EMBO reports (2013) 14 2, 172–177 doi:; DOI: 10.1038/embor.2012.217
Since the discovery of apoptosis, caspase activation has generally been used as a marker of apoptotic cells (reviewed in [1]). Caspases are cysteine proteases that exist in an inactive form and are activated by cleavage through intrinsic or extrinsic apoptotic signalling mechanisms. The core apoptotic machinery is conserved in evolution between the worm Caenorhabditis elegans, the fruit fly Drosophila melanogaster and mammals [1]. Indeed, a mammalian antibody against the active (cleaved) form of mammalian caspase 3 detects the active Drosophila effector caspase orthologues Dcp-1 and Drice, and is used as a read-out of the activity of the upstream initiator caspase Dronc (caspase 9 orthologue; [2]). Many studies equate cleaved-caspase 3 antibody staining with apoptotic cells, however it has emerged that caspase activation has non-apoptotic roles in both Drosophila and mammalian systems, including roles in differentiation and compensatory cell proliferation (reviewed in [3]). In this issue of EMBO reports, Cagan and colleagues [4] reveal a non-apoptotic role for caspase activation in cell migration in Drosophila.
Caspase-mediated cell migration
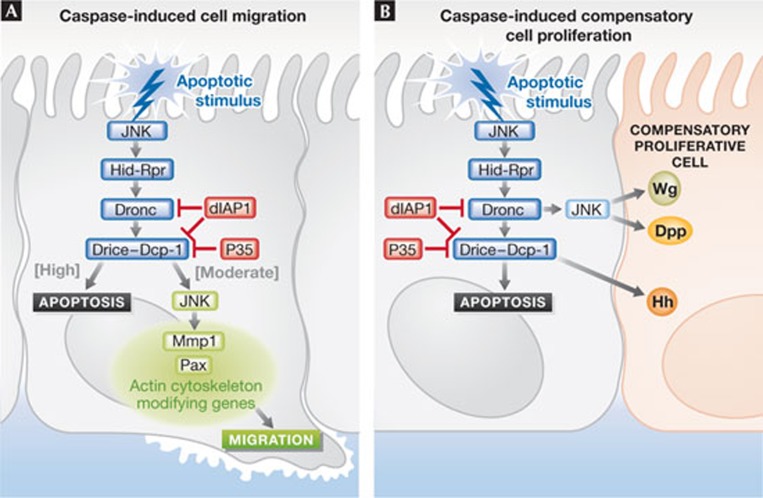
Cagan and colleagues [4] activated the apoptotic pathway in the anterior–posterior boundary in the developing Drosophila wing epithelium by expressing the apoptotic inducer Hid—the functional orthologue of mammalian SMAC/Diablo—whilst preventing cell death by inhibiting effector caspases through expression of the baculovirus caspase inhibitor P35. This resulted in the migration and invasion of the so-called ‘undead’ [3] GFP-marked Hid, P35-expressing cells away from the anterior–posterior boundary, along the basal plane of the wing epithelium (Fig 1A). The undead cells activated Dronc, as revealed by active-caspase 3 antibody staining, but did not show later signs of apoptosis, such as DNA fragmentation. The undead cells also activated the stress response Jun kinase (JNK) pathway, and expression of the JNK target Mmp1, which is involved in the degradation of the extracellular matrix and cell invasion. JNK and Mmp1 activity were required for the invasion, as blocking their activity prevented invasion. By reducing the expression of Dronc or Drice using RNA interference (RNAi), or with a Drice loss-of-function mutant, the authors showed that the activity of both these caspases was required for invasion of the undead cells. Indeed, co-expression of a constitutively active version of Dronc (ΔN-Dronc) with P35 was sufficient to induce an invasive phenotype. Moreover, reducing Dronc or Drice by using RNAi conferred an invasive phenotype to Hid-expressing cells, which would normally undergo apoptosis. However, reducing Drice was not as effective as Dronc knockdown, suggesting that other Drosophila effector caspases such as Dcp-1, Decay or Damm might also be involved. From these results the authors concluded that precise levels of caspase activity are required for cell invasion; high levels (hid expression alone) lead to apoptosis, whereas moderate levels (hid p35; hid droncRNAi; hid driceRNAi or hid ΔN-Dronc) induce invasion. If caspase activity is reduced further (hid diap1) invasion is prevented. Caspase activation-induced migration most likely occurs through activation of JNK. Caspases can function both upstream and downstream from JNK in a positive feedback loop. Moreover, the JNK pathway leads to the transcriptional upregulation of the apoptosis initiator genes hid and rpr, as well as genes required for cell migration and invasion, such as paxillin, mmp1 and actin cytoskeletal modifying genes (reviewed in [3,5]). Thus, at moderate levels of caspase activation, JNK levels might be optimal for inducing expression of cell migration and invasion genes, but not of hid and rpr— thereby preventing sufficient caspase activation to trigger apoptosis.
Figure 1.
Caspase-driven cell migration and compensatory proliferation. Comparison between (A) caspase-driven cell migration and (B) caspase-induced compensatory proliferation in Drosophila. Dpp, Decapentaplegic; Hh, Hedgehog; JNK, Jun kinase; Mmp1, matrix metalloproteinase 1; Pax, paxillin; Wg, Wingless.
The findings presented by Cagan and colleagues raise several important issues relevant to understanding the cellular response to caspase activation and relate to previous studies of cell invasion in Drosophila and mammalian cells. Several examples of cell invasion have been described in Drosophila as being dependent on JNK activation [5]. For example, a study from the Cagan lab showed that activation of the tyrosine kinase Src results in a JNK- and Mmp-dependent invasive phenotype, which is also conserved in the boundary cells of human squamous cell carcinomas [5]. Furthermore, expression of JNK pathway activators with oncogenic Ras (RasV12) promotes invasive tumorigenesis in both Drosophila and mammalian cells [6]. Indeed, a report showed that expression of ErbB2 in sporadic mammalian cells within acini cultured in a three-dimensional matrix, resulted in cell extrusion into the lumen of the acini and overproliferation, dependent on Ras–MAPK signalling and Mmp activation [7]. The activation state of caspases or their involvement in cell invasion in these systems has not been investigated, but the significance of this for cancer biology research clearly warrants these studies.
Cell proliferation and invasion
In multicellular organisms, activation of apoptosis triggers compensatory proliferation in surrounding cells to maintain tissue homeostasis [3]. When tissues are subjected to cellular stress that induces high levels of apoptosis—such as irradiation, heat-shock and expression of Hid—surviving cells undergo additional rounds of proliferation to compensate for the lost cells. In the proliferating Drosophila eye or wing epithelial tissues, Dronc coordinates cell death and compensatory proliferation through JNK, by inducing expression of the mitogens Decapentaplegic (Dpp; TGF-β family orthologue) and Wingless (Wg; Wnt orthologue), whereas in differentiating eye tissue, the effector caspases Drice and Dcp-1 activate the Hedgehog signalling pathway to induce compensatory proliferation. In other contexts, the Unpaired–Jak–Stat signalling pathway [8] and the Hippo tissue growth control pathway are involved in compensatory proliferation [3].
When P35 is expressed with Hid or in conjunction with irradiation to create undead cells, the continued production of Dpp and Wg from the undead cells induces hyperproliferation of the surrounding tissue (Fig 1B; [3]). It is possible that the secretion of these ligands might also have a role in the invasive phenotype observed by Cagan and colleagues, as their orthologues in mammalian cells, Wnt and TGF-β, have well-established roles in invasion and metastasis (reviewed in [9]). Indeed, a previous report by Morata and colleagues [10] showed that the undead phenotype resulting from irradiation and P35 expression in the posterior wing compartment of the fly, which induced compensatory proliferation of posterior cells, was associated with the invasion of undead cells into the anterior compartment. Although in this case, the tissue damage due to irradiation might have contributed to the invasive phenotype, mutations in dpp or wg prevented both the posterior compartment overgrowth and cell invasion [10]. This finding also raises the question of whether compensatory cell proliferation, induced by Wg, Dpp or other signalling pathways, might also be occurring in the system used by Cagan and colleagues and whether this might contribute to the invasive phenotype. Morata and colleagues [10] also observed that JNK activation is sufficient to induce cell proliferation and invasion through induction of Dpp and Wg if the level of caspase activation was reduced by a dronc mutation. It is important to determine whether this also occurs in the system used by Cagan and colleagues. Future research into this area will undoubtedly provide valuable insight into these issues.
Perspectives
In summary, the results of Cagan and co-workers [4] highlight the emerging view within the field that, in contrast to the dogma, cleaved caspase 3 is not an accurate read-out of apoptosis, and that assays for more downstream events of programmed cell death are needed to confirm that cells are indeed undergoing apoptosis. This report also opens up new lines of investigation into the role of caspases in previously documented models of cell invasion in Drosophila, as well as in mammalian cells and human cancer. Comparisons between caspase-mediated compensatory proliferation and cell migration raise the question of whether similar mechanisms might be involved in both processes. Furthermore, the study raises an important issue with respect to standard cancer therapy, in which radiation or drug treatment is used to induce cancer cell death. If the activation of caspases is not sufficient to induce apoptosis it might instead confer invasive and metastatic properties to the cancer cells, thereby promoting tumour progression rather than abating it.
Footnotes
The authors declare that they have no conflict of interest.
References
- Steller H (2008) Cell Death Differ 15: 1132–1138 [DOI] [PubMed] [Google Scholar]
- Fan Y, Bergmann A (2010) Cell Death Differ 17: 534–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo HD, Bergmann A (2012) Cold Spring Harb Perspect Biol 4: a008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudrapatna VA, Bangi E, Cagan RL (2013) EMBO Rep [Epub ahead of print] doi:; DOI: 10.1038/embor.2012.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanatos RK, Vidal M (2011) J Genet Genomics 38: 431–438 [DOI] [PubMed] [Google Scholar]
- Brumby AM et al. (2011) Genetics 188: 105–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CT, Brugge JS (2012) Nature 482: 410–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Pastor-Pareja JC, Xu T (2010) Nature 463: 545–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot LJ, Bhattacharya SD, Kuo PC (2012) Int J Biochem Mol Biol 3: 117–136 [PMC free article] [PubMed] [Google Scholar]
- Perez-Garijo A, Shlevkov E, Morata G (2009) Development 136: 1169–1177 [DOI] [PubMed] [Google Scholar]