disease is not aided by histopathological analysis: cyto-architectural features of the tumour cells, as well as the presence of traditionally adverse pathological features such as capsular invasion, vascular invasion or necrosis are of doubted prognostic value in this context. Even in malignant cases, the proliferating fraction may not exceed 2% of the whole tumour mass (Thompson, 2002), hence reducing the reliability of Ki-67 as a discriminator of malignancy. Moreover scoring methods for predicting malignant behaviour are not entirely reproducible (Agarwal et al, 2010) and lack correlation with genotype (Eisenhofer et al, 2012).
Genetic analysis, although useful, only gives partial information for risk stratification: for example, although it is known that SDH-B mutation carriers (representing up to 7% of the patients presenting with PCC/PGL disease) will suffer from a higher lifelong chance of recurrence or malignant dedifferentiation than compared with other paraganglioma syndromes (Brouwers et al, 2006), the course of the disease can still be highly variable with some mutation carriers presenting with highly aggressive and malignant disease, and others presenting with indolent or even no detectable disease (Gimenez-Roqueplo et al, 2006; Srirangalingam et al, 2008).
Genome-wide expression profile studies have shed some light upon the pathophysiology of PCC/PGL. VHL- and SDH-related tumours (dubbed Cluster 1) do not share the same transcriptomic signature with RET- and NF-1-related PCC/PGL (so-called Cluster 2). Sporadic tumours with no known germline mutations can cluster with either group. Tumours falling into Cluster 1 appear to be primarily driven by pseudo-hypoxic signals, whereas those in Cluster 2 PCC/PGL are associated with a dysregulation of the mammalian target of rapamycin (mTOR) pathway (Dahia et al, 2005). It is unclear at present if the clustering of these expression profiles could be useful to identify the risk of malignancy and therefore optimise the post-surgical care pathway of patients with PCC/PGL.
The investigation of the malignant phenotype is particularly limited by the lack of validated immortalised human cell lines as well as the lack of pre-clinical in vivo models of malignant PCC/PGL. Tissue biomarkers are therefore the only viable option to enable a better characterisation of malignancy, improve its earlier detection and potentially to identify pathways amenable to systemic treatment.
We therefore designed the present study in order to elucidate the role of mTOR and hypoxic pathway biomarkers in the histopathological identification of malignancy in PCC/PGL, to evaluate their distribution across the different familial PCC/PGL syndromes and to correlate tissue expression of these biomarkers with common clinico-pathological features.
Patients and methods
Patient characteristics
A total of 100 consecutive patients who received surgical treatment for PCC or PGL at Imperial College Healthcare NHS Trust between 1983 and 2011 were included. Formalin-fixed, paraffin-embedded specimens and matching haematoxylin and eosin (H&E) slides were retrieved from the local pathology archive. All the diagnostic H&E sections were reviewed to confirm the diagnosis and to ensure the accurate identification of the relevant pathological features, with examination of at least five sections per case, depending on the abundance of the sampled material at the time of diagnosis.
Complete clinical and follow-up information, including patient demographics, tumour size and stage at diagnosis, and site of presentation were retrieved by review of medical records. The presence of adverse pathological features, such as vascular and capsular invasion, and the presence of coagulative necrosis were identified following H&E slide review by a board-certified endocrine pathologist (RD), blinded to clinical outcomes. Patients were categorised according to the catecholamine secretory pattern of their tumour (adrenaline, noradrenaline, dopamine) depending on the results of at least three different measurements of their 24 h urinary catecholamine excretion. Malignancy was identified either by the presence of metastatic spread to lymph nodes or visceral sites either at the time of diagnosis or during follow up.
Genetic testing for the major PCC/PGL susceptibility genes (SDH-B, D, VHL and RET) was offered, from the year 2005, to patients whose age at diagnosis was <40 years as per local standard of care (Gimenez-Roqueplo et al, 2006). NF-1 syndrome was diagnosed from phenotypic criteria. For the purpose of our study, we identified those tumours arising in patients testing negative to germline mutations in PCC/PGL susceptibility genes (n=16) as sporadic. We included in this subgroup another 19 patients diagnosed before 2005 (i.e. before genetic screening was generally available) with sporadic disease based on clinical criteria: unilateral PCC, lack of family history for syndromic PCC/PGL disease and age at diagnosis >40 years.
As negative controls, we included 20 adrenal medulla specimens obtained from adrenalectomies performed for purposes unrelated to primary adrenal disease. Ethical approval was granted by the Hammersmith and Queen Charlotte's Local Research Ethics Committee (Ref. 08/H0707/143) in accordance with the Principles of the Declaration of Helsinki.
Tissue microarrays (TMAs) and immunohistochemistry
After review of H&E-stained sections, three 1 mm cores were identified from the most representative areas of the tumour tissue, then re-embedded into recipient TMA blocks using an MTA-1 Manual Tissue Microarrayer (Beecher Instruments, Sun Prairie, WI, USA). Normal adrenal medulla controls were processed using the same method. In all, 5-μm thick TMA sections were de-paraffinized in xylene and rehydrated in graded alcohols. Optimal heat-mediated antigen retrieval conditions were applied according to the primary antibody using a microwave oven at 900 W. Incubation in citrate buffer at pH 6.0 for 20 min was preferred for antibodies to mTOR (Cat. Nr. 2983, Cell Signaling, Hitchin, UK), VEGF-A (Cat. Nr. Sc-152; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), Hif-1α (Cat. Nr. Ab8366 AbCam, Cambridge, UK), Ki-67 (Clone MM1, Leica Microsystems, Milton Keynes, UK) and AKT (Cat. Nr. 4685, Cell Signaling), whereas antibodies to CaIX (Cat. Nr. NB100-417, Novus Biologicals, Cambridge, UK) were incubated in pH 9.0 EDTA buffer for 30 min. Before immunostaining, slides were cooled to room temperature and endogenous peroxidase activity was suppressed by incubation with a 3% solution of H2O2 for 5 min. The primary antibody against total mTOR was incubated at a 1 : 50 dilution, Ki-67 was diluted to 1 : 800, whereas all the other antibodies were used at a 1 : 1000 dilution.
TMA slides were then stained with a fully automated procedure on a Bond Max Autostainer (Leica Microsystems) using the Polymer-HRP system (BioGenex, Fremont, CA, USA) with subsequent development in diaminobenzidine and Mayer's haematoxylin counterstaining. Appropriately selected tissue sections were used according to the manufacturer's instruction as external positive controls during each reaction. Negative control reactions were performed omitting the primary antibodies from the dilution buffer. This resulted in a complete absence of staining in all cases. Two trained histopathologists (NN, MV) blinded to the clinical data scored all the cases and a consensus score was reached in any case of discrepancy. Tissue samples were scored using the immunohistochemical score (IHS), as described previously (Dhillon et al, 2010). Briefly, each sample can be assigned an IHS ranging from 0 to 300, based on the multiplication of the percentage of cells showing immunohistochemical expression (0–100) by the intensity of the signal (graded 1–3). Every core was assessed individually and the mean of three readings was calculated in each case. The pattern of staining (cytoplasmic, membranous, nuclear, diffuse) was also described in each case. An IHS cutoff value of ≥150 was used to discriminate high versus low expression, as described previously (Bowman et al, 2002).
Statistical analysis
Pearson's χ2 or Fisher's exact tests were used to investigate any significant associations between categorical variables as appropriate. Differences in medians were assessed using the Mann–Whitney U Test or the Kruskal–Wallis test as appropriate. Associations and differences in medians were conventionally considered statistically significant when the resulting p value was <0.05. Binomial multivariate logistic regression analysis was carried out to identify independent predictors of malignancy: a stepwise backward procedure was used, and variables with a p value >0.10 were removed from the model. The accuracy of each tested biomarker in discriminating the metastatic phenotype was estimated using area under the curve in receiver operating characteristic (ROC) curve analysis. Statistical analysis was performed using SPSS statistical package version 19.0 (IBM SPSS Inc., New York, NY, USA) and GraphPad Prism 5.0d (GraphPad Software, La Jolla, CA, USA).
Results
Clinical Demographics
A summary of the clinicopathological features describing our patient cohort is provided in Table 1. The 100 patients included in our study were followed up for a median interval of 3 years from the time of diagnosis (range 6 months–30 years). Radiologically or histologically proven malignancy was identified in 10 patients: 3 patients had metastatic disease at presentation, whereas 7 developed malignant recurrence during follow up. The median age at diagnosis was 40 years. Eighty percent of patients had evidence of catecholamine secretion at diagnosis. Median tumour size was 4.7 cm (range 1–14 cm). A total of 29 patients were confirmed to have syndromic PCC/PGL following genotype analysis, with 9 patients testing positive for germline mutations in SDH-B, 2 for SDH-D, 3 for VHL, 2 for NF-1 and 13 for RET. The distribution of genotypes across metastatic cases was as follows: three were sporadic (negative for all the mutations tested), two patients tested positive for mutations in SDH-B, one for SDH-D, one for VHL and one for NF-1. In another two patients, genotype data were unavailable: in one case the age at diagnosis was 37 years (hence making inherited PCC/PGL disease potentially plausible), whereas in the second patient the age at diagnosis was 73 years, making the possibility of having inherited PCC/PGL disease less likely. All the patients received surgery as the primary treatment for their tumour. This was followed by external beam radiotherapy or systemic chemotherapy in six and two cases, respectively, of malignant recurrent disease. Iodine-131-meta-iodobenzylguanidine therapy was administered in four cases.
Table 1. Patient characteristics.
| Baseline characteristic | n=100 |
|---|---|
|
Gender | |
| Male | 46 |
| Female |
54 |
|
Age in years | |
| <40 | 50 |
| ⩾40 |
50 |
|
Disease site | |
| PCC monolateral | 59 |
| PCC bilateral | 5 |
| PGL (extra-adrenal) |
36 |
|
Behaviour | |
| Benign | 90 |
| Metastatic |
10 |
|
Catecholamine secretion | |
| Adrenaline | 28 |
| Noradrenaline | 54 |
| Dopamine | 15 |
| Adrenaline+noradrenaline | 21 |
| Adrenaline+dopamine | 8 |
| Noradrenaline+dopamine | 11 |
| Silent | 20 |
| Missing |
13 |
|
Tumour necrosis | |
| Absent | 76 |
| Present |
16 |
|
Capsular invasion | |
| Absent | 79 |
| Present |
13 |
|
Vascular invasion | |
| Absent | 84 |
| Present |
8 |
|
Presentation | |
| Primary | 96 |
| Recurrent |
4 |
|
Genotype (germline mutations) | |
| SDH-B | 9 |
| SDH-D | 2 |
| NF-1 | 2 |
| VHL | 3 |
| RET | 12 |
Abbreviations: NF-1=neurofibromatosis-1; PCC=phaeochromocytomas; PGL=paragangliomas; RET=rearranged during transfection; SDH=succinate dehydrogenase; VHL=von Hippel–Lindau.
Relationship between the expression of the candidate biomarkers and patient characteristics
The expression of the candidate biomarkers in benign, metastatic tumours and normal adrenal medulla controls is reported in Figure 1. Representative sections of PCC/PGL stained for each biomarker are shown in Figure 2.
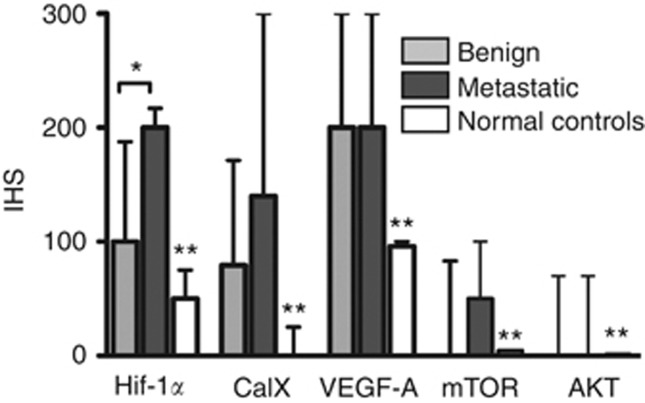
Figure 1.
The expression of hypoxia inducible factor 1α (Hif-1α), carbonic anhydrase IX (CaIX), vascular endothelial growth factor A (VEGF-A), mammalian target of rapamycin (mTOR) and AKT in benign, metastatic phaeochromocytomas (PCC)/paragangliomas (PGL) and normal adrenal medulla controls by immunohistochemistry. Immunohistoscore (IHS) values are presented as medians±interquartile ranges. An asterisk marks a statistically significant difference in median IHS values across indicated columns, whereas double asterisks mark a statistically significant difference in median IHS values between normal controls and tumour samples for each biomarker.
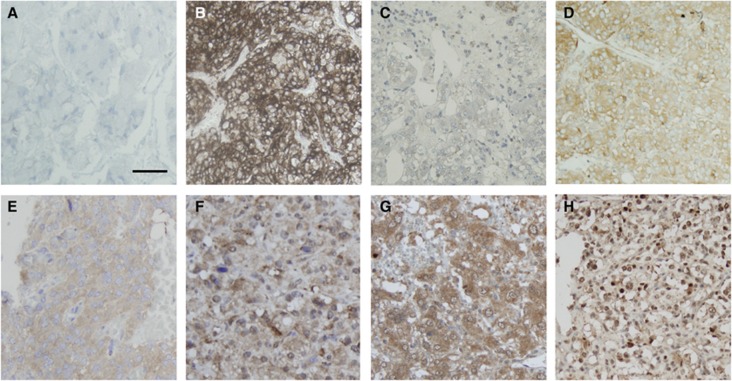
Figure 2.
Expression of the candidate biomarkers in PCC/PGL by immunohistochemistry. Representative sections of tumours showing (A) a negative tumour core . Panel (B) shows strong membranous expression of CaIX in a VHL PCC, not replicated in (C) the matching normal medulla sample. Panel (D) shows a case of positive granular cytoplasmic expression of CaIX, typical of all the non-VHL cases (both sporadic and familial) tested in our series. Panels (E) and (F) are representative of mTor and AKT expression. Samples showing Hif-1α cytoplasmic and nuclear expression are reported in panels G and H, respectively. Magnification × 200, Bar=100 μm.
In PCC/PGLs, IHS values for Hif-1α ranged from 0–300 (median 120, mean 107), and 35% were categorised as showing high expression (defined as IHS ⩾150). Both nuclear and cytoplasmic positivity were taken into account owing to the cytoplasmic positivity previously observed in PCC/PGL (Favier et al, 2009). Elevated Hif-1α expression with IHS⩾150 was associated with the presence of tumour necrosis (P=0.01) and higher CaIX expression (P<0.001). Normal adrenal medulla cores displayed a significantly lower expression of Hif-1α compared with PCC/PGL (median 62, mean 55, range 0–100, P<0.001).
The median CaIX IHS was 76 (mean of 78, range 0–300), with 26% of the tumours demonstrating high expression of CaIX. CaIX expression was also more commonly seen in PGL compared with PCC (P=0.03) and absent in healthy normal adrenal medulla controls (P<0.001). Both membranous and cytoplasmic staining were observed, with a predilection for strong membranous staining (IHS 300) in all the three patients with VHL-related PCC (P=0.03; Figure 2C). Matching adrenal medulla from the same VHL patients was available in two cases and did not express CaIX in both cases (Figure 2D). A fourth case of VHL-PCC was included to validate our findings and showed again strong membranous staining, whereas matching normal adrenal medulla displayed weak cytoplasmic staining. None of the VHL mutant tumours displayed convincingly different histological features exclusive to the VHL genotype (Koch et al, 2002).
The expression of mTOR and AKT was exclusively cytoplasmic and found to be elevated in only 5% of the cases. mTOR median IHS was 0 (mean 33, range 0–300) and median AKT IHS was 0 (mean 28, range 0–250). Normal adrenal medulla did not express both markers (P<0.001).
The median VEGF IHS was 200 in PCC/PGL (mean 178, range 75–275), with 64% of the tumours showing high VEGF expression. A significantly lower degree of VEGF expression was noted in normal adrenal medulla samples (median 91, mean 84, range 0–220, P<0.001).
The expression of Ki-67 was described as being <2% in all cases of PCC/PGL except for a single malignant tumour at 10%. All the normal controls were Ki-67 negative.
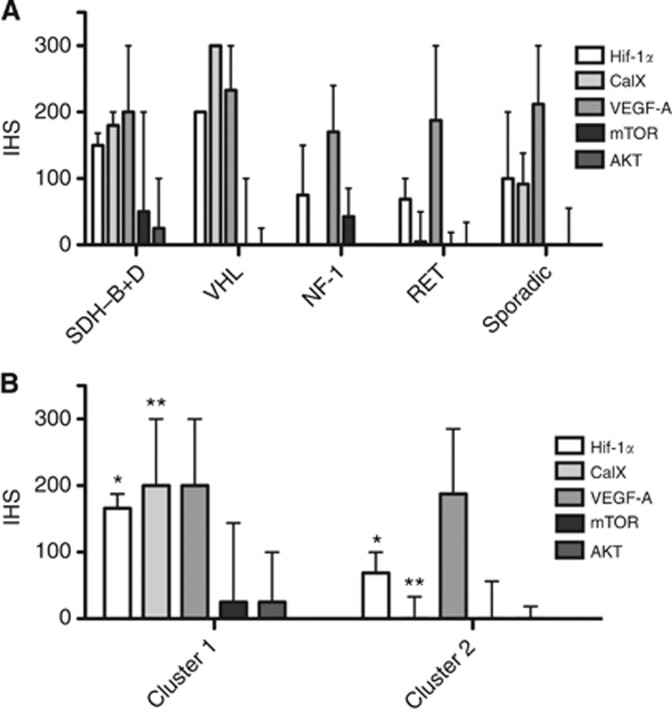
The differential expression of the tested biomarkers in sporadic cases of PCC/PGL (n=35) and across the diverse genotypes of inherited PCC/PGL disease is reported in Figure 3. ‘Cluster 1' tumours from patients with known mutations in SDH-B, D and VHL genes (n=14) were grouped together, whereas ‘Cluster 2' included tumours from patients with NF-1 and RET PCC/PGL (n=15). Cluster 1 tumours had significantly higher Hif-1α expression (median 170, mean 150, range 90–200) than Cluster 2 tumours (median 70, mean 70, range 0–200, P=0.01). CaIX was also significantly upregulated in Cluster 1 tumours (median 200, mean 170, range 0–300) as opposed to Cluster 2 tumours (median 0, mean 30, range 0–170, P=0.004). By contrast, VEGF expression was high in both Cluster 1 (median 200, mean 210, range 100–300) and Cluster 2 tumours (median 180, mean 190, range 100–300, P=0.81; Figure 3A).
Figure 3.
The relationship between the expression of the tested biomarker and the genetic background of syndromic PCC/PGL compared with cases testing negatively for the main susceptibility loci. Panel (A) shows the distribution of the tested biomarkers across sporadic and familial PCC/PGL. In panel (B), syndromic cases are grouped by ‘transcriptomic cluster' as described by Dahia et al (2005). The distribution of IHS values across the various categories are presented as medians±interquartile ranges. Statistically significant differences in medians across the tested groups are marked with asterisks as per Figure 1.
Clinico-pathological predictors of metastatic spread
Hif-1α emerged as the only biomarker to be significantly upregulated in metastatic (median IHS 200, range 0–266) as opposed to benign tumours (median IHS 100, range 0–300, P=0.02). The relationship between clinical variables and metastatic phenotype is reported in Table 2, where Hif-1α overexpression (P=0.007) together with tumour necrosis (P=0.001), tumour size >5 cm (P=0.05), capsular (P=0.02) and vascular invasion (P<0.001) emerged as statistically significant univariate predictors of metastatic spread.
Table 2. Clinicopathological predictors of metastatic behaviour in PCC/PGL.
| Variable | Benign | Metastatic | P |
|---|---|---|---|
| Gender, M/F |
38/48 |
5/5 |
0.73 |
| Age, <40/>40 |
46/43 |
4/6 |
0.48 |
| Site, adrenal/extra-adrenal |
60/27 |
4/6 |
0.08 |
| Catecholamine secretion, absent/present |
20/56 |
0/9 |
0.08 |
| Capsular invasion, absent/present |
72/9 |
5/4 |
0.02* |
| Vascular invasion, absent/present |
79/2 |
3/6 |
<0.001* |
| Tumour necrosis, absent/present |
72/9 |
3/6 |
0.001* |
| Tumour size, <5/>5 cm |
59/24 |
3/6 |
0.05* |
| mTOR expression, low/high |
82/4 |
10/0 |
0.99 |
| Hif-1α expression, low/high |
61/26 |
2/7 |
0.007* |
| CaIX expression, low/high |
58/21 |
5/5 |
0.12 |
| VEGF-A expression, low/high |
30/57 |
5/5 |
0.33 |
| AKT expression, low/high |
79/3 |
10/0 |
0.99 |
| Ki-67 expression, <2%/>2% | 82/5 | 9/1 | 0.49 |
Abbreviations: CaIX=carbonic anhydrase IX; F=female; Hif-1α=hypoxia inducible factor 1 alpha; M=male; mTOR= PCC=phaeochromocytomas; PGL=paragangliomas; SDH=succinate dehydrogenase; VEGF=vascular endothelial growth factor-A; VHL=von Hippel–Lindau.
Categorisation of adrenaline, noradrenaline or dopamine secretion was carried out using clinically employed cutoff values. Associations reaching statistical significance (P<0.05) are marked with an asterisk.
Multivariate logistic regression analysis confirmed Hif-1α overexpression (odds ratio (OR) 9.8, 95% confidence interval (CI) 1.0–121.0, P=0.06), tumour necrosis (OR 6.0, 95%CI 1.0–47.0 P=0.06) and vascular invasion (OR 65.0, 95%CI 5.6–748.0 P<0.001) as being independent predictors of metastasis in our data set. Tumours displaying vascular invasion were significantly bigger in size (median 7.0 cm, range 5–10) compared with those that did not (median 4.5 cm, range 1–14, P=0.03).
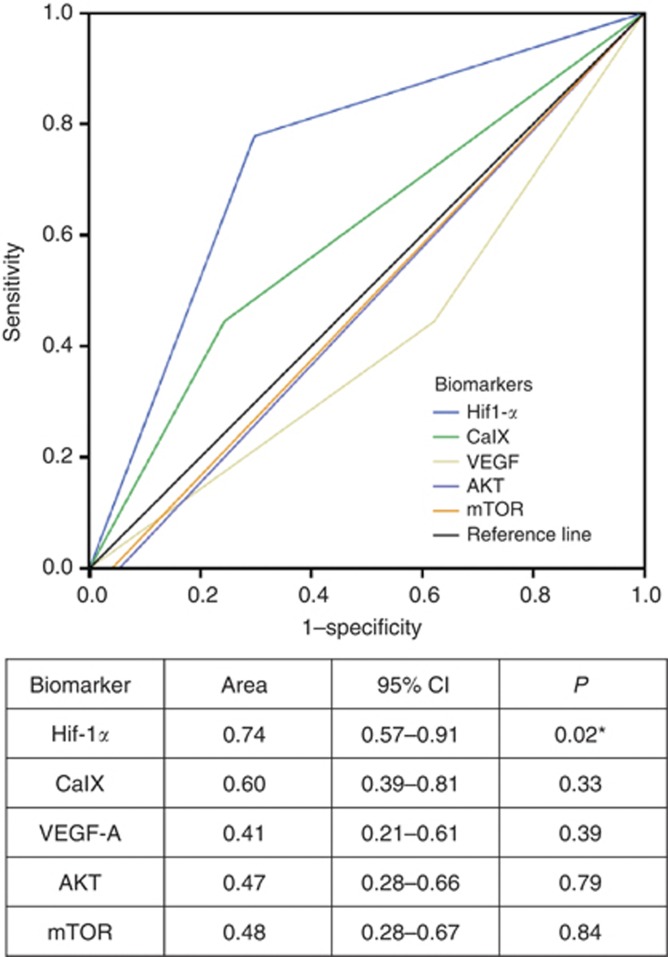
As shown in Figure 4, when comparatively tested with the other tissue biomarkers using ROC curve analysis, Hif-1α overexpression with IHS≥150 emerged as the most accurate immunomarker in the prediction of metastatic behaviour with an area under the ROC curve value of 0.74 (95%CI 0.57–0.91, P=0.02), where 1.0 denotes a perfectly discriminatory test and 0.5 denotes a non-discriminatory test.
Figure 4.
Receiver operating characteristic (ROC) curve analysis showing the superior accuracy of Hif-1α in the prediction of metastatic behaviour.
Discussion
Identification of the molecular pathways driving the malignant phenotype remains elusive in PCC/PGL. Despite an increasing number of published studies attempting to qualify novel predictors of malignancy, no tissue biomarker has, to date, been implemented in the routine post-surgical follow up of patients with PCC/PGL (Ahlman, 2006). In fact, because of the contradictory conclusions arising from some of these studies, the true clinical value of most biomarkers remains controversial.
In this study, we have assembled a large series of PCC/PGL tumours, with clinical and genotype data, together with normal adrenal medulla samples as a control. We utilised TMA technology to efficiently and uniformly probe and investigate the immunohistochemical expression of key candidate biomarkers of malignancy. In our quest for molecular determinants of malignancy, we focused on five candidate biomarkers for which a predominant pathogenic role had been previously explored in PCC/PGL, either as hallmarks of hypoxia and angiogenesis such as Hif-1α and its downstream effectors CaIX and VEGF or as markers of unrestrained proliferative potential involving activation of the mTOR and AKT pathway (Gao et al, 2008).
We found that the extent of activation of the hypoxic response as measured by Hif-1α immunoreactivity is a prognostic trait that qualifies metastatic behaviour in primary PCC/PGL tissue independently from other adverse histological features, such as capsular invasion or tumour volume.
More importantly, Hif-1α expression, necrosis and the presence of vascular invasion outperformed Ki-67 expression as predictors of metastasis in our study, suggesting that malignant progression of PCC/PGL can act independently from tumour proliferation. This finding holds major implications for the management of PCC/PGL as treatment decisions and follow-up intervals are often based on tumour cell proliferation rate and confirms the previously reported inability of Ki-67 expression to identify malignancy (Strong et al, 2008).
Moreover, the lack of independent prognostic power found for some of the histological features that are traditionally thought to predict an adverse course of the disease may limit the reliability of histopathological scores such as the Kimura index (Kimura et al, 2005) that incorporates proliferation count as well as extracapsular spread as parameters to stratify post-surgical risk of malignant progression.
Inconsistent results have also emerged as to the role of vascular invasion and necrosis in prognosticating malignancy, with one study reporting vascular invasion as being the only significant prognostic factor between the two (Gao et al, 2008), while another study reported diametrically opposed results, with necrosis being more predictive of malignancy (Strong et al, 2008). However, genotype subclassification was not reported in either study, which may, at least in part, explain the different conclusions reached by the authors.
Hif-1α, a master regulator of the hypoxic response, is pivotal in orchestrating the transcriptional programmes for cell proliferation, angiogenesis and extracellular matrix remodelling (Giatromanolaki and Harris, 2001). In PCC/PGL, Hif-1α stability is greatly increased as a result of inactivating mutations in the mitochondrial tumour-suppressor genes such as SDH subunits, whose disruption ultimately results in impaired Hif-1α hydroxylation (Gottlieb and Tomlinson, 2005). Hif-1α stabilisation is also typical of VHL-related PCC, where pVHL deficiency reduces the clearance of Hif-1α through the ubiquitin–proteasome pathway. In one described case, a mutation in the prolyl hydroxylase gene PHD2, which hydroxylates Hif-1α and designates it for destruction, has been associated with congenital erythrocytosis and the development of a paraganglioma (Ladroue et al, 2008). Hif-1α stabilisation, by whichever mechanism, leads to unopposed activation of the hypoxic response genes in a phenomenon termed pseudohypoxia (Kim et al, 2010).
This is not the first report to suggest that Hif-1α-driven expression of target genes involved in the angiogenic response such as VEGF may be involved in the progression of PCC/PGL in vitro and in vivo (Middeke et al, 2002; Takekoshi et al, 2004). Previous studies have documented an association between VEGF expression and malignancy after gene expression profiling of a very small case series comprising only 20 cases, with 9 malignant tumours (Favier et al, 2002), and another used immunohistochemistry of paraffin-embedded samples in a single-centre, retrospective study of 105 tumours, only 8 of which were malignant (Salmenkivi et al, 2003). This observation, therefore, lacks independent validation, a process that is mandatory in order to fully assess the clinical applicability of any given prognostic biomarker (Wagner and Srivastava, 2012). Our study confirms VEGF to be significantly overexpressed in PCC/PGL compared with normal controls; however, the lack of a significant difference in VEGF expression between benign and malignant phenotypes leads us to conclude that VEGF overexpression is associated with tumorigenesis but not necessarily with malignancy.
We infer that the association of Hif-1α expression with malignancy may be independent of VEGF expression. In our study, Hif-1α-positive tumours displayed a significant upregulation of CaIX expression, a transmembrane enzyme believed to confer survival advantage to proliferating tumour cells through its protective effect against intracellular acidosis (Swietach et al, 2007). Although CaIX was not associated with malignancy in our study, we speculate whether its expression, which is largely dependent upon Hif-1α transcriptional activity (Kaluz et al, 2009) could, at least in part, be responsible for the downstream pro-oncogenic effects of Hif-1α, a hypothesis that warrants exploration in mechanistic studies.
It is known that the mTOR/AKT and pseudohypoxic molecular pathways interact in several tissues (Majumder et al, 2004), but the extent of interaction between hypoxia and mTOR signalling in promoting malignant transformation has not been previously tested in PCC/PGL. In our study, we found a relatively low level of mTOR protein expression in sporadic tumours and across the various types of inherited PCC/PGL disease, with a relatively higher degree of expression in SDHx-associated tumours. mTOR expression is therefore not restricted to tumours with preserved mitochondrial function but is also present in pseudohypoxic PCC/PGL, therefore suggesting cross-talk between the two pathways. Interestingly, our data prove that mTOR is not upregulated as a result of malignant de-differentiation. Notably, the treatment of malignant PCC/PGL with mTOR inhibitors has been unsuccessful (Druce et al, 2009), possibly because of the preferential activation of the mTORC-2 complex in a subset of tumours (Favier et al, 2009). As we only focused on total mTOR protein, we are unable to delineate the role of the m-TOR interacting proteins in PCC/PGL, a point that warrants further investigation in future studies. Likewise, we found that AKT overexpression is not typical of malignant PCC/PGL, confirming the findings of a previous smaller study (Fassnacht et al, 2005).
We also analysed the expression of our candidate biomarkers with respect to genotype (familial syndromes vs sporadic). We found that the expression of hypoxic response genes such as Hif-1α and CaIX is greater in tumours associated with a pseudohypoxic aetiology (Cluster 1) in contrast to those associated with mTOR dysregulation (Cluster 2), consistent with the ‘cluster' pathogenic hypothesis (Nolting & Grossman, 2012) (Figure 3B). We observed upregulation of Hif-1α in some sporadic tumours (Figure 3A), suggesting that the role of pseudohypoxia in the pathogenesis of PCC/PGL is not restricted to tumours with genetically driven mitochondrial dysfunction and again consistent with the findings of Dahia et al (2005) who showed that sporadic tumours may cluster with either 1 or 2. Interestingly, our data also show that VEGF expression is not influenced by genotype or transcriptomic cluster, suggesting that PCC/PGL tumour cells may upregulate VEGF even when pVHL or SDH function is intact, and that perhaps VEGF expression is part of a final common pathway for the pathogenesis of PGL/PCC of all aetiologies.
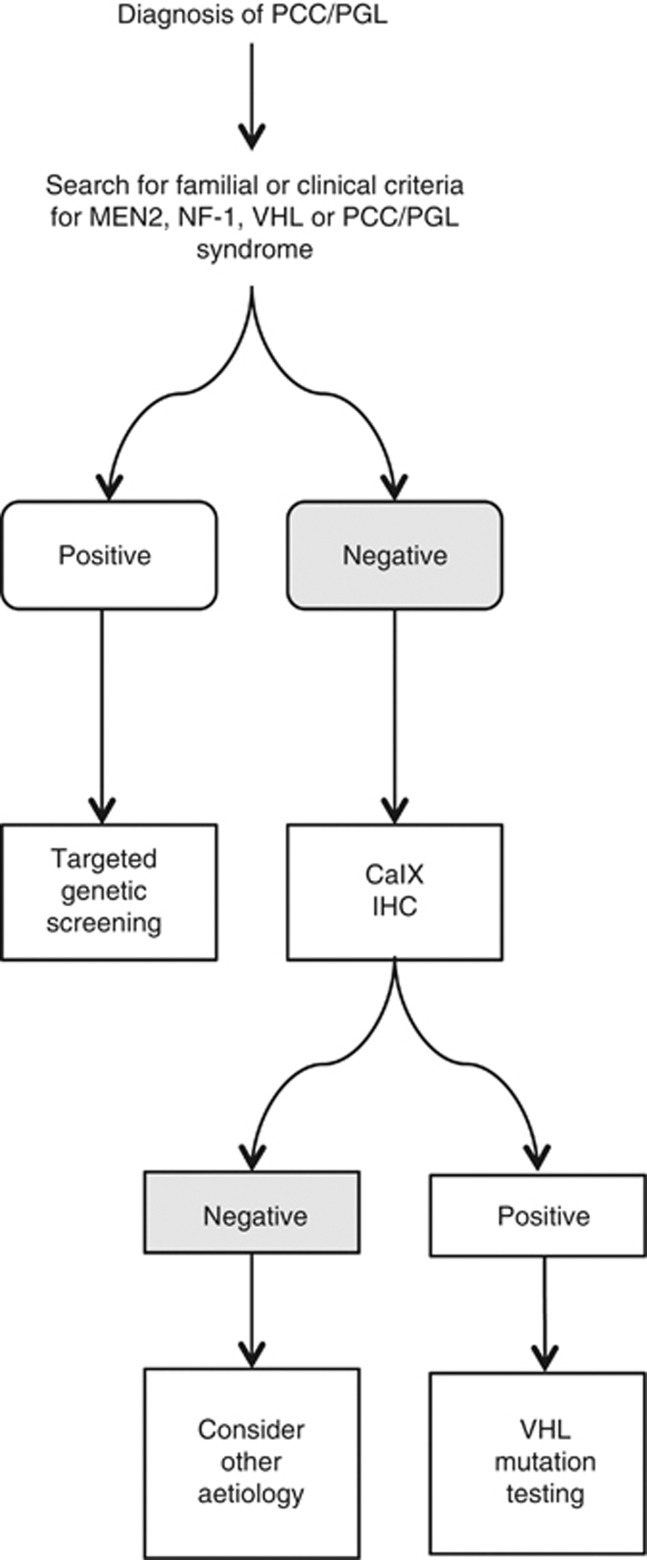
A perhaps more interesting finding holding potential diagnostic implications comes from the observation that all VHL-related PCC, but not matching adrenal medulla from the same patients, displayed strong membranous CaIX staining, a peculiar pattern that was not replicated in other cases, where a granular cytoplasmic staining was seen. It is also notable that the normal adrenal medulla in the same patients did not demonstrate this staining. Based on this finding we hypothesise that a membranous CaIX expression pattern may be a distinctive feature of VHL PCCs. CaIX immunostaining could be prospectively included in an algorithm (Figure 5) to guide genetic testing, similar to the concept that has been advocated for SDH-B and -A immunohistochemistry tests (Gill et al, 2010; Korpershoek et al, 2011). This is of importance in VHL subtype 2C patients who tend to develop PCC in the absence of other features of VHL disease. In this particular subpopulation, VHL disease can remain undiagnosed, with up to 11% of apparently sporadic PCC actually harbouring germline VHL mutations, 13% of which occur de novo (Brukamp and Haase 2006). Even when familial origin is suspected, such as in the case of bilateral presentation of PCC, pathological features which suggest a specific aetiology to the disease are desirable to differentiate VHL-related tumours from RET- and NF-1-related tumours (Koch et al, 2002). Further validation of this finding's diagnostic performance is required in a larger series of VHL-associated PCC/PGL, before CaIX can enter the clinical arena especially in light of a previously published report showing that CaIX may be differentially expressed in a small series of VHL mutant tumours (Blank et al, 2010).
Figure 5.
Possible algorithm incorporating CaIX immunohistochemical test (CaIX IHC) for genetic testing for PCC/PGL. A positive CaIX immunohistochemistry result should direct the clinician or the geneticist to perform a confirmatory VHL mutation test first, avoiding indiscriminate testing for all the four susceptibility loci.
Our study is limited by its single-centre, retrospective design and by the relatively small sample size utilised, where only 10 metastatic cases were represented. Qualification of prognostic markers should be ideally carried out in prospectively collected cohorts, with equal representation of benign and malignant phenotypes. The rarity of PCC/PGL and particularly of malignant cases, however, makes this unfeasible in practice. A relatively small sample size and under-representation of malignant disease are common traits to almost all studies focusing on biomarkers of malignancy in PCC/PGL; however, this study is not inferior in power to the majority of previously published ones, where the proportion of malignant cases was at best 15% (Salmenkivi et al, 2001; Strong et al, 2008; Blank et al, 2010). As a consequence of the acknowledged limitations, our results should be interpreted as exploratory, and a process of external validation of our findings using an independently collected cohort is warranted before our prognostic model can be applied in the clinic.
Among the potential confounding factors influencing our analysis, it is arguable that the over-representation of SDHx mutant tumours amid the malignant cases, where Hif-1α activity would be expected to be increased as a result of the underlying mitochondrial dysfunction characterising these tumours, could have contributed to the association between high Hif-1α expression and malignancy, therefore biasing our observations. However, subanalysis of the genotype proves that SDHx mutations, although the most prevalent, were not the exclusive aetiology of the metastatic cases included in our cohort. Moreover, in a previous study by Blank et al (2010) only 1 of the 5 cases of familial SDHB exhibited positive HIF-1α staining, supporting our contention that HIF-1a expression is not a universal feature of SDHx disease.
In our study, we did not focus on Hif-2α, a larger isoform that shares substantial structural and functional homologies with the cognate transcription factor Hif-1α, including the common transcriptional control of VEGF (Loboda et al, 2010). However, some of the downstream effects of Hif-2α can differ substantially from Hif-1α, including promotion of cell-cycle progression through c-Myc stabilisation and cyclin D1 expression (Covello et al, 2006). Hif-2α is expressed in PCC/PGL, with a predominance in VHL-driven tumours as opposed to SDH-deficient ones (Pollard et al, 2006). The complex functional interplay between the two Hif isoforms has not been fully elucidated in PCC/PGL and, due to the intimate relationship between Hif-2α and cell-cycle regulation, warrants further investigation in the context of malignant transformation.
Despite the acknowledged limitations and the exploratory nature of our findings that indeed require further validation, some conclusions may be drawn from our study. Firstly, patients with low Hif-1α expression, lack of necrosis and vascular invasion could be offered a less stringent follow up, allowing for the provision of a more personalised pathway of care. Secondly, our findings may guide drug development for PCC/PGL towards anti-angiogenic strategies, now further enriched by the availability of direct Hif-1α- (Semenza, 2007) and CaIX-targeted inhibitors (Zatovicova et al, 2010; Lavecchia et al, 2011). Thirdly, our insights regarding membranous CaIX expression as a specific marker of VHL-related PCC may further streamline the process of genetic testing for inherited PCC/PGL disease, contributing to a more judicious use of scarce resources.
Acknowledgments
Investigative Medicine is funded by the MRC, BBSRC, NIHR, an Integrative Mammalian Biology (IMB) Capacity Building Award, and a FP7-HEALTH-2009-241592 EuroCHIP grant, and by funding from the NIHR Imperial Biomedical Research Centre within the Academic Health Sciences Centre. We acknowledge the support of the Wellcome Trust McMichael Clinical Research Facility.
Author-contributions
All the authors have contributed to study conception and design, analysis and interpretation of data or drafting and revising the manuscript critically for important intellectual content. All the authors have approved the final version to be published.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Agarwal A, Mehrotra PK, Jain M, Gupta SK, Mishra A, Chand G, Agarwal G, Verma AK, Mishra SK, Singh U. Size of the tumor and pheochromocytoma of the adrenal gland scaled score (PASS): can they predict malignancy. World J Surg. 2010;34 (12:3022–3028. doi: 10.1007/s00268-010-0744-5. [DOI] [PubMed] [Google Scholar]
- Ahlman H. Malignant pheochromocytoma: state of the field with future projections. Ann NY Acad Sci. 2006;1073:449–464. doi: 10.1196/annals.1353.049. [DOI] [PubMed] [Google Scholar]
- Allibhai Z, Rodrigues G, Brecevic E, Neumann HP, Winquist E. Malignant pheochromocytoma associated with germline mutation of the SDHB gene. J Urol. 2004;172 (4 Pt 1:1409–1410. doi: 10.1097/01.ju.0000137892.89141.6a. [DOI] [PubMed] [Google Scholar]
- Blank A, Schmitt AM, Korpershoek E, van Nederveen F, Rudolph T, Weber N, Strebel RT, de Krijger R, Komminoth P, Perren A. SDHB loss predicts malignancy in pheochromocytomas/sympathethic paragangliomas, but not through hypoxia signalling. Endocr Relat Cancer. 2010;17 (4:919–928. doi: 10.1677/ERC-09-0316. [DOI] [PubMed] [Google Scholar]
- Bowman A, Gabra H, Langdon SP, Lessells A, Stewart M, Young A, Smyth JF. CA125 response is associated with estrogen receptor expression in a phase II trial of letrozole in ovarian cancer: identification of an endocrine-sensitive subgroup. Clin Cancer Resh. 2002;8 (7:2233–2239. [PubMed] [Google Scholar]
- Brouwers FM, Eisenhofer G, Tao JJ, Kant JA, Adams KT, Linehan WM, Pacak K. High frequency of SDHB germline mutations in patients with malignant catecholamine-producing paragangliomas: implications for genetic testing. J Clin Endocrinol Metab. 2006;91 (11:4505–4509. doi: 10.1210/jc.2006-0423. [DOI] [PubMed] [Google Scholar]
- Brukamp E, Haase VH.2006Von Hippel–Lindau Disease, Phaeochromocytoma and Hypertension Advanced therapy in hypertension and vascular diseaseMohler ER, Townsend RR, (eds).660–668.. B.C. Decker: Hamilton, Ontario, Canada [Google Scholar]
- Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20 (5:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, Kung AL, Sanso G, Powers JF, Tischler AS, Hodin R, Heitritter S, Moore F, Dluhy R, Sosa JA, Ocal IT, Benn DE, Marsh DJ, Robinson BG, Schneider K, Garber J, Arum SM, Korbonits M, Grossman A, Pigny P, Toledo SP, Nose V, Li C, Stiles CD. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1 (1:72–80. doi: 10.1371/journal.pgen.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLellis RA, Lloyd RV, Heitz P, Eng C. Pathology and Genetics of Tumours of Endocrine Organs. IARC: Lyon; 2004. [Google Scholar]
- Dhillon T, Mauri FA, Bellezza G, Cagini L, Barbareschi M, North BV, Seckl MJ. Overexpression of the mammalian target of rapamycin: a novel biomarker for poor survival in resected early stage non-small cell lung cancer. J Thorac Oncol. 2010;5 (3:314–319. doi: 10.1097/JTO.0b013e3181ce6604. [DOI] [PubMed] [Google Scholar]
- Druce MR, Kaltsas GA, Fraenkel M, Gross DJ, Grossman AB. Novel and evolving therapies in the treatment of malignant phaeochromocytoma: experience with the mTOR inhibitor everolimus (RAD001) Horm Metab Res. 2009;41 (9:697–702. doi: 10.1055/s-0029-1220687. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Tischler AS, de Krijger RR. Diagnostic tests and biomarkers for pheochromocytoma and extra-adrenal paraganglioma: from routine laboratory methods to disease stratification. Endocr Pathol. 2012;23 (1:4–14. doi: 10.1007/s12022-011-9188-1. [DOI] [PubMed] [Google Scholar]
- Fassnacht M, Weismann D, Ebert S, Adam P, Zink M, Beuschlein F, Hahner S, Allolio B. AKT is highly phosphorylated in pheochromocytomas but not in benign adrenocortical tumors. J Clin Endocrinol Metab. 2005;90 (7:4366–4370. doi: 10.1210/jc.2004-2198. [DOI] [PubMed] [Google Scholar]
- Favier J, Briere JJ, Burnichon N, Riviere J, Vescovo L, Benit P, Giscos-Douriez I, De Reynies A, Bertherat J, Badoual C, Tissier F, Amar L, Libe R, Plouin PF, Jeunemaitre X, Rustin P, Gimenez-Roqueplo AP. The Warburg effect is genetically determined in inherited pheochromocytomas. PloS one. 2009;4 (9:e7094. doi: 10.1371/journal.pone.0007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favier J, Plouin PF, Corvol P, Gasc JM. Angiogenesis and vascular architecture in pheochromocytomas: distinctive traits in malignant tumors. Am J Pathol. 2002;161 (4:1235–1246. doi: 10.1016/S0002-9440(10)64400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Kong F, Xu Z. Development of differential diagnosis for benign and malignant pheochromocytomas. Int J Urol. 2008;15 (9:771–777. doi: 10.1111/j.1442-2042.2008.02111.x. [DOI] [PubMed] [Google Scholar]
- Giatromanolaki A, Harris AL. Tumour hypoxia, hypoxia signaling pathways and hypoxia inducible factor expression in human cancer. Anticancer Res. 2001;21 (6B:4317–4324. [PubMed] [Google Scholar]
- Gill AJ, Benn DE, Chou A, Clarkson A, Muljono A, Meyer-Rochow GY, Richardson AL, Sidhu SB, Robinson BG, Clifton-Bligh RJ. Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma-pheochromocytoma syndromes. Hum Pathol. 2010;41 (6:805–814. doi: 10.1016/j.humpath.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Burnichon N, Amar L, Favier J, Jeunemaitre X, Plouin PF. Recent advances in the genetics of phaeochromocytoma and functional paraganglioma. Clin Exp Pharmacol Physiol. 2008;35 (4:376–379. doi: 10.1111/j.1440-1681.2008.04881.x. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Lehnert H, Mannelli M, Neumann H, Opocher G, Maher ER, Plouin PF. Phaeochromocytoma, new genes and screening strategies. Clin Endocrinol (Oxf) 2006;65 (6:699–705. doi: 10.1111/j.1365-2265.2006.02714.x. [DOI] [PubMed] [Google Scholar]
- Gimm O, DeMicco C, Perren A, Giammarile F, Walz MK, Brunaud L. Malignant pheochromocytomas and paragangliomas: a diagnostic challenge. Langenbeck's Arch Surg/ Deutsche Gesellschaft fur Chirurgie. 2012;397 (2:155–177. doi: 10.1007/s00423-011-0880-x. [DOI] [PubMed] [Google Scholar]
- Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat Rev Cancer. 2005;5 (11:857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- Harari A, Inabnet WB. Malignant pheochromocytoma: a review. Am J Surg. 2011;201 (5:700–708. doi: 10.1016/j.amjsurg.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Kaluz S, Kaluzova M, Liao SY, Lerman M, Stanbridge EJ. Transcriptional control of the tumor- and hypoxia-marker carbonic anhydrase 9: A one transcription factor (HIF-1) show. Biochim Biophys Acta. 2009;1795 (2:162–172. doi: 10.1016/j.bbcan.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Rini BI, Hansel DE. Von Hippel–Lindau syndrome. Adv Exp Med Biol. 2010;685:228–249. doi: 10.1007/978-1-4419-6448-9_22. [DOI] [PubMed] [Google Scholar]
- Kimura N, Watanabe T, Noshiro T, Shizawa S, Miura Y. Histological grading of adrenal and extra-adrenal pheochromocytomas and relationship to prognosis: a clinicopathological analysis of 116 adrenal pheochromocytomas and 30 extra-adrenal sympathetic paragangliomas including 38 malignant tumors. Endocr Pathol. 2005;16 (1:23–32. doi: 10.1385/ep:16:1:023. [DOI] [PubMed] [Google Scholar]
- Koch CA, Mauro D, Walther MM, Linehan WM, Vortmeyer AO, Jaffe R, Pacak K, Chrousos GP, Zhuang Z, Lubensky IA. Pheochromocytoma in von Hippel–Lindau disease: distinct histopathologic phenotype compared to pheochromocytoma in multiple endocrine neoplasia type 2. Endocr Pathol. 2002;13 (1:17–27. doi: 10.1385/ep:13:1:17. [DOI] [PubMed] [Google Scholar]
- Korpershoek E, Favier J, Gaal J, Burnichon N, van Gessel B, Oudijk L, Badoual C, Gadessaud N, Venisse A, Bayley JP, van Dooren MF, de Herder WW, Tissier F, Plouin PF, van Nederveen FH, Dinjens WN, Gimenez-Roqueplo AP, de Krijger RR. SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab. 2011;96 (9:E1472–E1476. doi: 10.1210/jc.2011-1043. [DOI] [PubMed] [Google Scholar]
- Ladroue C, Carcenac R, Leporrier M, Gad S, Le Hello C, Galateau-Salle F, Feunteun J, Pouyssegur J, Richard S, Gardie B. PHD2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med. 2008;359 (25:2685–2692. doi: 10.1056/NEJMoa0806277. [DOI] [PubMed] [Google Scholar]
- Lavecchia MJ, Diez RP, Colinas PA. A combined theoretical and spectroscopic study of 4,6-di-O-acetyl-2,3-dideoxy-D-erythro-hex-2-enopyranosyl sulfamide: a novel glycosyl carbonic anhydrase IX inhibitor. Carbohydr Res. 2011;346 (3:442–448. doi: 10.1016/j.carres.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors--similar but not identical. Mol Cells. 2010;29 (5:435–442. doi: 10.1007/s10059-010-0067-2. [DOI] [PubMed] [Google Scholar]
- Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10 (6:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- Manger WM, Eisenhofer G. Pheochromocytoma: diagnosis and management update. Curr Hypertens Rep. 2004;6 (6:477–484. doi: 10.1007/s11906-004-0044-2. [DOI] [PubMed] [Google Scholar]
- Middeke M, Hoffmann S, Hassan I, Wunderlich A, Hofbauer LC, Zielke A. In vitro and in vivo angiogenesis in PC12 pheochromocytoma cells is mediated by vascular endothelial growth factor. Exp Clin Endocrinol Diab. 2002;110 (8:386–392. doi: 10.1055/s-2002-36424. [DOI] [PubMed] [Google Scholar]
- Nolting S, Grossman AB. Signaling pathways in pheochromocytomas and paragangliomas: prospects for future therapies. Endocr Pathol. 2012;23 (1:21–33. doi: 10.1007/s12022-012-9199-6. [DOI] [PubMed] [Google Scholar]
- Petri BJ, van Eijck CH, de Herder WW, Wagner A, de Krijger RR. Phaeochromocytomas and sympathetic paragangliomas. Br J Surg. 2009;96 (12:1381–1392. doi: 10.1002/bjs.6821. [DOI] [PubMed] [Google Scholar]
- Pollard PJ, El-Bahrawy M, Poulsom R, Elia G, Killick P, Kelly G, Hunt T, Jeffery R, Seedhar P, Barwell J, Latif F, Gleeson MJ, Hodgson SV, Stamp GW, Tomlinson IP, Maher ER. Expression of HIF-1alpha, HIF-2alpha (EPAS1), and their target genes in paraganglioma and pheochromocytoma with VHL and SDH mutations. J Clin Endocrinol Metab. 2006;91 (11:4593–4598. doi: 10.1210/jc.2006-0920. [DOI] [PubMed] [Google Scholar]
- Reisch N, Peczkowska M, Januszewicz A, Neumann HP. Pheochromocytoma: presentation, diagnosis and treatment. J Hypertens. 2006;24 (12:2331–2339. doi: 10.1097/01.hjh.0000251887.01885.54. [DOI] [PubMed] [Google Scholar]
- Salmenkivi K, Haglund C, Ristimaki A, Arola J, Heikkila P. Increased expression of cyclooxygenase-2 in malignant pheochromocytomas. J Clin Endocrinol Metab. 2001;86 (11:5615–5619. doi: 10.1210/jcem.86.11.8052. [DOI] [PubMed] [Google Scholar]
- Salmenkivi K, Heikkila P, Liu J, Haglund C, Arola J. VEGF in 105 pheochromocytomas: enhanced expression correlates with malignant outcome. APMIS. 2003;111 (4:458–464. doi: 10.1034/j.1600-0463.2003.1110402.x. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov Today. 2007;12 (19-20:853–859. doi: 10.1016/j.drudis.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Srirangalingam U, Walker L, Khoo B, MacDonald F, Gardner D, Wilkin TJ, Skelly RH, George E, Spooner D, Monson JP, Grossman AB, Akker SA, Pollard PJ, Plowman N, Avril N, Berney DM, Burrin JM, Reznek RH, Kumar VK, Maher ER, Chew SL. Clinical manifestations of familial paraganglioma and phaeochromocytomas in succinate dehydrogenase B (SDH-B) gene mutation carriers. Clin Endocrinol (Oxf) 2008;69 (4:587–596. doi: 10.1111/j.1365-2265.2008.03274.x. [DOI] [PubMed] [Google Scholar]
- Strong VE, Kennedy T, Al-Ahmadie H, Tang L, Coleman J, Fong Y, Brennan M, Ghossein RA. Prognostic indicators of malignancy in adrenal pheochromocytomas: clinical, histopathologic, and cell cycle/apoptosis gene expression analysis. Surgery. 2008;143 (6:759–768. doi: 10.1016/j.surg.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26 (2:299–310. doi: 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- Takekoshi K, Isobe K, Yashiro T, Hara H, Ishii K, Kawakami Y, Nakai T, Okuda Y. Expression of vascular endothelial growth factor (VEGF) and its cognate receptors in human pheochromocytomas. Life Sci. 2004;74 (7:863–871. doi: 10.1016/j.lfs.2003.07.036. [DOI] [PubMed] [Google Scholar]
- Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26 (5:551–566. doi: 10.1097/00000478-200205000-00002. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Srivastava S. New paradigms in translational science research in cancer biomarkers. Transl Res. 2012;159 (4:343–353. doi: 10.1016/j.trsl.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatovicova M, Jelenska L, Hulikova A, Csaderova L, Ditte Z, Ditte P, Goliasova T, Pastorek J, Pastorekova S. Carbonic anhydrase IX as an anticancer therapy target: preclinical evaluation of internalizing monoclonal antibody directed to catalytic domain. Curr Pharm Des. 2010;16 (29:3255–3263. doi: 10.2174/138161210793429832. [DOI] [PubMed] [Google Scholar]