Abstract
Hereditary channelopathies, that is, mutations in channel genes that alter channel function and are causal for the pathogenesis of the disease, have been described for several members of the transient receptor potential channel family. Mutations in the TRPV4 gene, encoding a polymodal Ca2+ permeable channel, are causative for several human diseases, which affect the skeletal system and the peripheral nervous system, with highly variable phenotypes. In this review, we describe the phenotypes of TRPV4 channelopathies and overlapping symptoms. Putative mechanisms to explain the puzzle, and how mutations in the same region of the channel cause different diseases, are discussed and experimental approaches to tackle this surprising problem are suggested.
Keywords: transient receptor potential, cation channels, skeletal disease, neuropathy
See the Glossary for abbreviations used in this article.
Glossary.
- AIP4
E3 ubiquitin ligase
- BSCL2
Berardinelli–Seip congenital lipodystrophy gene 2, encoding the protein seipin
- CHO
Chinese hamster ovary cell line
- DCTN1
gene encoding axonal transport protein dynactin 1
- DYNC1H1
gene encoding the cytoplasmic protein dynein 1 heavy chain 1
- GARS
gene encoding glycyl-tRNA synthase
- HECT
homologous to E6-AP carboxyl terminus family ubiquitin ligase AIP4
- HEK293
human embryonic kidney 293 cell line
- HSPB1/3/8
genes encoding heat-shock proteins required for protein folding
- IGHMBP2
immunoglobulin helicase (mu)-binding protein 2
- KIF1B
gene encoding the kinesin-like protein KIF1B
- Kir4.1
inwardly rectifying K+ channel
- MAP7
microtubule-associated protein 7
- MFN2
gene encoding mitofusin 2
- PDZ
postsynaptic density protein (PSD95), Drosophila disc large tumour suppressor (Dlg1) and zonula occludens 1 protein (zo-1) domain
- PLEKHG5
pleckstrin homology domain-containing, family G member 5
- TRPM
members of melastatin subfamily of TRP
- TRPV
members of vanilloid subfamily of TRP
- SETX
gene encoding RNA helicase senataxin
- SMN1
gene encoding SMN, a protein necessary for the survival of motor neurons
- SOX9
a high-mobility-group domain transcription factor
Introduction
The TRPV4 gene is prone to many mutations that cause malfunctions of the encoded Ca2+ permeable cation channel, which in turn mediate a surprising number of diseases. One group comprises skeletal dysplasias, such as brachyolmia, spondylometaphyseal dysplasia Kozlowski (SMDK), spondylo-epimetaphyseal dysplasia Maroteaux pseudo-Morquio type 2 (SEDM-PM2), metatropic dysplasia and parastremmatic dysplasia. Common features of these diseases are a short trunk with vertebral platyspondyly and scoliosis. Familial digital arthropathy brachydactyly (FDAB) only affects fingers and toes.
Mutations in TRPV4 have also been identified in motor and sensory neuropathies—such as congenital distal spinal muscle atrophy, scapuloperoneal spinal muscle atrophy and Charcot–Marie–Tooth disease type 2C (CMT2C)—genetically heterogeneous inherited disorders caused by the degeneration of motor neurons and peripheral nerves. TRPV4 neuropathies show a striking phenotypic variability. This phenotypical diversity of the mutations, which are often located in the same domains as the channel proteins, is one of the most exciting puzzles in the pathophysiology of ion channels in general and transient receptor potential (TRP) channels in particular. Understanding the underlying mechanisms of these channel malfunctions not only is essential for treatment of these diseases but also might help to elucidate the function of these proteins and their involvement in different signalling cascades.
TRPV4—some basics
TRPV4 is broadly expressed and is one of the best-characterized TRP channels [1]. It could be compared to Proteus, a God of the sea in Greek mythology, who has characteristics of flexibility, versatility, mutability and adaptability, emerges in many shapes and can appear in frightening forms.
TRPV4 is a polymodally gated TRP channel that can be activated by diverse stimuli including moderate heat, cell swelling, endogenous chemicals such as anandamide, arachidonic acid and its epoxyeicosatrienoic acid metabolites, as well as by a growing number of exogenous chemical ligands [2,3,4,5,6,7,8,9,10,11,12]. Known exogenous ligands include (i) natural, plant-derived compounds, such as bisandrographolide A (BAA), from an extract of the Indian herbaceous plant Andrographis paniculata [13], citric acid [14] and apigenin (4′,5,7-trihydroxyflavone), a flavone found in many plants [15]; (ii) phorbol esters such as 4α-phorbol 12,13-didecanoate (4α-PDD), 4α-phorbol 12,13-dihexanoate (4α-PDH) and phorbol 12-myristate 13-acetate [7,11]; and (iii) synthetic ligands, such as GSK1016790A, RN-1747 and RN-1734 [10,12]. Ruthenium red is still frequently used as a TRPV4 antagonist, but it is known to also inhibit many other TRP channels [16]. Much more selective is the hydra compound HC-067047 (see also the TRPV4 database; http://www.iuphar-db.org/DATABASE/ObjectDisplayForward?familyId=78&objectId=510; [17]).
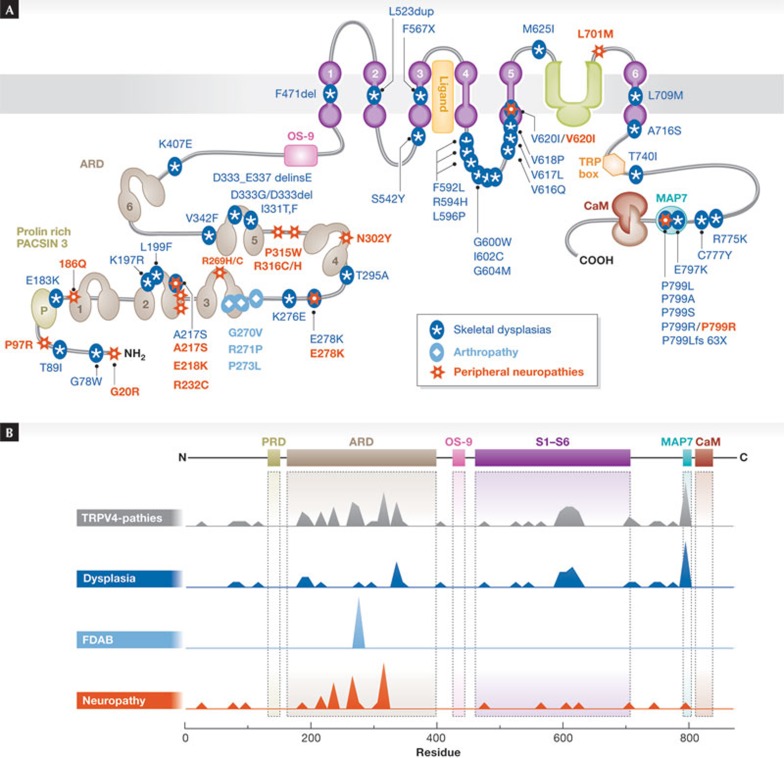
Although the crystal structure of the channel has not yet been fully determined, we do have some insight into its three-dimensional structure. A single TRPV4 subunit consists of six transmembrane segments (S1–S6), and four such subunits assemble to form a functional TRPV4 channel. S5, S6 and the interconnecting loop form the central cation-permeable pore, while S3 and S4 seem to constitute an agonist-binding pocket, in particular for phorbol compounds, and might also contribute to channel gating. The amino- and carboxytermini face the cytoplasm and contain a variety of functional domains (Fig 1; [18,19]). For example, as in other members of the TRPV subfamily, the N-terminus of TRPV4 features a characteristic ankyrin repeat domain (ARD), consisting of six ankyrin repeats (ANK). These repeats form a double-helical structure and are connected by extraordinary long folds, called ‘fingers’, which might be important for decisive protein–protein interactions [1,20,21]. A proline-rich domain (PRD) that has been implicated in the mechanosensitivity of the TRPV4 channel precedes the first ANK. Within this PRD—delineated by Pro 132 and Pro 152—the central proline residue, at positions 142, 143 and 144, interacts with pacsin 3, a protein implicated in vesicular membrane transport, endocytosis and cytoskeleton reorganization [22,23]. The ARD and the juxtamembrane region form an interaction site for OS-9, an endoplasmic-recticulum-resident protein involved in protein-folding and ubiquitination [24]. The TRPV4 C-terminal tail contains additional functional domains such as a TRP box, a calmodulin-binding site and a binding site for cytoskeletal proteins such as MAP7, actin and tubulin [25,26,27]. The last four amino acids of the C-terminus Asp-Ala-Pro-Leu are similar to a PDZ-binding-like motif that contributes to interaction of TRPV4 with a huge variety of PDZ-domain proteins (see the 37 interaction partners at the TRIP Database 2.0; http://trpchannel.org/; [28]).
Figure 1.
Domain structure and disease-causing mutations in TRPV4. A schematic view of the TRPV4 channel indicating several domains and mutants that cause skeletal dysplasias, neuropathies and the arthropathy FDAB. (A) Secondary structure of TRPV4, with putative functional domains. (B) Mutation frequency along the amino acid sequence of TRPV4 for all TRPV4-dependent diseases, as well as for skeletal dysplasias, FDAB and neuropathies. FDAB, familial digital arthropathy brachydactyly.
The expression pattern of TRPV4 is relatively broad. In the brain, TRPV4 is expressed in neurons of the vascular organ of the lamina terminalis, anterior hypothalamic structures and ependymal cells of the choroid plexus in the lateral ventricles, where it is required as an osmosensor [2]. It has also been detected in motor, spinal ventral root and dorsal root ganglion neurons, in which it might have a role in nociception [29]. Other prominent sites of TRPV4 expression, mainly with sensory functions—mechanosensing, volume regulation and osmosensing—are the bladder urothelium, kidney epithelium, vascular endothelium, inner ear, pulmonary aortic smooth muscle, cardiac fibroblasts, myocytes and skeletal muscle fibres. TRPV4 has been put forward as a regulator of the skin-barrier function of keratinocytes [30,31,32,33] and of oxidative metabolism in adipocytes [34], and as an important player in the development of chondrocytes [35,36].
Our understanding of the physiological role of TRPV4 in cells and organs has increased significantly from the study of TRPV4-deficient mice. These mice are viable and fertile, but have various peculiar phenotypes, including a larger bladder capacity due to an impaired stretch sensor in the bladder wall [37], thicker bones due to impaired osteoclast differentiation [38], reduced water intake due to altered osmosensation [39,40], compromised vascular endothelial function [5,41,42,43,44,45’ and some sensory defects, such as hearing alteration [46], impaired pressure sensation [14] and compromised pain sensing [1,47]. Recently, the first selective TRPV4 antagonist with in vivo activity has been described; the HC067047 inhibits bladder hyperactivity in mice and rats without obvious side effects and prevents heart failure-induced pulmonary oedema [17,48].
TRPV4 mutations in human channelopathies
By definition, hereditary channelopathies are diseases caused by mutations in ion channel genes and the resulting altered channel functions. Considering that ion channels are highly specialized and sophisticated molecular devices, it is not surprising that any change to their structure might significantly modify their activity. However, given the relatively mild nature of the phenotype of the TRPV4 knockout mice and the lack of obvious undesirable side-effects of TRPV4 antagonism in mice and rats, it comes as a surprise that mutations in TRPV4 are the direct cause of several disabling or even lethal human diseases. Two main diverging groups of diseases have been described as causally linked to mutations in the TRPV4 gene, namely skeletal dysplasias with short stature, platyspondyly and defects in bone ossification as the most common symptoms, distal neuropathies with mainly motor defects in the distal limbs but also in the respiratory system and the vocal cord, and sometimes with sensory defects [49]. In addition, mutations in the trpv4 gene have been identified as the cause of FDAB, a progressive osteoarthropathy. All known disease-causing mutations are indicated in Fig 1 and listed in Table 1.
Table 1. TRPV4 mutations leading to skeletal diseases and neuropathies.
| Mutation | BO3 | SEDM-PM2 | SMDK | PD | MD | FDAB | CDSMA | SPSMA | CMT2C | Region | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| G20R | − | − | − | − | − | − | + | − | − | N-terminus | [72] |
| G78W | − | − | − | − | + / FA | − | − | − | − | N-terminus | [54,56] |
| T89I | − | − | − | − | + | − | − | − | − | N-terminus | [56] |
| P97R | − | − | − | − | − | − | + | − | − | N-terminus | [63] |
| E183K | − | + | − | − | − | − | − | − | − | ARD1; Loop1 | [51,56] |
| R186Q | − | − | − | − | − | − | − | − | + | ARD1 | [96] |
| K197R | − | − | − | − | + | − | − | − | − | ARD2 | [21,56] |
| L199F | − | − | − | − | + | − | − | − | − | ARD2 | [21,56,93] |
| A217S | − | − | + | − | − | − | − | + | − | ARD2 | [94] |
| E218K | − | − | − | − | − | − | − | − | + | ARD2 | [72] |
| R232H | − | − | − | − | − | − | − | − | + | ARD2; Loop2 | [57] |
| R232C | − | − | − | − | − | − | + | + | + | ARD2; Loop2 | [57,63,69,72,95] |
| R269C | − | − | − | − | − | − | + | + | + | ARD3 | [21,57,69,71,96] |
| R269H | − | − | − | − | − | − | + | + | + | ARD3 | [57,65,69,71,72,84] |
| G270V | − | − | − | − | − | + | − | − | − | ARD3 | [73] |
| R271P | − | − | − | − | − | + | − | − | − | ARD3 | [73] |
| F272L | − | − | − | − | − | + | − | − | − | ARD3 | [73] |
| K276E | − | − | − | − | +/ FA | − | − | − | − | ARD3; Loop3 | [54] |
| E278K | − | − | + | − | + | − | − | + | − | ARD3; Loop3 | [21,72,93,94] |
| T295A | − | − | − | − | + | − | − | − | − | ARD4 | [56,93] |
| N302Y | − | − | − | − | − | − | − | − | + | ARD4 | [72] |
| R315W | − | − | − | − | − | − | + | + | + | ARD4; Loop4 | [57,68,69,70,97] |
| R316C | − | − | − | − | − | − | + | + | + | ARD4; Loop4 | [57,65,70] |
| R316H | − | − | − | − | − | − | − | − | + | ARD4; Loop4 | [70] |
| I331T | − | − | − | − | + | − | − | − | − | ARD5 | [21,93] |
| I331F | − | − | + | − | + | − | − | − | − | ARD5 | [21,93] |
| D333G | − | − | + | − | − | − | − | − | − | ARD5 | [21,93] |
| D333-E337delinsE | − | − | − | − | + | − | − | − | − | ARD5 | [55] |
| V342F | − | − | − | − | + | − | − | − | − | ARD5; Loop5 | [21,93] |
| K407E | − | − | − | − | + | − | − | − | − | ARD6–S1 | [98] |
| F471del | − | − | − | − | +/ FA | − | − | − | − | S1 | [93] |
| L523dup | − | − | + | − | − | − | − | − | − | S2 | [98] |
| S542Y | + | − | − | − | − | − | − | − | − | S2–S3 loop | [68] |
| Y567X | − | − | − | − | − | − | − | − | + | S3 | [72] |
| F592L | − | − | − | − | + | − | − | − | − | S4–S5 loop | [93] |
| R594H | − | − | + | + | − | − | − | − | − | S4–S5 loop | [51,93] |
| L596P | − | − | + | − | − | − | − | − | − | S4–S5 loop | [93] |
| G600W | − | − | + | − | − | − | − | − | − | S4–S5 loop | [93] |
| Y602C | + | + | − | − | − | − | − | − | − | S4–S5 loop | [51] |
| I604M | − | − | − | − | +/ FA | − | − | − | − | S4–S5 loop | [55] |
| R616Q | + | − | − | − | − | − | − | − | − | S4–S5 loop | [52] |
| F617L | − | − | + | − | + | − | − | − | − | S4–S5 loop | [55,98] |
| L618Q | − | − | − | − | + /FA | − | − | − | − | S4–S5 loop | [55] |
| V620I | + | − | − | − | − | − | − | + | − | S4–S5 loop | [52,69] |
| M625I | − | − | + | − | − | − | − | − | − | S5 pore | [93] |
| T701I | − | − | − | − | − | − | − | − | + | S5 pore | [72] |
| L709M | + | − | + | − | − | − | − | − | − | S6 | [93] |
| A716S | − | − | + | − | − | − | − | − | − | S6, cytoplasmic | [53,68,93] |
| T740I | − | − | − | − | + /FA | − | − | − | − | C-terminus | [54] |
| R775K | − | − | − | − | + | − | − | − | − | C-terminus | [93] |
| C777Y | − | − | + | − | − | − | − | − | − | C-terminus | [93] |
| E797K | − | + | + | + | + | − | − | − | − | C-terminus | [51,55,56,93,94,99] |
| P799S | − | − | − | − | + | − | − | − | − | C-terminus | [93,98] |
| P799A | − | − | − | − | + | − | − | − | − | C-terminus | [93] |
| P799R | − | + | − | + | − | − | − | − | + | C-terminus | [94,99] |
| P799L | − | + | − | − | + | − | − | − | − | C-terminus | [53,93,98] |
| P799Lfs63X | − | + | − | − | − | − | − | − | + | C-terminus | [51] |
Metatropic dysplasia (MD) is combined with fetal akinesia (FA). ARD, ankyrin repeat domain; BO3, autosomal dominant skeleto-dysplasia brachyolmia type 3; CDSMA, congenital distal spinal muscle atrophy; CMT2C, Charcot–Marie–Tooth disease type 2C; FDAB, familial digital arthropathy brachydactyly; PD, parastremmatic dysplasia; SEDM-PM2, spondylo-epimetaphyseal dysplasia Maroteaux pseudo-Morquio type 2; SMDK, spondylometaphyseal dysplasia Kozlowski type; SPSMA, scapuloperoneal spinal muscular atrophy.
TRPV4-mediated skeletal diseases
Skeletal dysplasias are a heterogeneous group of over 200 diseases characterized by abnormalities of bone and cartilage growth, resulting malformations and disproportions in the skeleton. In recent years, a wide spectrum of mutations in the trpv4 gene have been identified as the cause of various forms of dwarfism characterized by a short trunk, as well as of FDAB, a skeletal dysplasia.
TRPV4-associated skeletal dysplasias. In 1929, Morquio first described a type of familial osseous bone dystrophy, with four siblings characterized predominantly by a shortening of the trunk [50]. Since this first description, Morquio syndrome has been used as a common denominator for a heterogeneous group of bone dystrophies characterized by having a short trunk and being of short stature due to generalized platyspondyly—flattening of the vertebrae. Genetically, the diseases summarized under Morquio syndrome are caused by defects in genes such as N-acetylgalactosamine 6-sulphatase, β-galactosidase and dymeclin—a protein with unclear function that seems to be important in the secretory pathway [51]. The pseudo-Morquio disease type 2, which was renamed as spondylo-epiphyseal dysplasia (SED) and later as SEDM-PM2 (OMIM 184095; [51]), has been identified as one of several bone dysplasias caused by mutations in the TRPV4 gene. SEDM-PM2 patients have short stature, shortened hands and feet, platyspondyly, diffuse osteoporosis and arthrosis. Other TRPV4-dependent dysplasias include autosomal dominant brachyolmia type 3 (BO3; OMIM 113500), SMDK (OMIM 184252), parastremmatic dysplasia (OMIM 168400), and non-lethal and lethal metatropic dysplasia (OMIM 156530; see Sidebar A for a summary of the symptoms; [51,52,53,54,55]). Lethal metatropic dysplasia is probably the most severe TRPV4-related disease, with arthrogryposis (from the Greek for ‘curved or hooked joints’), which is characterized by multiple joint contractures, hip dislocation, clubfoot and muscle weakness, and fetal akinesia as the most severe, lethal symptoms [54]. Characteristic of these TRPV4-mediated dysplasias are the short trunk, scoliosis and brachydactyly, which are neither associated with ocular changes, as opposed to the corneal clouding often observed in Morquio types A and B lysosomal storage diseases, nor with neurodevelopmental abnormalities, as opposed to the mental retardation in pseudo-Morquio disease type I. In total, more than 50 mutations in TRPV4 have been reported to cause brachyolmias. These mutations are spread over the whole gene (Fig 1; Table 1), although two hot spots have been observed, Pro 799 in exon 15 and Arg 594 in exon 11 [56].
Sidebar A | TRPV4-mediated skeletal diseases.
Autosomal dominant brachyolmia type 3
Autosomal dominant brachyolmia type 3 is an autosomal dominant disorder, primarily characterized by brachyolmia, which in Greek means ‘short trunk’. Patients are normal length at birth, but develop short trunk and short stature during childhood, mainly due to platyspondyly (flattening (platy-) of the vertebrae (spondyl-) throughout the axial skeleton). Other typical features include kyphoscoliosis (abnormal curvature of the spine in both coronal and sagittal planes) and slightly short limbs.
Spondylo-epimetaphyseal dysplasia Maroteaux pseudo-Morquio type 2
Spondylo-epimetaphyseal dysplasia Maroteaux pseudo-Morquio type 2 (SEDM-PM2) is an autosomal dominant dysplasia (abnormality of development) that affects the vertebrae as well as the epiphyses (rounded ends of long bones) and metaphyses (area below the epiphysis including the growth plate). Patients are normal length at birth, but develop short trunk and short stature, and generally have kyphoscoliosis, brachydactyly (shortening of finger and toes), short femoral necks with dysplastic epiphyses, genu valgum (‘knock-knees’) and osteoporosis.
Spondylometaphyseal dysplasia Kozlowski type
Spondylometaphyseal dysplasia Kozlowski type (SMDK) is an autosomal dysplasia that mainly affects the vertebrae and the metaphyses. As in SEDM-PM2 and autosomal dominant brachyolmia type 3, patients are a normal length at birth, but develop short stature during childhood, but compared with SEDM-PM2 and autosomal dominant brachyolmia type 3, SMDK patients display a more severe phenotype, including severe scoliosis and extremely short stature due to shortening of both trunk and femur. The disease is often associated with mild brachydactyly, metaphyseal abnormalities in the pelvis and premature degenerative joint disease.
Parastremmatic dysplasia
Parastremmatic dwarfism is an autosomal dominant dysplasia, characterized typically by a severe distortion and twisting of the limbs and extreme dwarfism. The disease is congenital (apparent at birth) and patients develop progressive kyphoscoliosis, distortion and bowing of the extremities and contractures of the large joints during the first years of life. The malformations are attributed to a deficit in bone mineralization.
Metatropic dysplasia
Metatropic dysplasia is an autosomal dysplasia that can occur in mild, severe and even lethal forms. Non-lethal metatropic dysplasia is congenital, and patients initially have relatively short limbs compared with their trunk. Radiological markers are severe platyspondyly, severe metaphyseal enlargement and shortening of long bones. As children get older, they develop kyphoscoliosis, such that arms and legs become longer compared with their torso. The term metatropic, from the Greek ‘metatropos’ (changing patterns), refers to this changing skeletal phenotype. The lethal form of metatropic dysplasia is often associated with fetal akinesia, for example, absence of movements, severe joint contractures and respiratory complications leading to perinatal lethality.
Familial digital arthropathy brachydactyly
Familial digital arthropathy brachydactyly is a relatively mild autosomal dominant skeletal disease with no dysplasia symptoms. The earliest changes appear during the first decade of life and involve irregularities in the finger joints of hands and feet. Adults show deformations of interphalangeal, metacarpophalangeal and metatarsophalangeal joints and have a deforming and painful osteoarthritis. Other parts of the skeleton are unaffected.
TRPV4 in FDAB. FDAB (OMIM 606835) represents another mild TRPV4-related bone disease, distinct from dysplasias. FDAB patients seem to be normal at birth, but during the first decade of life they develop irregularities in the joints of finger and toes. In adults, all joints are affected by painful and deforming osteoarthritis. However, in contrast with the brachyolmias, no malformations of other parts of the skeleton have been observed in FDAB patients. At this point, FDAB-causing mutations in TRPV4 are restricted to finger 3 of the ARD (mutations G270V, R271P, F273L; Fig 1; Table 1).
TRPV4 neuropathies
Since the first description of a motor-sensory neuropathy by Charcot, Marie and Tooth in 1886 [57], many related but genetically different forms have been identified. TRPV4 has emerged as a major causative player in hereditary neuropathies. TRPV4-axonal neuropathies are a group of disorders that can either present as a predominantly motor axonal peripheral neuropathy, or can be associated with distinct sensory disturbances. In some forms, these neuropathies also have other symptoms, including vocal cord paresis, hearing defects or bladder hyperactivity. TRPV4-related diseases show autosomal dominant inheritance, but some disease-causing mutations have incomplete and variable disease penetration.
TRPV4 in distal hereditary spinal muscle atrophies. Spinal muscle atrophies (SMAs) have been known about since the nineteenth century and are characterized by loss of neurons in the anterior horn of the spinal cord. This leads to muscle weakness, wasting and degeneration of the motor neurons in the ventral horn, and is often combined with multiple pulmonary and orthopaedic symptoms. The most frequent form of SMA, responsible for more than 90% of cases, is caused by mutations in SMN1, which encodes the survival motor neuron protein (SMP) in humans. SMP-related SMA primarily affects the proximal muscles and is autosomal recessive [58,59].
Congenital distal SMAs (CDSMAs; OMIM 600175), also known as congenital distal hereditary motor neuropathies (CDHMNs or dHMNs), include phenotypically similar but clinically distinct disorders that mainly affect the distal limbs. These neuropathies, which are not caused by mutations in SMN1, are rare and lack a major sensory component, which sets them apart from neuropathies with combined motor and sensory dysfunctions. The TRPV4 gene is one of at least 12 causative genes that have been identified—the others include HSPB1, HSPB3, HSPB8, GARS, BSCL2, IGHMBP2, DCTN1, ATP7, SETX, DYNC1H1 and PLEKHG5. However, in 80% of cases the underlying genetic cause is unknown [60,61]. The products of the causative genes have been implicated in diverse cellular functions, including protein (mis)folding (HSPB1, HSPB3 and HSPB8), RNA metabolism (GARS and SETX), axonal transport (DCTN1) and ion transport (TRPV4 and the Cu2+ transporting ATPase ATP7A; [60]). The CDSMA caused by mutations in the TRPV4 gene represents the only TRPV4-dependent neuropathy that is not associated with sensory symptoms and is not progressive (see Sidebar B for a summary of TRPV4 neuropathies; [62]). The autosomal dominant disease is characterized by muscle atrophy, weakness and wasting, which is mainly restricted to the lower limbs and limits the mobility of the patients. Additional symptoms include abnormal curvature of the spine (hyperlodosis and kyphoscoliosis), talipes equinovarus (clubfoot), tongue fasciculation as well as knee and hip contractures. Some patients have severe distal motor neuropathy and arthrogryposis at birth. At this point, six different TRPV4 mutations leading to CDSMAs have been identified that are all restricted to the N-terminal cytosolic tail (Fig 1; Table 1; [57,63]).
Sidebar B | TRPV4-mediated neuropathies.
Congenital distal spinal motor neuropathy
Congenital distal spinal motor neuropathy (CDSMA) is an autosomal dominant disease characterized by a congenital and non-progressive deficiency of lower motor neurons. This leads to muscle weakness, muscle atrophy and areflexia in the lower part of the body. Patients typically have talipes equinovarus (clubfoot) and flexion contractures of the knees and hips, but no sensory deficits.
Scapuloperoneal spinal muscular atrophy
Scapuloperoneal spinal muscular atrophy (SPSMA) is an autosomal dominant disease characterized by a congenital reduction of muscles in the peroneus and scapular (shoulder blade) muscles, the latter causing the typical appearance of ‘scapular winging'. Furthermore, patients often have vocal cord paresis, which might cause breathing difficulties, a hoarse voice, progressive distal weakness and mild sensory deficits.
Charcot–Marie–Tooth disease type 2C
Charcot–Marie–Tooth disease type 2C (CMT2C) is an autosomal dominant disorder of the peripheral nervous system and is an axonal form of Charcot–Marie–Tooth disease, which is a general denominator for a heterogeneous group of neuropathies that affect both motor neuron and sensory neuron function. CMT2C is characterized by progressive muscle weakness and atrophy, in particular of the distal muscles, combined with a loss of touch sensation. Other common symptoms are bilateral sensorineural hearing loss, bladder urgency and incontinence and vocal cord paresis.
TRPV4 in scapuloperoneal spinal muscular atrophy. Scapuloperoneal SMA (SPSMA; OMIM 181405) is a clinically separate SMA characterized initially by scapuloperoneal atrophy, weakness and scapular winging, which progresses to distal muscle wasting in the lower limbs and is often associated with vocal cord paralysis and respiratory stridor. In 1992, SPSMA was first analysed in a large New England kindred, where it was characterized by an autosomal dominant inheritance pattern and large heterogeneity in disease expression and progression in different branches of the family [64]. Some affected family members had scapular winging, absence of tendon reflexes and scoliosis; others also had cranial nerve defects that led to facial weakness. Importantly, SPSMA patients often have clear but mild sensory defects, for example, a reduced detection of weak mechanical stimuli such as vibration [57].
By using a genetic linkage analysis in a French-Canadian family with SPSMA, Deng and colleagues identified a heterozygous mutation in the TRPV4 gene locus that occurs in exon 6 and results in the amino acid substitution of arginine by cysteine, R316C [65]. At this point, eight TRPV4 mutations leading to SPSMA have been identified, located in either the N-terminus or the transmembrane region (Fig 1; Table 1).
TRPV4 in CMT2C. CMT, also known as hereditary motor and sensory neuropathy (HMSN), is the most common inherited neuromuscular disorder, affecting at least 1 in 2,500. It actually comprises a large group of diseases characterized by progressive muscle wasting and loss of sensory function due to progressive degeneration of peripheral sensory and motor neurons. So far, mutations in over 50 genes have been shown to cause CMT, which occurs in autosomal dominant, autosomal recessive and X-linked forms. On the basis of detailed motor nerve conduction velocity, most cases of CMT can be classified into two main types. The demyelinating form (CMT1) is associated with a reduced motor nerve conduction velocity (<38 m s−1), whereas in the axonal form (CMT2) conduction velocity is mostly not affected [66].
The most common form of CMT, CMT1A, involves the duplication of a chromosomal region that includes the gene PMP22, which encodes a Schwann cell glycoprotein that is a major component of myelin. Many genes, often with apparently unrelated functions, have been identified as the cause of different forms of CMT2, including genes encoding molecular motors (KIF1B in CMT2A1), proteins involved in protein folding (HSPB8 in CMT2L) and proteins with mitochondrial function (MFN2 in CMT2A2; [67]).
Mutations in TRPV4 are the cause of CMT2C (OMIM 606071), an autosomal dominant, axonal neuropathy with vocal cord paralysis and diaphragmatic weakness, but with normal motor nerve conduction velocity. Many of the affected individuals have sensorineural hearing loss and bladder urgency, as well as respiratory symptoms, due to stridor, that can substantially shorten life expectancy [68,69]. Deng and colleagues extended their genetic analysis to a CMT2 family in which an arginine-to-histidine change was found at codon 269 of TRPV4 [65]. Other mutations include R315W and R316Cm, which have also been detected in patients with CDSMA and SPSMA [70,71].
At least 13 TRPV4 mutations causing CMT2C have been identified, spread over the protein (Fig 1; Table 1; [72]).
Mixed skeletal dysplasia and neuropathy disease forms
It is intriguing that mutations in many genes with distinct cellular functions result in diseases with similar phenotypes—for example, mutations in at least a dozen genes, including TRPV4, cause SMA—and even more mind-boggling that mutations in a single gene (here, TRPV4) can cause phenotypically distinct diseases, such as skeletal dysplasias and peripheral neuropathies.
A further complication is that detailed clinical analyses of patients with TRPV4 mutations revealed that there is not always a clear separation of skeletal dysplasia and neuropathy symptoms, as skeletal and neurological phenotypes can occur simultaneously [54]. As a general rule, patients with TRPV4 mutations that cause milder forms of short trunk skeletal dysplasias or FDAB rarely have neurological symptoms such as peripheral neuropathy or vocal cord paresis [73]. Rare cases of severe metatropic dysplasia with fetal akinesia, that is, the prenatal and postnatal absence of limb movements, are an exception to the rule [54].
By contrast, patients with TRPV4 mutations that lead primarily to peripheral neuropathies also tend to have skeletal malformations. In many cases, these skeletal malformations seem to have a distinct aetiology compared with the brachyolmias. For example, CDSMA or CMT2C patients frequently have arthrogryposis, talipes or scoliosis, but mostly lack signs of platyspondyly, which is the prime characteristic of TRPV4-dependent skeletal dysplasias [57]. Nevertheless, there are rare cases of CDSMA and CMT2C patients with classical symptoms of autosomal dominant brachyolmia, including short trunk and platyspondyly [68,69].
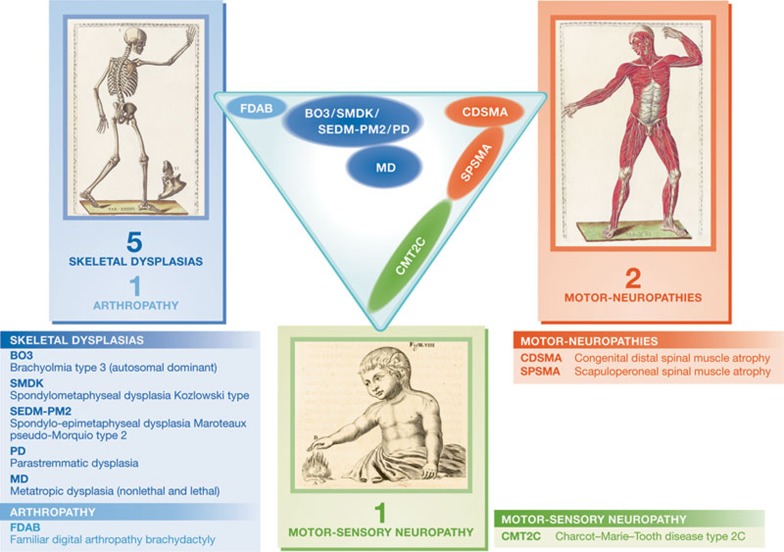
A further complication, especially in the case of TRPV4-dependent diseases, is that there is significant phenotypic noise, in that the same mutation, even within the same pedigree, can lead to variable degrees of disease manifestation or even the absence of symptoms. The exact disease manifestation depends not only on the specific mutation in the TRPV4 gene, but also on other genetic and environmental factors. Therefore, strict classification of patients with TRPV4 channelopathies into subclasses of skeletal dysplasias or neuropathies might need to be revised. For instance, a term such as ‘TRPV4-pathy’ could be used as a general clinical term to describe patients with mutations in the TRPV4 gene, which can manifest as a variable combination of skeletal, motor and neuronal symptoms (Fig 2).
Figure 2.
Spectrum of TRPV4-pathies. Qualitative description of the occurrence of skeletal, motor and sensory system dysfunctions in clinically distinct TRPV4-dependent diseases. Top: Bartholomeo Eustachi, Tabulae anatomicae, Rome, P. Junchus, 1783. Bottom: René Descartes, De homine figuris, Leiden, P. Leffen and F. Moyard, 1662. Illustrations are from the public domain of the US National Library of Medicine.
From mutation to disease
Although there is no doubt that mutations in the TRPV4 gene can cause skeletal dysplasias and neurodegenerative diseases, we have little or no understanding of the pathophysiological mechanisms underlying these diseases. Disappointingly, at this point, we have no clear answers to several key questions including: (i) What are the stimuli that activate and regulate TRPV4 function in vivo in cell types relevant for the diseases, such as peripheral neurons, osteoblasts, osteoclasts or chondrocytes? (ii) What are the effects of the disease-causing mutations on TRPV4 function in these and other cell types? (iii) How does altered TRPV4 function in these cells lead to the different disease symptoms? (iv) Which other (cellular) factors influence disease manifestations? (see also Sidebar C). Obtaining clear answers to these questions is a prerequisite to understanding the aetiology of these diseases and to develop potential pharmacological means to prevent or cure TRPV4 channelopathies.
Sidebar C | In need of answers.
What disposes the TRPV4 gene to such an exceptionally high number of disease-causing mutations compared with all other TRP superfamily members?
What is/are the relevant stimulus/stimuli for TRPV4 activation in vivo?
What is the correct in vivo functional phenotype of the disease-causing mutants? Gain-of-function, loss-of-function or alteration-of-function?
How can mutations within the same protein, and even within the same region of the protein, lead to such different disease phenotypes?
Why are other tissues and organs with a high expression of TRPV4— for example, bladder and vascular tissues—not or only mildly affected in patients?
Is TRPV4 a valid molecular target for the pharmacological treatment of the TRPV4-dependent diseases?
Experimental approaches
Functional expression of disease mutants. To test for the functional consequences of disease-causing mutations in the TRPV4 gene, several studies have transiently overexpressed a variety of mutant channels in cell lines such as HEK293 and CHO, and in Xenopus oocytes. Channel activity was assessed under basal conditions and following stimulation with known stimuli—mechanical and osmotic stress, heat and chemical agonists—by using whole-cell patch-clamp recordings and intracellular calcium imaging. In many cases, mutations associated with skeletal dysplasias or neuropathies were reported to cause an apparent gain of channel function, as indicated by higher basal or stimulated calcium levels, or larger basal and stimulated whole-cell currents [74,75,76]. By contrast, the FDAB mutations were found to have reduced activity, suggesting a loss of channel function [73]. However, there does not seem to be an unambiguous correlation between channel activity after overexpression and disease pattern or severity. For example, in a single study, SMDK patients with similar clinical features seemed to have either gain- or loss-of-function mutations [53]. In addition, in studies from different laboratories, heterologous overexpression of the same mutant (R269H) indicated either a gain- or loss-of-function phenotype, suggesting that cell type and possibly methodological aspects might affect the outcome of this type of assay [65,70,71,76]. The picture is complex.
One of the problems with these studies is that they look at the function of channels consisting of purely mutant channel subunits. However, the TRPV4-dependent diseases have a dominant inheritance pattern, which implies that patients carry both a mutant and a wild-type allele. Given that functional TRPV4 channels are tetramers, patients are expected to express TRPV4 channels with variable stoichiometry of wild-type and mutant subunits. Assuming that all subunits are equally expressed and assemble randomly into tetrameric channels, one would expect that the TRPV4 channels in the cell of a patient would consist of 6.25% pure wild-type channels, 6.25% pure mutant channels and that most functional channels (87.5%) would be hetero-multimeric channels consisting of a mixture of subunits. At this point, the properties of such hetero-multimeric channels have not been studied. However, it is possible that they might have properties that differ strongly from either pure wild-type or pure mutant homotetramers, which might be important to our understanding of altered channel function in patients. Functional evaluation of hetero-multimers with controlled stoichiometry of wild-type and mutant subunits is possible, for example, by construction and expression of tandem constructs [77]. It should be noted that a large subset of disease-causing mutations are located in the ARD of TRPV4, a region that itself is involved in subunit oligomerization [78].
Biochemical and structural approaches. Although, as discussed above, a crystal structure of an entire TRP channel is still lacking, work has resulted in the purification and crystallization of the ARD of several TRPV channels, including TRPV4. This revealed that TRPV4 activity is regulated by binding of ATP and calmodulin to the ARD, and that ATP increases the stability of the ARD. It further suggested that finger 3 of the ARD might act as a switch to regulate channel activity [21]. This study also revealed that several disease-causing mutations in the ARD affect thermal stability and ATP binding, which might contribute to altered channel function in disease. Further progress in TRP channel crystallography, finally leading to the determination of the atomic structure of the full TRPV4 channel or a closely related homologue, will provide a much better understanding of the structural and functional consequences of the various disease-causing mutants.
Animal models. At this point, there is a lack of animal models for TRPV4-dependent human diseases. As described above, general deletion of TRPV4 expression in Trpv4 knockout mice leads to a variety of mild symptoms, including a slight thickening of the bones, which has been attributed to a defect in osteoclast differentiation [21]. However, the phenotypes of the Trpv4 knockout mouse are not informative for our understanding of the human diseases, which are due to alteration rather than elimination of TRPV4 function. In a study, the gain-of-function mutants R616Q and V620I were introduced into the osteoclast lineage of Trpv4 knockout mice. This resulted in decreased femoral bone mass and bone mineral density, but further skeletal malformations were not reported [79]. However, this animal model certainly does not reflect the TRPV4 heterozygosity found in patients, and should therefore, as with the heterologous expression experiments, be interpreted with caution. Intriguingly, general overexpression of wild-type mouse TRPV4 in zebrafish embryos induced abnormalities, including dysplasia-like phenotypes such as a curved and shortened body axis, but also malformations of the eye and brain [24]. It can be expected that further improvement of animal models, in particular the development of knockin animals for different disease-causing mutants, will provide better models for studying the aetiology of TRPV4-mediated diseases.
Potential mechanisms of disease
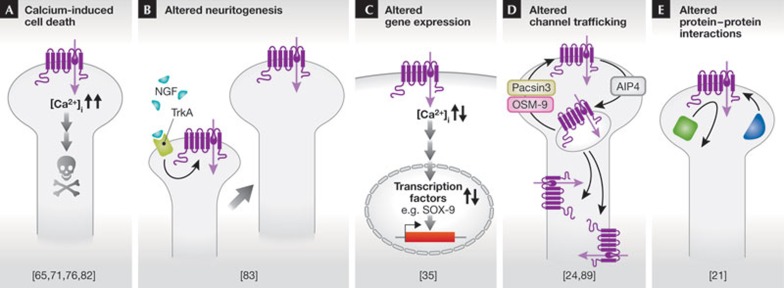
At this point, we can only speculate about the (patho)physiological mechanisms that link mutations in TRPV4 to a puzzling variety of skeletal, motor and sensory symptoms in patients. Further puzzles concern the differences in the onset of the disease. Some affected individuals are born with defects. Some develop the disease during their lifetime—mostly in mid-adulthood. Finally, some carriers of mutations that cause severe disease in other patients (R269C, R315W and R323C) do not have any disease symptoms, implying a ‘non-penetration’ of the disease. It is intriguing to unravel these puzzles, which is a prerequisite for the rational development of treatments. In the following text, we discuss potential and mutually non-exclusive mechanisms, whereby altered channel function might lead to disease (Fig 3).
Figure 3.
Five potential mechanisms coupling mutations in TRPV4 to disease pathology. See text for more details. AIP4, E3 ubiquitin ligase; NGF, nerve growth factor; TrkA, tropomyosin receptor kinase A.
In addition, it should be noted that we cannot fully exclude the possibility that TRPV4 might have functions other than that of an ion channel. Indeed, there are several examples in the literature of TRP channels that have additional capabilities, such as the enzymatic activity in the C-terminal cytosolic domains of TRPM2, TRPM6 and TRPM7 [18] or TRPM8 acting as a G-protein-coupled receptor [80]. As with TRPM7, other TRP channels might be regulated by caspase-mediated cleavage inducing wide-ranging effects on cell death and differentiation [81].
TRPV4-induced Ca2+ overload and toxicity. Overexpression studies of Trpv4 mutations involved in skeletal dysplasia and neuropathy indicated increased basal intracellular Ca2+ levels, presumably due to gain-of-function. As intracellular Ca2+ signals are crucial for processes such as cell survival and cell death, cells expressing such mutations might have difficulties in maintaining a non-toxic Ca2+ concentration. Indeed, increased basal Ca2+ influx in cell types expressing gain-of-function Trpv4 mutants was shown to cause cell toxicity and increased cell death, which has been proposed as a mechanism for motor neuron degeneration in TRPV4-dependent neuropathies [76,82]. As selective TRPV4 antagonists have been developed with no obvious signs of toxicity in animal models [17], pharmacological blockade of TRPV4 has been suggested as a potential therapeutic strategy [74]. However, many skeletal dysplasia mutations also have a gain-of-function phenotype, without causing detectable motor neuron degeneration. Moreover, other brachyolmia mutations, in addition to the three mutants identified in FDAB, are apparently loss-of-function mutations [53]. Thus, whilst many disease-causing TRPV4 mutants seem to be associated with increased Ca2+ influx, it is unlikely that Ca2+-induced cell toxicity is a general pathophysiological mechanism.
Altered TRPV4-dependent neuritogenesis. Evidence was presented that TRPV4 might be important in the outgrowth of neurites (neuritogenesis) of peripheral neurons in response to human nerve growth factor. Neurotrophic factor-derived neuritogenesis was found to be regulated by the phospholipase A2-mediated TRPV4 pathway, and it was suggested that altered neuritogenesis, rather than neuronal death, might underlie the pathology of sensory and motor nerves in TRPV4-dependent neuropathies [83].
Altered TRPV4-dependent gene expression. Altered gene expression downstream from TRPV4 function represents another potential pathogenic mechanism. For example, SOX9 is a transcription factor that is essential for chondrocyte differentiation and chondrocyte-specific gene expression. It induces chondrogenesis in mesenchymal cells. Chondrocyte differentiation and proliferation in the bone growth plate results in the formation of four layers, representing successive stages of differentiation—resting, proliferating, prehypertrophic, hypertrophic and terminal hypertrophic chondrocytes. Terminal hypertrophic chondrocytes undergo apoptosis and are replaced by bone from the joint action of osteoblasts and multinuclear osteoclasts. SOX9 is an important regulator in this process [84]. In a search for genes that activate SOX9-dependent transcription, TRPV4 was identified as an enhancer of SOX9-dependent reporter activity, probably through a Ca2+/calmodulin-dependent pathway. Moreover, the expression pattern of TRPV4 was similar to the expression patterns of chondrogenic marker genes such as type II collagen and aggrecan. Activation of TRPV4 also promotes synthesis of sulphated glycosaminoglycan [35]. Therefore, dysregulation of SOX9 activity downstream from mutated TRPV4 channel activity might lead to defects of the SOX9-dependent initiation of bone formation in the growth plate, which might represent a potential mechanism for TRPV4-dependent skeletal dysplasias [85].
Dysregulated cellular trafficking of TRPV4. A possible but not yet investigated mechanism, whereby the disease-causing mutations might affect TRPV4 function, is altering cellular trafficking of the channel. Such alterations can affect the abundance of TRPV4 at the plasma membrane and influence the spatial distribution of the channel—cell body against axon in peripheral neurons and apical against basolateral membrane in polarized osteoclasts. They also cause accumulation of TRPV4 in intracellular organelles, all of which have important effects on cellular responses to TRPV4 gating.
In this respect, it is intriguing to note that many disease-causing mutations affect arginine residues, such as Arg 269 (in neuropathies) and Arg 271 (in FDAB). It can be speculated that these residues belong to specific motifs that regulate channel trafficking, similar to the bi-arginine (RXR) retention and retrieval motif involved in trafficking and multimerization of ATP-sensitive K+ channels and cystic fibrosis transmembrane conductance regulator [86,87]. Surface expression of TRPV4 is further enhanced by pacsin 3, a cytosolic protein that binds to the N-terminal PRD [22]. OS9, an endoplasmic-reticulum-associated protein, interacts with the cytosolic N-terminal tail of TRPV4 close to the juxtamembrane region between ARD and S1, thereby impeding the release of TRPV4 from the endoplasmic reticulum and reducing TRPV4 level at the plasma membrane [24,88]. Finally, plasma membrane levels of TRPV4 are also controlled by ubiquitination. TRPV4 ubiquitination is strongly increased by the HECT family ubiquitin ligase AIP4. AIP4 promotes the endocytosis of TRPV4 and decreases its level at the membrane, thereby reducing basal channel activity [89]. Further research must clarify whether some of the disease-causing mutants influence these trafficking processes.
Dysregulated protein–protein interactions. There is no straightforward explanation for the fact that TRPV4 mutations with apparently comparable gain-of-function can cause either skeletal malformations with no neurological symptoms, or neuropathies without skeletal malformations. The specificity of the distinct disease patterns suggests that the underlying mutations affect TRPV4 function in a cell-specific manner, possibly by interacting with cell-type-specific regulatory proteins or protein complexes. For example, it has been shown that the membrane protein MLC1, which is mutated in megalencephalic leukoencephalopathy with subcortical cysts 1 (OMIM 604004), forms a complex in astrocytes with a Na–K-ATPase, the potassium channel Kir4.1, syntrophin, aquaporin 4 and TRPV4 [90]. It is tempting to speculate that TRPV4 mutations affect formation of this or similar supramolecular complexes, which might constitute a crucial step in the pathophysiology of the diseases. On the basis of structural data, it has been suggested that mutations in the convex surface of the ARD are associated with neuropathies, whereas those in the concave surface cause bone dysplasia. This might suggest that the opposite surfaces of the ARD have distinct—and possibly cell-type-specific—interacting partners [21].
Conclusions
The spectrum of TRPV4-mediated channelopathies is characterized by many mutants leading to diverse phenotypes and different diseases. Importantly, TRPV4 is also involved in a plethora of acquired diseases. It should be mentioned, although not discussed in this review, that a human TRPV4 variant (P19S) probably predisposes the carriers to acquired chronic obstructive pulmonary disease and sensitizes them to air pollution—for example, diesel exhaust particles. This TRPV4 variant reduces airway clearance due to decreased cilial activity, which is thought to be a TRPV4-dependent mechanism [91]. It also causes hyponatraemia, affecting the systemic water balance [92].
Finally, we can only tentatively refer to possible mechanisms by which TRPV4 might induce this large variety of pathological phenotypes. Such mechanisms include defects in Ca2+ homeostasis in chondrocytes, in motor and sensory neurons, altered neuritogenesis, dysfunctional gene expression during chondrocyte differentiation, defective channel trafficking and dysregulation of protein–protein interactions. It remains a challenge for the future to unravel the aetiology of the TRPV4 diseases, which requires a much better understanding of the role of TRPV4 in complex cell functions. TRPV4 provides an intriguing paradigm to study complex channel function from the molecular level to the level of human diseases and back.
Acknowledgments
We thank the members of the Laboratory of Ion Channel Research for helpful discussions. Our work was supported by grants from the Belgian Federal Government (IUAP P6/28 and P7/13), the Research Foundation-Flanders (G.0565.07 and G.0A61.13) and the Research Council of the KU Leuven (GOA 2009/07, EF/95/010 and PF-TRPLe).
Footnotes
The authors declare that they have no conflict of interest.
References
- Everaerts W, Nilius B, Owsianik G (2010) The vallinoid transient receptor potential channel Trpv4: from structure to disease. Prog Biophys Mol Biol 103: 2–17 [DOI] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD (2000) OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2: 695–702 [DOI] [PubMed] [Google Scholar]
- Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B (2004) Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA 101: 396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J et al. (2005) Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res 97: 908–915 [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B (2002) Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277: 47044–47051 [DOI] [PubMed] [Google Scholar]
- Watanabe H et al. (2002) Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277: 13569–13577 [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Janssens A, Wondergem R, Droogmans G, Nilius B (2003) Modulation of TRPV4 gating by intra- and extracellular Ca2+. Cell Calcium 33: 489–495 [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B (2003) Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438 [DOI] [PubMed] [Google Scholar]
- Thorneloe KS et al. (2008) N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent TRPV4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther 326: 432–442 [DOI] [PubMed] [Google Scholar]
- Klausen TK et al. (2009) Modulation of the transient receptor potential vanilloid channel TRPV4 by 4alpha-phorbol esters: a structure-activity study. J Med Chem 52: 2933–2939 [DOI] [PubMed] [Google Scholar]
- Vincent F et al. (2009) Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun 389: 490–494 [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Janssens A, Voets T, Nilius B (2007) Determinants of 4alpha-phorbol sensitivity in transmembrane domains 3 and 4 of the cation channel TRPV4. J Biol Chem 282: 12796–12803 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M (2003) Impaired pressure sensation with mice lacking TRPV4. J Biol Chem 278: 22664–22668 [DOI] [PubMed] [Google Scholar]
- Ma X, He D, Ru X, Chen Y, Cai Y, Bruce IC, Xia Q, Yao X, Jin J (2011) Apigenin, a plant-derived flavone, activates transient receptor potential TRPV4 cation channel. Br J Pharmacol 166: 349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, Bödding M, Droogmans G, Nilius B (2002) Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem 277: 33704–33710 [DOI] [PubMed] [Google Scholar]
- Everaerts W et al. (2010) Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci USA 107: 19084–19089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G (2011) The transient receptor potential family of ion channels. Genome Biol 12: 218–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsianik G, D'hoedt D, Voets T, Nilius B (2006) Structure–function relationship of the TRP channel superfamily. Rev Physiol Biochem Pharmacol 156: 61–90 [PubMed] [Google Scholar]
- Phelps CB, Huang RJ, Lishko PV, Wang RR, Gaudet R (2008) Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels. Biochemistry 47: 2476–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada H, Procko E, Sotomayor M, Gaudet R (2012) Structural and biochemical consequences of disease-causing mutations in the ankyrin repeat domain of the human TRPV4 channel. Biochemistry 51: 6195–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuajungco MP, Grimm C, Oshima K, D'Hoedt D, Nilius B, Mensenkamp AR, Bindels RJ, Plomann M, Heller S (2006) PACSINs bind to the TRPV4 cation channel: PACSIN 3 modulates the subcellular localization of TRPV4. J Biol Chem 281: 18753–18762 [DOI] [PubMed] [Google Scholar]
- D'hoedt D, Owsianik G, Prenen P, Cuajungco MP, Grimm G, Heller S, Voets T, Nilius B (2007) Stimulus-specific modulation of the cation channel TRPV4 by PACSIN 3. J Biol Chem 283: 6272–6280 [DOI] [PubMed] [Google Scholar]
- Wang Y, Fu X, Gaiser S, Kottgen M, Kramer-Zucker A, Walz G, Wegierski T (2007) OS-9 regulates the transit and polyubiquitination of TRPV4 in the endoplasmic reticulum. J Biol Chem 282: 36561–36570 [DOI] [PubMed] [Google Scholar]
- Strotmann R, Schultz G, Plant TD (2003) Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem 278: 26541–26549 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hirao A, Mizuno A (2003) Microtubule-associated protein 7 increases the membrane expression of transient receptor potential vanilloid 4 (TRPV4). J Biol Chem 278: 51448–51453 [DOI] [PubMed] [Google Scholar]
- Goswami C, Kuhn J, Heppenstall PA, Hucho T (2010) Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PLoS ONE 5: e11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Elias A, Lorenzo IM, Vicente R, Valverde MA (2008) IP3 receptor binds to and sensitizes TRPV4 channel to osmotic stimuli via a calmodulin-binding site. J Biol Chem 283: 31284–31288 [DOI] [PubMed] [Google Scholar]
- Eid SR (2011) Therapeutic targeting of TRP channels—the TR(i)P to pain relief. Curr Top Med Chem 11: 2118–2130 [DOI] [PubMed] [Google Scholar]
- Shen J, Harada N, Kubo N, Liu B, Mizuno A, Suzuki M, Yamashita T (2006) Functional expression of transient receptor potential vanilloid 4 in the mouse cochlea. Neuroreport 17: 135–139 [DOI] [PubMed] [Google Scholar]
- Adapala RK, Thoppil R, Luther DJ, Paruchuri S, Meszaros JG, Chilian WM, Thodeti CK (2013) TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J Mol Cell Cardiol 54: 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Horn NA, Huynh T, Kelava L, Lansman JB (2012) Evidence TRPV4 contributes to mechanosensitive ion channels in mouse skeletal muscle fibers. Channels (Austin) 6: 246–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida N et al. (2012) Importance of transient receptor potential vanilloid 4 (TRPV4) in epidermal barrier function in human skin keratinocytes. Pflugers Arch 463: 715–725 [DOI] [PubMed] [Google Scholar]
- Ye L et al. (2012) TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell 151: 96–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S et al. (2007) Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J Biol Chem 282: 32158–32167 [DOI] [PubMed] [Google Scholar]
- Lewis R, Asplin KE, Bruce G, Dart C, Mobasheri A, Barrett-Jolley R (2011) The role of the membrane potential in chondrocyte volume regulation. J Cell Physiol 226: 2979–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevaert T et al. (2007) Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117: 3453–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama R et al. (2008) TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab 8: 257–265 [DOI] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM (2003) Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci USA 100: 13698–13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W (2008) Molecular mechanisms of TRPV4-mediated neural signaling. Ann NY Acad Sci 1144: 42–52 [DOI] [PubMed] [Google Scholar]
- Earley S, Heppner TJ, Nelson MT, Brayden JE (2005) TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279 [DOI] [PubMed] [Google Scholar]
- Marrelli SP, O'Neil R, G, Brown RC, Bryan RM (2006) PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol Heart Circ Physiol 292: H1390–H1397 [DOI] [PubMed] [Google Scholar]
- Saliez J et al. (2008) Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117: 1065–1074 [DOI] [PubMed] [Google Scholar]
- Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I (2008) Role of cytochrome P450-dependent TRPV4 activation in flow-induced vasodilatation. Cardiovasc Res 80: 445–452 [DOI] [PubMed] [Google Scholar]
- Sonkusare SK, Bonev AD, Ledoux L, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT (2012) Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Suzuki M, Mizuno A, Hara A (2005) Hearing impairment in TRPV4 knockout mice. Neurosci Lett 382: 304–308 [DOI] [PubMed] [Google Scholar]
- Cortright DN, Szallasi A (2009) TRP channels and pain. Curr Pharm Des 15: 1736–1749 [DOI] [PubMed] [Google Scholar]
- Thorneloe KS et al. (2012) An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med 4: 159ra148. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G (2010) Channelopathies converge on TRPV4. Nat Genet 42: 98–100 [DOI] [PubMed] [Google Scholar]
- Morquio L (1929) Sur une forme de dystrophie osseuse familiale. Archives de Médecine des Infants 32: 129–135 [Google Scholar]
- Nishimura G et al. (2010) Spondylo-epiphyseal dysplasia, Maroteaux type (pseudo-Morquio syndrome type 2), and parastremmatic dysplasia are caused by TRPV4 mutations. Am J Med Genet A 152A: 1443–1449 [DOI] [PubMed] [Google Scholar]
- Rock MJ et al. (2008) Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nat Genet 40: 999–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow D et al. (2009) Mutations in the gene encoding the calcium-permeable ion channel TRPV4 produce spondylometaphyseal dysplasia, Kozlowski type and metatropic dysplasia. Am J Hum Genet 84: 307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger S et al. (2011) Fetal akinesia in metatropic dysplasia: The combined phenotype of chondrodysplasia and neuropathy? Am J Med Genet A 155A: 2860–2864 [DOI] [PubMed] [Google Scholar]
- Camacho N, Krakow D, Johnykutty S, Katzman PJ, Pepkowitz S, Vriens J, Nilius B, Boyce BF, Cohn DH (2010) Dominant TRPV4 mutations in nonlethal and lethal metatropic dysplasia. Am J Med Genet A 152A: 1169–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura G, Lausch E, Savarirayan R, Shiba M, Spranger J, Zabel B, Ikegawa S, Superti-Furga A, Unger S (2012) TRPV4-associated skeletal dysplasias. Am J Med Genet C Semin Med Genet 160C: 190–204 [DOI] [PubMed] [Google Scholar]
- McEntagart M (2012) TRPV4 axonal neuropathy spectrum disorder. J Clin Neurosci 19: 927–933 [DOI] [PubMed] [Google Scholar]
- Dubowitz V (1995) Disorders of the Lower Motor Neurone: The Spinal Muscular Atrophies. London, UK: Saunders [Google Scholar]
- Main M, Kairon H, Mercuri E, Muntoni F (2003) The Hammersmith functional motor scale for children with spinal muscular atrophy: a scale to test ability and monitor progress in children with limited ambulation. Eur J Paediatr Neurol 7: 155–159 [DOI] [PubMed] [Google Scholar]
- Rossor AM, Kalmar B, Greensmith L, Reilly MM (2011) The distal hereditary motor neuropathies. J Neurol Neurosurg Psychiatry 83: 6–14 [DOI] [PubMed] [Google Scholar]
- Drew AP, Blair IP, Nicholson GA (2011) Molecular genetics and mechanisms of disease in distal hereditary motor neuropathies: insights directing future genetic studies. Curr Mol Med 11: 650–665 [DOI] [PubMed] [Google Scholar]
- Vlam L, Schelhaas HJ, van Blitterswijk M, van Vught PW, de Visser M, van der Kooi AJ, van der Pol WL, van den Berg LH (2012) Mutations in the TRPV4 gene are not associated with sporadic progressive muscular atrophy. Arch Neurol 69: 790–791 [DOI] [PubMed] [Google Scholar]
- Fiorillo C et al. (2012) TRPV4 mutations in children with congenital distal spinal muscular atrophy. Neurogenetics 13: 195–203 [DOI] [PubMed] [Google Scholar]
- DeLong R, Siddique T (1992) A large New England kindred with autosomal dominant neurogenic scapuloperoneal amyotrophy with unique features. Arch Neurol 49: 905–908 [DOI] [PubMed] [Google Scholar]
- Deng HX et al. (2010) Scapuloperoneal spinal muscular atrophy and hereditary motor and sensory neuropathy type IIC are allelic disorders caused by mutations in TRPV4. Nat Genet 42: 165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind CE, Shy ME (2011) Genetics of neuropathies. Semin Neurol 31: 494–505 [DOI] [PubMed] [Google Scholar]
- Berciano J (2011) Peripheral neuropathies: molecular diagnosis of Charcot-Marie-Tooth disease. Nat Rev Neurol 7: 305–306 [DOI] [PubMed] [Google Scholar]
- Chen DH, Sul Y, Weiss M, Hillel A, Lipe H, Wolff J, Matsushita M, Raskind W, Bird T (2010) CMT2C with vocal cord paresis associated with short stature and mutations in the TRPV4 gene. Neurology 75: 1968–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimon M et al. (2010) Dominant mutations in the cation channel gene transient receptor potential vanilloid 4 cause an unusual spectrum of neuropathies. Brain 33: 1798–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer-Grumbach M et al. (2010) Alterations in the N-terminal ankyrin domain of TRPV4 cause congenital and scapuloperoneal spinal muscular atrophy, and hereditary motor and sensory neuropathy 2C. Nat Genet 42: 160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landouré G et al. (2010) Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat Genet 42: 170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett KA et al. (2012) Comprehensive analysis of the TRPV4 gene in a large series of inherited neuropathies and controls. J Neurol Neurosurg Psychiatry 83: 1204–1209 [DOI] [PubMed] [Google Scholar]
- Lamande SR et al. (2011) Mutations in TRPV4 cause an inherited arthropathy of hands and feet. Nat Genet 43: 1142–1146 [DOI] [PubMed] [Google Scholar]
- Baets J, De Jonghe P (2011) TRPV4 neuropathies: Calcium channel inhibition as a therapeutic target? Neurology 76: 856–857 [DOI] [PubMed] [Google Scholar]
- Loukin S, Zhou X, Su Z, Saimi Y, Kung C (2010) Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force. J Biol Chem 285: 27176–27181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CJ et al. (2011) TRPV4 mutations and cytotoxic hypercalcemia in axonal Charcot-Marie-Tooth neuropathies. Neurology 76: 887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens A, Voets T (2011) Ligand stoichiometry of the cold- and menthol-activated channel TRPM8. J Physiol 589: 4827–4835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arniges M, Fernandez-Fernandez JM, Albrecht N, Schaefer M, Valverde MA (2006) Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. J Biol Chem 281: 1580–1586 [DOI] [PubMed] [Google Scholar]
- Masuyama R, Mizuno A, Komori H, Kajiya H, Uekawa A, Kitaura H, Okabe K, Ohyama K, Komori T (2012) Calcium/calmodulin-signaling supports TRPV4 activation in osteoclasts and regulates bone mass. J Bone Miner Res 27: 1708–1721 [DOI] [PubMed] [Google Scholar]
- Klasen K, Hollatz D, Zielke S, Gisselmann G, Hatt H, Wetzel CH (2012) The TRPM8 ion channel comprises direct Gq protein-activating capacity. Pflugers Archiv 463: 779–797 [DOI] [PubMed] [Google Scholar]
- Desai BN et al. (2012) Cleavage of TRPM7 releases the kinase domain from the ion channel and regulates its participation in Fas-induced apoptosis. Dev Cell 22: 1149–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecto F, Shi Y, Huda R, Martina M, Siddique T, Deng HX (2011) Mutant TRPV4-mediated toxicity is linked to increased constitutive function in axonal neuropathies. J Biol Chem 286: 17281–17291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y et al. (2012) Axonal neuropathy-associated TRPV4 regulates neurotrophic factor-derived axonal growth. J Biol Chem 287: 6014–6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornak U, Mundlos S (2003) Genetic disorders of the skeleton: a developmental approach. Am J Hum Genet 73: 447–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Leddy HA, Liedtke W (2010) Transient receptor potential vanilloid 4. Ann NY Acad Sci 1192: 404–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY (1999) A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 22: 537–548 [DOI] [PubMed] [Google Scholar]
- Owsianik G, Cao L, Nilius B (2003) Rescue of functional DeltaF508-CFTR channels by co-expression with truncated CFTR constructs in COS-1 cells. FEBS Lett 554: 173–178 [DOI] [PubMed] [Google Scholar]
- Verma P, Kumar A, Goswami C (2010) TRPV4-mediated channelopathies. Channels (Austin) 4: 319–328 [DOI] [PubMed] [Google Scholar]
- Wegierski T, Hill K, Schaefer M, Walz G (2006) The HECT ubiquitin ligase AIP4 regulates the cell surface expression of select TRP channels. EMBO J 25: 5659–5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti A et al. (2012) Megalencephalic leukoencephalopathy with subcortical cysts protein 1 functionally cooperates with the TRPV4 cation channel to activate the response of astrocytes to osmotic stress: dysregulation by pathological mutations. Hum Mol Genet 21: 2166–2180 [DOI] [PubMed] [Google Scholar]
- Li J et al. (2011) TRPV4-mediated calcium influx into human bronchial epithelia upon exposure to diesel exhaust particles. Environ Health Perspect 119: 784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W et al. (2009) A loss-of-function nonsynonymous polymorphism in the osmoregulatory TRPV4 gene is associated with human hyponatremia. Proc Natl Acad Sci USA 106: 14034–14039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J et al. (2010) Novel and recurrent TRPV4 mutations and their association with distinct phenotypes within the TRPV4 dysplasia family. J Med Genet 47: 704–709 [DOI] [PubMed] [Google Scholar]
- Cho TJ et al. (2012) TRPV4-pathy manifesting both skeletal dysplasia and peripheral neuropathy: A report of three patients. Am J Med Genet A 158A: 795–802 [DOI] [PubMed] [Google Scholar]
- Astrea G, Brisca G, Fiorillo C, Valle M, Tosetti M, Bruno C, Santorelli FM, Battini R (2012) Muscle MRI in TRPV4-related congenital distal SMA. Neurology 78: 364–365 [DOI] [PubMed] [Google Scholar]
- Landoure G et al. (2012) Exome sequencing identifies a novel TRPV4 mutation in a CMT2C family. Neurology 79: 192–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni S, Harlalka G, Offiah A, Shuper A, Crosby AH, McEntagart M (2011) Striking phenotypic variability in familial TRPV4-axonal neuropathy spectrum disorder. Am J Med Genet A 155A: 3153–3156 [DOI] [PubMed] [Google Scholar]
- Andreucci E et al. (2011) TRPV4 related skeletal dysplasias: a phenotypic spectrum highlighted byclinical, radiographic, and molecular studies in 21 new families. Orphanet J Rare Dis 6: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Shin SH, Auh CK, Chun JS (2012) Human skeletal dysplasia caused by a constitutive activated transient receptor potential vanilloid 4 (TRPV4) cation channel mutation. Exp Mol Med 44: 707–722 [DOI] [PMC free article] [PubMed] [Google Scholar]



 Thomas Voets & Bernd Nilius
Thomas Voets & Bernd Nilius