Abstract
Targeted gene delivery, transfection efficiency and toxicity concerns remain a challenge for effective gene therapy. In this study, we dimerized the HIV-1 TAT peptide and formulated a nanoparticle vector (dTAT NP) to leverage the efficiency of this cell penetrating strategy for tumor-targeted gene delivery in the setting of intratracheal administration. Expression efficiency for dTAT NP-encapsulated luciferase or angiotensin II type 2 receptor (AT2R) plasmid DNA (pDNA) was evaluated in Lewis Lung carcinoma (LLC) cells cultured in vitro or in vivo in orthotopic tumor grafts in syngeneic mice. In cell culture, dTAT NP was an effective pDNA transfection vector with negligible cytotoxicity. Transfection efficiency was further increased by addition of calcium and glucose to dTAT/pDNA NP. In orthotopic tumor grafts, immunohistochemical analysis confirmed that dTAT NP successfully delivered pDNA to the tumor, where it was expressed primarily in tumor cells along with the bronchial epithelium. Notably, gene expression in tumor tissues persisted at least 14 days after intratracheal administration. Moreover, bolus administration of dTAT NP-encapsulated AT2R or TRAIL pDNA markedly attenuated tumor growth. Taken together, our findings offer a preclinical proof of concept for a novel gene delivery system that offers an effective intratracheal strategy for administering lung cancer gene therapy.
Keywords: Angiotensin II type 2 receptor, cationic peptide nanoparticles, lung adenocarcinoma, targeted gene therapy, apoptosis, transfection
Introduction
Lung cancer is the leading cause of cancer-related morbidity and mortality in the United States, although its prognosis has improved due to advances in diagnostic and surgical techniques and early surveillance. The American Cancer Society estimates that 221,130 persons in the United States developed lung cancer in 2011, with 156,940 deaths (1). Lung cancer-dependent deaths constituted 15% (men) and 12% (women) of estimated total cancer-related deaths in 2011 (1). From 1995 to 2001, the relative 5-year survival ratio of patients with lung or bronchus cancer was still quite low (15%), with minimal improvement since the 1970's (12%). Therefore, novel treatment strategies for lung cancer are urgently needed.
In order to develop successful gene therapy systems, it is essential to improve gene transfection efficiency while minimizing toxicity and enhancing stability in vivo (2-7). Adenoviral vectors are effective, as they allow strong transgene expression in a variety of tissues, including tumor tissue (8, 9). However, their clinical efficacy is limited because they tend to be rapidly cleared from circulation (10, 11). Viral vectors are also potentially pathogenic; cases of viral infection have been reported (12). Therefore, nonviral vectors should be more promising as gene delivery vehicles: they are safe, easy to synthesize, cost-effective, and have a low degree of immunogenicity compared to viral vectors (13). Because naked plasmid DNA (pDNA) does not easily penetrate cellular membranes (14), nonviral gene delivery systems may include agents to improve intracellular delivery and promote transfection; pDNA complexed with cationic lipids (lipoplexes) and polymers (polyplexes) are the most commonly employed nonviral gene delivery vehicles (15-21).
The HIV-1 TAT peptide, which represents a protein transduction domain (22, 23) and a nuclear localization sequence (24), has been reported to show unusual translocation abilities by directly crossing biological membranes independent of receptors and temperature (25). The TAT peptide has been suggested to be an effective vector offering potential for translatable gene delivery; TAT-Ca/pDNA complexes were stable, maintaining particle size and transfection efficiency even in the presence of 10% of FBS (26) .
Angiotensin II (Ang II), an octapeptide hormone, is the key effector in the renin-angiotensin system. Ang II has two well-defined receptors: Ang II type 1 and type 2 receptors (AT2R) (27). AT2R, the second major receptor isoform, is primarily expressed in the mesenchyme of the fetus and to a limited extent in adult tissues (28). AT2R is known to inhibit cell proliferation and stimulate apoptosis in cardiovascular and neuronal tissues in vitro (29). Our previous studies revealed that AT2R deficiency significantly altered chemical carcinogen-induced tumorigenesis in mouse colon (30) and lung (31). A recent study indicates that host AT2R deficiency stimulates the growth of murine pancreatic carcinoma grafts (32). These results suggest that AT2R expression plays an important role in tumor growth.
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a naturally occurring cytokine that acts by binding as a homotrimer to Death Receptor (DR)-4 or -5 and recruiting an adaptor receptor, such as FADD or caspase-8. Since activated caspase-8, in turn, activates a caspase pathway that induces extrinsic apoptotic cell death (33), TRAIL is known to be a strong anti-cancer gene candidate (34). In fact, TRAIL gene therapy has been tested in multiple mouse cancer models with success (35, 36). Targeted gene delivery to cancer tissue should reduce side effects in healthy tissue and enable TRAIL as a candidate gene for gene therapy.
In the present study, we have developed a modified TAT peptide by connecting two TAT peptides in tandem (dTAT). Here we show that dTAT NP incorporating luciferase or AT2R pDNA (dTAT/pLUC or dTAT/pAT2R) administered via intratracheal spray can be detected primarily in the tumor tissues of the lung and in bronchial epithelium. Bolus administration of dTAT/pAT2R or of dTAT/pTRAIL, which was used as a positive control for the apoptosis inducer, significantly attenuated the growth of lung carcinoma grafts in syngeneic mice. Therefore, dTAT NP are a realistic gene delivery system, and the dTAT and an apoptosis inducer gene NP can be used as a powerful and less toxic therapeutic for lung cancer.
Materials and methods
Materials
Plasmid DNA (pDNA) encoding human AT2R (agtr2 pcDNA3.1+) was obtained from the UMR cDNA Resource Center (University of Missouri-Rolla). The pDNA encoding human TRAIL (trail pCMV-SPORTS 6) was purchased from Open Biosystems. Firefly luciferase pGL3 (pLUC) and pcDNA3.1+ were from Promega and Invitrogen, respectively. The dTAT peptide was purchased from Biomatik Corp.. Branched polyethylenimine (PEI, 25 kDa) was obtained from Aldrich . Lewis Lung Carcinoma (LLC, CRL-1642), A549 (CCL-185), and BEAS-2B (CRL-9609) cells were from the American Type Culture Collection. These cells were not specifically characterized for this study. Dulbecco's Modified Eagle's Medium (DMEM) and Ham's F-12 medium were from Fisher Scientic. Fetal bovine serum (FBS) was purchased from Equitech-bio, Inc.. Penicillin-streptomycin and trypsin-EDTA were from Invitrogen. MTS reagent [tetrazolium compound; 3-(45-dimethylthiazol-2-yl)-5- (3-carboxymethoxyphenyl) -2-(4-sulfophenyl)-2H-tetrazolium, inner salt] was from Promega. Other chemicals were analytical grade.
Cell culture
LLC,A549, and BEAS-2B cells were grown in DMEM, Ham's F-12 medium, and BEBM media (BEGM kit, Lonza), respectively, supplemented with 10% v/v FBS and 1% v/v penicillin/streptomycin at 37° C in a humidified air atmosphere containing 5% CO2.
Preparation of dTAT/pDNA NP
The dTAT/pDNA NP were prepared by mixing via pipette 10 μL pDNA (0.1 μg pDNA/μL) and 15 μL dTAT (1 μg dTAT/μL) solutions. The resultant dTAT/pDNA solution was stabilized by adding either 25 μL of 10% glucose, 0.2M NaCl, or 0.2M KCl solution for in vitro studies. For in vivo mouse studies, 25 μL of 10% glucose was added to the dTAT/pDNA solution. Finally, 15 μL 0.3M CaCl2 was added to the stabilized solution. The final solution was mixed vigorously by pipette. Before use, dTAT/pDNA NP were allowed to equilibrate for 20 min at 4° C.
Preparation of PEI/pDNA NP
PEI/pLUC NP were prepared by adding 10 μl pGL3 solution (0.1 μg/μL) to 15 μL PEI solution (N/P ratio 10) while pipetting, followed by 20 min incubation at 4° C. The N/P ratio refers to the molar ratio of amine groups in the cationic polymer, which represent the positive charges, to phosphate groups in the plasmid DNA, which represent the negative charges. NP were prepared immediately prior to each individual experiment.
In vitro cell transfection studies
LLC cells were trypsinized, counted, and diluted to a concentration of approximately 80,000 cells/mL. Then 0.1 mL of that dilution was added to each well of a 96-well plate, and the cells were incubated in a humidified atmosphere at 5% CO2 at 37°C for 24 h. Immediately before transfection, cells were washed once with PBS and 100 μl sample (20% of NP to 80% of serum free cell culture medium) was added to each well. Cells were incubated with the NP for 5 h. The transfection agent was then aspirated and 100 μL of fresh serum-containing medium was added, followed by further incubation. Luciferase expression was determined at 24 h after transfection by the Luciferase Assay System from Promega according to the manufacturer's recommended protocol. The light units were normalized against protein concentration in the cell extracts, which was measured using the BCA™ Protein Assay (Thermo Scientific). Transfection results were expressed as relative light units (RLU) per mg of cellular protein.
Assessment of cytotoxicity (MTS Assay)
Cytotoxicity of polymers was determined by the CellTiter 96® Aqueous Cell Proliferation Assay (Promega). LLC cells were grown as described in the transfection experiments. Cells were treated with the samples for ~24 h. The medium was then removed and replaced with a mixture of 100 μL fresh culture medium and 20 μL MTS reagent solution. The cells were incubated for 3 h at 37°C in the 5% CO2 incubator. The absorbance of each well was measured at 490 nm using a microtiter plate reader (SpectraMax, M25, Molecular Devices Corp.) to determine cell viability.
Cell viability analysis
The MTT assay was performed to examine the effect on LLC cell proliferation in vitro of dTAT/pAT2R or of high concentration glucose, NaCl, or KCl in the dTAT/pDNA solution. In brief, 700 LLC cells were seeded in 96 well plates 24 h prior to the addition of dTAT/pAT2R, glucose, or salt solution. The cancer cells were treated with dTAT/pAT2R alone, dTAT/pAT2R with additional glucose or salt solution, or glucose or salt solution alone in serum free-DMEM (0.25 or 0.5μg of dTAT/pDNA per well) at 37°C for 5 h, and then the medium was replaced with DMEM containing 10% FBS. After 48h incubation at 37°C, the MTT assay was carried out as previously described (37). The same procedure was used to examine the potential adverse effect of dTAT alone (1.87 or 3.74 μg dTAT/mL) on the viability of LLC, A549, and BEAS-2B cells. In this study, the MTT assay was carried out after 1, 3, or 5 days incubation in serum-containing DMEM or BEBM medium.
Gene expression analysis using real-time PCR
Transfection of pAT2R into LLC cells was confirmed by real-time PCR. 5000 LLC cells were seeded in 24 well plates 24 h prior to the addition of dTAT/pAT2R. The cancer cells were treated with dTAT/pAT2R in serum free-DMEM (1 or 2μg of dTAT/pDNA per well) at 37°C for 5 h and then were allowed to grow in DMEM containing 10% FBS at 37°C for 48 h. Then, gene expression was analyzed as previously described (37). AT2R primers were 5’ –ACTTCGGGCTTGTGAACATC-3’ (forward), and 5’ –TAAATCAGCCACAGCGAGGT– 3’ (reverse); 18S ribosome RNA primers were 5’ –TCGCTCCACCAACTAAGAAC– 3’ (forward) and 5’ –GAGGTTCGAAGACGATCAGA– 3’ (reverse).
In vivo studies
All animal experiments were done under strict adherence with Kansas State University Institutional Animal Care and Use Committee protocols. Wild-type female C57BL/6 mice obtained from the Jackson Laboratory were housed in a clean facility and held for 10 days to acclimatize. Experimental design I: LLC cells were seeded at 30000 cells per well in a 24-well plate and incubated 24 hrs in 10% FBS-containing medium. Medium was then changed to medium containing either the dTAT/pLUC (1ug of luciferase pDNA/well) or blank dTAT and incubated for 5 hrs. After changing the media back to NP-free, 10% FBS-containing media, cells were further incubated. Five days after treatment, 100, 1000, or 10000 cells were subcutaneously injected into the backs of the mice. At various time points up to 1 week, animals were imaged with a Caliper IVIS Lumina II biophotonic imager. Images were collected using a 6 min exposure time.
In Experimental design II and III, each mouse was injected via the tail vein with 2 × 106 LLC cells suspended in 200 μl of PBS. Experimental design II: Seven days after LLC cell injection, these mice were injected intratracheally using an intratracheal sprayer (Penn-Century Inc.) with 50μl of PBS (n=12) or 50μl of dTAT/pLUC (containing 0.7 μg pDNA, n=12). On days 3, 7, 10, and 14 after intratracheal administration of the dTAT/pLUC, mice were sacrificed, and lungs were dissected for histological analysis of tumor multiplicity and size. In addition, luciferase expression in the lung was analyzed immunohistochemically. Experimental design III: On day 7 after LLC injection, these mice were intratracheally treated using the sprayer with 50μl of PBS (n=6), dTAT alone (n=5), dTAT/pAT2R (n=5), or dTAT/pTRAIL (n=5). After sacrifice on day 15 after LLC inoculation, lungs were fixed in 10% buffered formalin and used for histological and immunohistochemical analysis.
Histological analysis
Fixed lung tissues were sectioned at 4 μm and stained with hematoxylin and eosin (H&E) for histological examination. Quantitative evaluation of tumor nodules in the lungs was performed as previously described (38).
Immunohistochemistry for luciferase, Ki-67, and AT2R in the tumor nodules
After deparaffinization and rehydration of the tissue sections, immunohistochemistry was carried out with antibodies to luciferase (1:1000, Novus Biologicals), Ki-67 (1:100, Abcam) and AT2R (1:100, Abcam), followed by incubation with biotin-conjugated antibodies against goat IgG or rabbit IgG (1:50, Vector Laboratories), reacted with the avidin-biotin peroxidase complex reagent (Vector Laboratories), visualized with 3, 3’-diaminobenzodine tetrahydrochloride (Sigma). To determine the Ki-67 labeling (proliferative) index, 10 nodules were selected randomly by light microscopy, and the number of Ki-67 positive cells in each area was counted. The index was assessed as the percentage of Ki-67-positive cells/tumor cells.
TUNEL assay
TUNEL assay was carried out using the DeadEndTM colorimetric TUNEL system (Promega) as previously described (38). The fold change was calculated by dividing the percentage of TUNEL-positive tumor cells in the treated tumors by those in untreated tumors.
Statistical analysis
All data are reported as mean ± SE. Statistical significance was assessed by one-way ANOVA. Group comparisons were deemed significant for 2-tailed P values below 0.05.
Results
dTAT/pLUC NP caused efficient gene transfection with low cytotoxicity in vitro
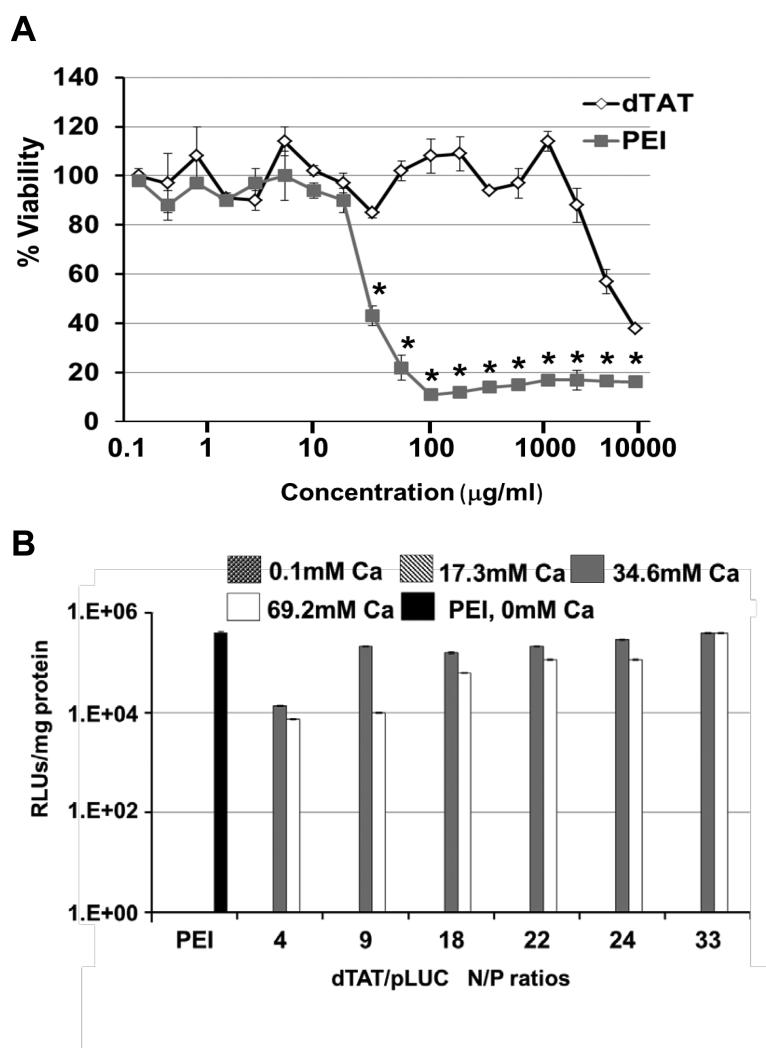
In studies reported here, dTAT and pLUC complexes were thoroughly mixed by pipetting, and CaCl2 was added to decrease the NP size through “soft” crosslinks of dTAT and pDNA (26). Here, the reduction in the size of dTAT/pLUC NP likely led to some of the noted increase in transfection. A CaCl2 concentration of 69.2 mM consistently produced small (75-110 nm) and stable dTAT NP with a single particle population (polydispersity < 0.21).
To investigate whether the dTAT affected the viability of LLC cells, the effect of free dTAT peptide or branched PEI (25 kDa) was analyzed by using a membrane translocalization signal (MTS) assay. LLC cells were incubated with up to 10 mg/mL of dTAT or PEI for ~24 hrs. Cytotoxicity profiles of dTAT peptides showed moderate cytotoxicity (IC50 ~4075 μg/mL, Fig. 1A), whereas branched PEI was strongly cytotoxic (IC50 ~28 μg/mL). Although the cell viability of LLC, A549, and BEAS-2B was slightly decreased by treatment with dTAT alone solution (1.86 or 3.72 μg/mL) at days 3 and 5, there was no statistically significant difference among the groups (Supplemental Fig. 1).
Figure 1.
A, Cytotoxicity profiles of PEI and dTAT. Cell viability is expressed as a function of polymer concentration. Results are presented as mean ± SD (n = 3).*, p < 0.05, as compared to the cytoxicity of the dTAT group at the same concentration. B, The transfection efficiency of PEI and dTAT NP with different concentrations of added CaCl2. Results are presented as mean ± SD (n = 3).
Luciferase gene expression was measured 48 h after transfection in order to study the ability of dTAT NP to transfect LLC cells (Fig. 1B). Different N/P ratios of the dTAT or branched PEI (N/P 10) NP were studied using different concentrations of CaCl2 (0, 17.3, 34.6, and 69.2 mM) as a condensing agent after NP formation. Most dTAT NP showed a high level of gene expression at and above 34.6 mM of added CaCl2 for the various N/P ratios when compared to branched PEI, which had excellent transfection efficiency only in the absence of CaCl2. The study revealed that the highest transfection efficiency by dTAT was achieved at 69.2 mM CaCl2 and an N/P ratio of 33. It is important to note that gene expression was not detectable for dTAT/pLUC at CaCl2 levels up to 17.3 mM.
dTAT-based AT2R gene transfection attenuated growth of lung cancer cells in vitro
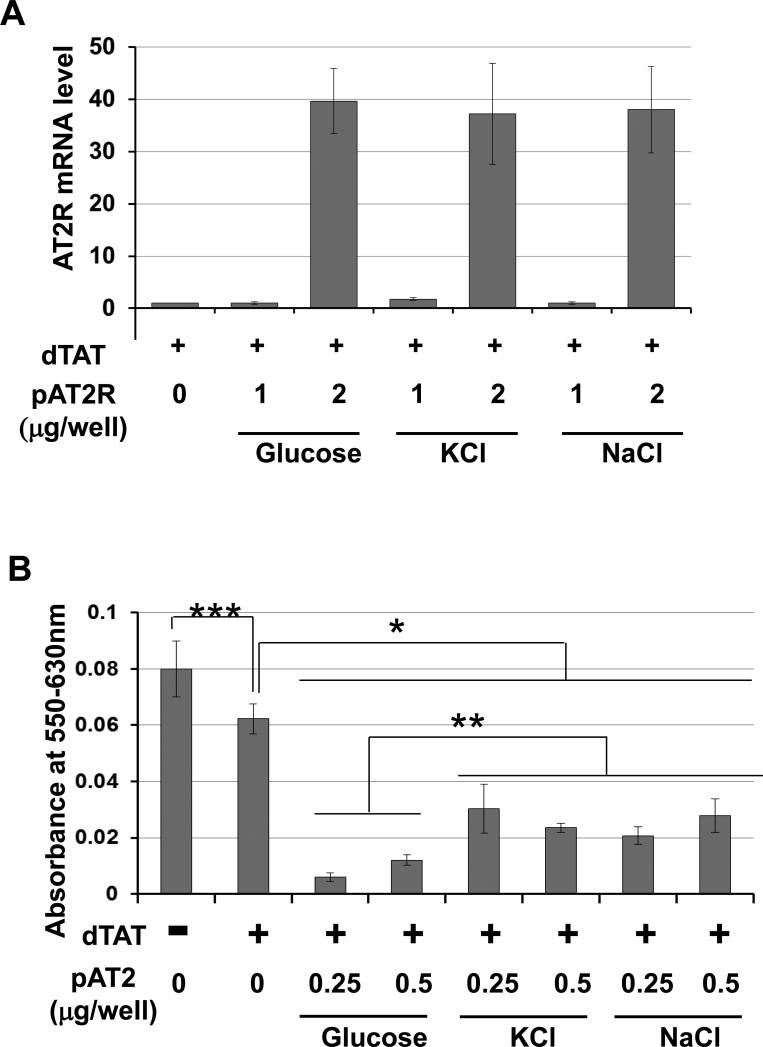
To examine agents that effectively stabilize dTAT/pDNA NP and cause effective transfection, dTAT/pAT2R solution was mixed with either glucose, KCl, or NaCl solution prior to condensing complexes with CaCl2, and the efficacy of AT2R expression and cell viability was evaluated. Real-time PCR revealed that all of the agents caused effective DNA transfection at 2 μg dTAT/pAT2R per well (Fig. 2A). As shown in Fig. 2B, dTAT/pAT2R transfection significantly attenuated viability of LLC cells compared with dTAT alone. Among the agents mixed with the dTAT/pAT2R solution, the glucose-stabilized dTAT/pAT2R decreased cell viability most effectively, while treatment using dTAT alone slightly inhibited tumor cell growth (Fig. 2B). Incubation of LLC cells with the solution containing glucose, NaCl, or KCl alone without dTAT or pDNA had no adverse effect on cell viability (Supplemental Fig. 2). Taken together, these results suggest that glucose was the most effective agent for dTAT/pDNA NP transfection, but by itself had no effect on tumor growth in vitro.
Figure 2.
Addition of glucose to the dTAT/pDNA NP caused cell growth attenuation most efficiently. (A) Real time PCR confirmed either glucose, KCl or NaCl is effective in dTAT/pAT2R transfection (1 or 2μg pAT2R per well of the 24 well plate. AT2R mRNA expression was determined two days after the treatment. Bar graphs indicate the average of two independent triplicate determinations. (B) The dTAT/pAT2R including glucose showed the strongest growth attenuation effect. In this study, each well contains 0.25 or 0.5 μg of pAT2R in the 96 well plate. Cell viability was determined two days after the treatment. Bar graphs indicate the average of two independent triplicate determinations. *, p < 0.05, as compared to the level of dTAT alone group. ** p < 0.05, as compared to the level of glucose group. *** p < 0.05, as compared to the level of PBS-control group.
In vivo imaging easily detects dTAT/pLUC NP transfected lung carcinoma cells in mice
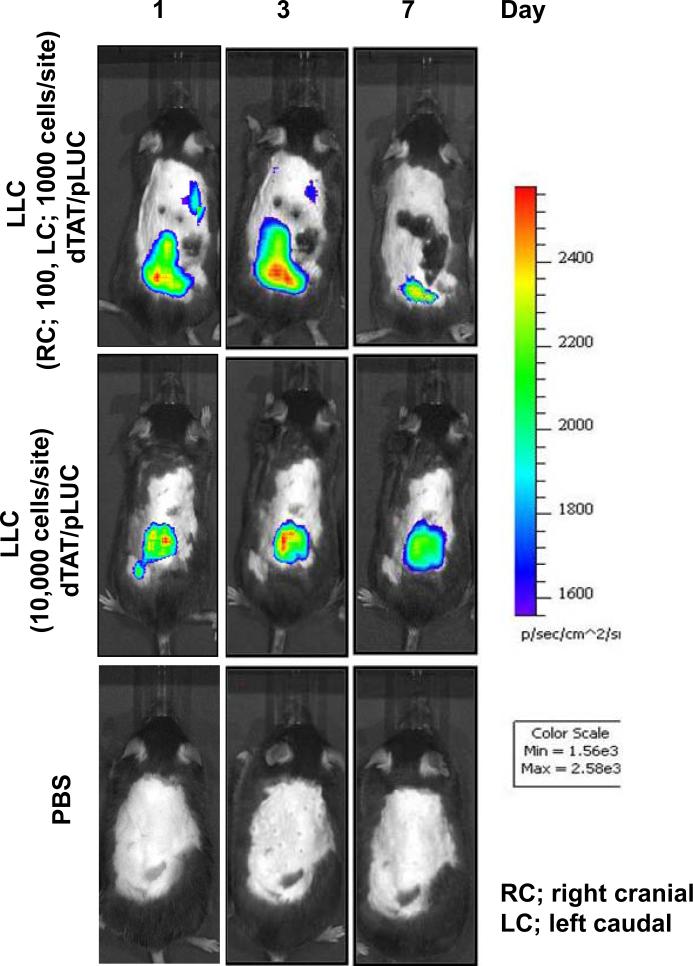
Since the magnitude of gene expression is one key to successful gene therapy, gene expression was evaluated using an in vivo imaging system after dTAT/pLUC transfected LLC cells were transplanted into the backs of mice. In this experiment, luciferase expression was detected by in vivo imaging 24h after transplantation with a minimum number of 100 luciferase transfected cells. Luciferase expression by dTAT/pLUC was easily detectable for at least 7 days after transplantation when over 1000 luciferase transfected cells were transplanted (Fig. 3). These results clearly indicate that dTAT NP-based gene transfection would be detectable for an in vivo mouse study.
Figure 3.
Bioluminescence IVIS images of firefly luciferase-expressing LLC cells transplanted subcutaneously in the backs of the mice. LLC cells were transfected with either dTAT/pLUC or dTAT NP alone in vitro and various cell numbers were transplanted into the backs of C57BL/6 mice as indicated in the figure.
Administration of dTAT/pLUC NP via intratracheal spray caused luciferase expression preferentially in lung tumor cells
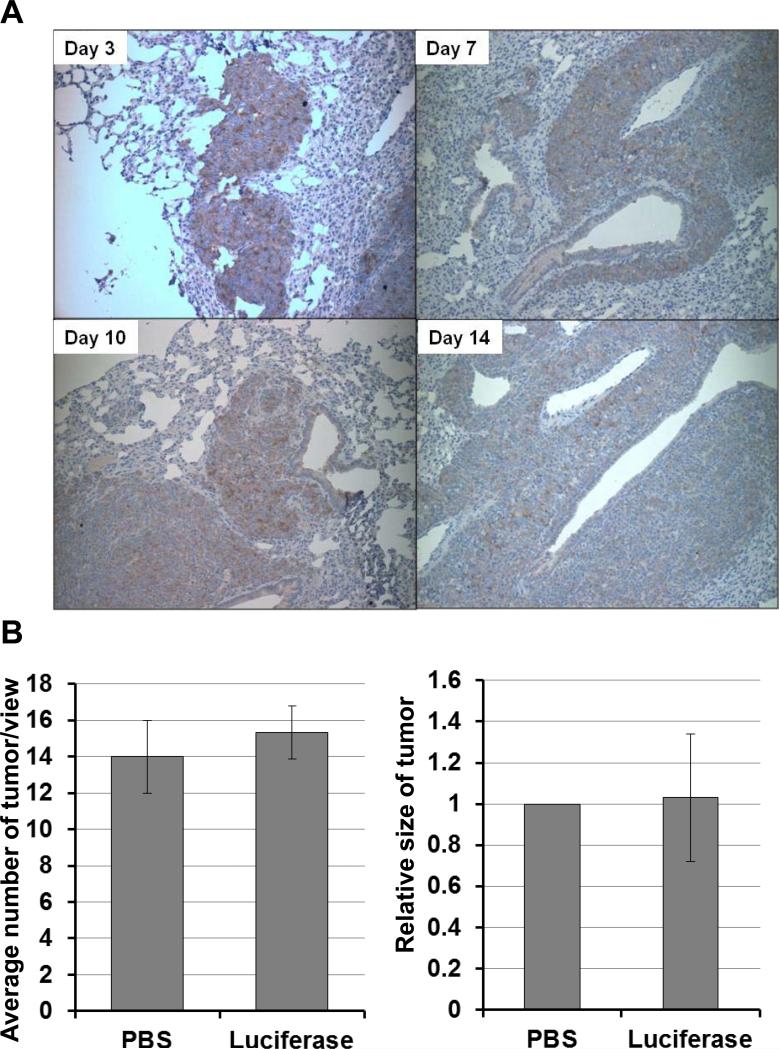
The effectiveness of intratracheally administered dTAT/pLUC NP, the luciferase expression sites, and the effect on tumor growth were quantitatively determined using LLC lung tumor bearing mice. Immunohistochemical detection of luciferase expression in the lung indicated that the primary expression sites are tumor cells and bronchioloalveolar epithelium (Fig. 4A). Strong luciferase expression was detected at 3 days after the intratracheal spray of dTAT/pLUC; this expression lasted until 14 days after the spray without losing much intensity, indicating that dTAT NP-based gene transfection is effective in vivo. In addition, this dTAT/pLUC transfection via intratracheal spray did not show any effects on tumor growth (Fig.4B). Histological examination of tumors in H&E stained sections showed a large number of LLC tumor nodules in mouse lungs treated with either dTAT/pLUC or PBS. The average number of tumors per view and the size of the tumors in both groups were not significantly different between the two groups (Fig. 4B).
Figure 4.
Determination of luciferase expression in LLC tumor-bearing lung treated with dTAT/pLUC via intratracheal spray. A, Specific localization of luciferase expression in LLC tumor-bearing mouse lung by immunohistochemistry.. The tumor cells were immunostained with luciferase antibody in the LLC lung nodule at various time points up to 2 weeks after the administration of dTAT/pLUC. B, the average tumor numbers in three view areas at 40x and tumor size of ten tumor nodules in each treatment group at 100x were expressed in the bar graph.
Administration of dTAT/pAT2R or pTRAIL NP via intratracheal spray caused significant growth attenuation of lung tumors
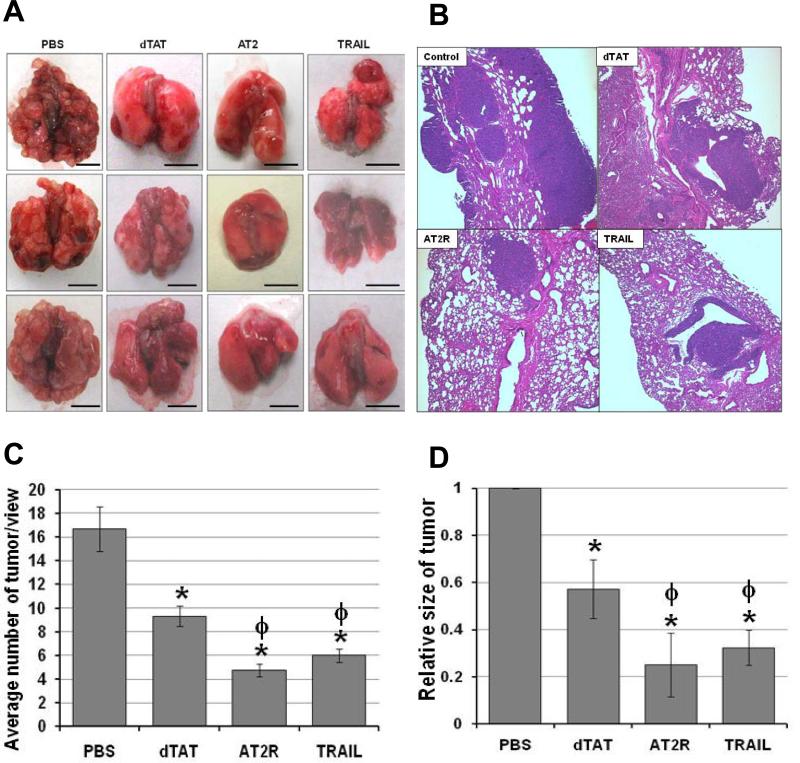
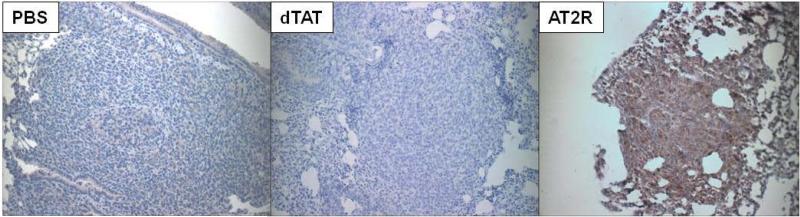
Since dTAT NP-based in vivo pDNA transfection was very effective and intratracheal spray was shown to deliver the dTAT/pLUC into the lung tumors effectively, the effect of dTAT NP-based in vivo transfection of endogenous apoptosis inducer genes, AT2R and TRAIL, was examined using orthotopic LLC lung tumor bearing mice. In this study, LLC cells (2 × 106) were inoculated via the tail vein. One week after cancer cell inoculation, during which time a preliminary study revealed that LLC grafts had started growing as microtumors, dTAT/pAT2R or dTAT/pTRAIL (1 μg DNA/50 μl solution) was sprayed once intratracheally. These treatments significantly decreased lung tumor prevalence as compared to PBS or dTAT/pLUC treated mice (Fig. 5A). The dTAT/pAT2R and pTRAIL also significantly decreased the macroscopic lung tumor multiplicity (Fig. 5A). Histological examination of tumors in H&E stained sections clearly showed a large number and size of LLC tumor nodules in mouse lungs treated with PBS, whereas only a small number of tumors were detected in dTAT/pAT2R treated mouse lungs (Fig. 5B-D). As expected, dTAT/pTRAIL attenuated both tumor size and the number of tumors significantly (Fig. 5). Interestingly, administering dTAT alone also attenuated tumor multiplicity and decreased tumor size (Fig. 5C). The AT2R expression site in the lung was determined immunohistochemically. As shown in Fig. 6, an intense immunoreactivity for AT2R was observed in the tumor cells in the dTAT/pAT2R treated group, but not in other groups (Fig. 6). These microscopic observations suggested that an intratracheal dTAT/pAT2R spray significantly attenuated lung tumor growth by expressing AT2R in the tumor cells. Accordingly, these data support the assertion that intratracheal spray of dTAT/pAT2R is an effective modality for targeted lung cancer gene therapy.
Figure 5.
Macroscopic and microscopic analysis of LLC tumors in C57 BL/6 mouse lungs. . A and B, macroscopic and microscopic views of the lung from a dTAT alone, dTAT/pAT2R or dTAT/pTRAIL treated mouse. Scale bars in macroscopic figures indicate 5mm. C and D, the average tumor numbers in 3 view areas at 40xand tumor size of 10 tumor areas at 100x in each treatment group were expressed in the bar graphs, respectively. * p < 0.05, as compared to the level of PBS control. ϕ, p < 0.05, as compared to the level of dTAT alone group.
Figure 6.
Specific localization of AT2R in LLC tumor-bearing mouse lung. Immunohistochemical image of the lung from a dTAT/pAT2R treated mouse shows AT2R expression in the tumor cells.
Determination of cell proliferation and apoptotic index in the tumors
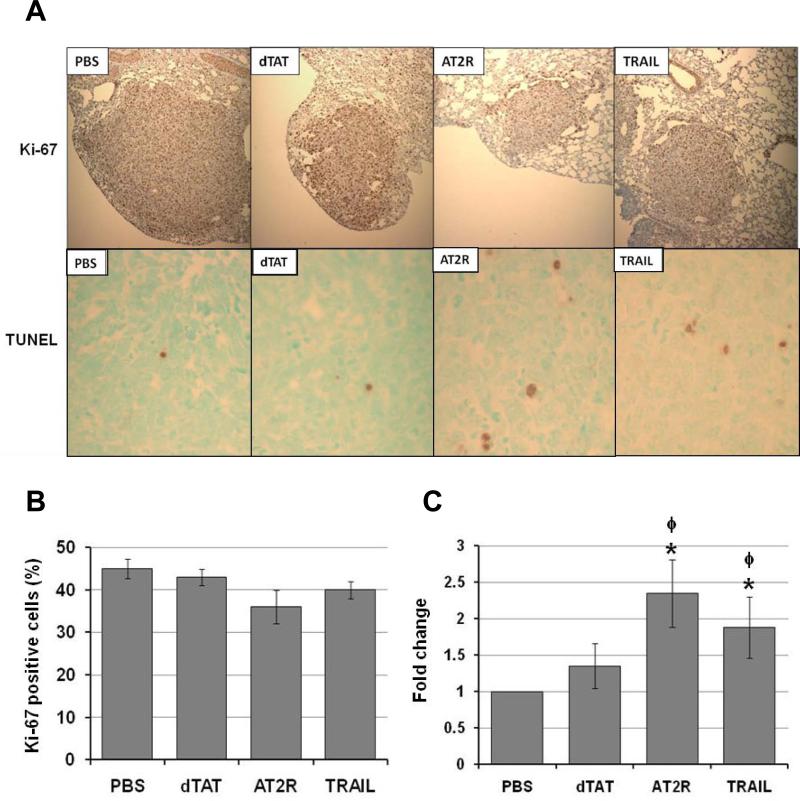
To evaluate the effect of the treatments on the proliferative and apoptotic activities of tumor cells, numbers of Ki-67 and TUNEL positive cells in tumor tissues were determined. Immunohistochemical analysis revealed that while the number of Ki-67 positive cells was slightly decreased in the dTAT/pAT2R or pTRAIL treated tumors, this difference was not significant (Fig. 7A and B). In contrast, both dTAT/pAT2R and dTAT/pTRAIL increased apoptosis. The TUNEL positive cells were significantly increased in tumors of mice treated with dTAT/pAT2R and pTRAIL relative to those treated with PBS or dTAT (Fig. 7A and C). Treatment with dTAT alone did not significantly alter either cell proliferation or apoptosis. These results indicate that treatment with dTAT/pAT2R increased apoptosis of tumor cells and thus decreased tumor multiplicity and the tumor size.
Figure 7.
Immunohistochemical analysis of cell division (A and B) and apoptosis (A and C) in LLC graft tumors in C57 BL/6 mouse lungs treated with either PBS, dTAT, dTAT/pAT2R or dTAT/pTRAIL. A, microscopic images of immunohistochemistry for Ki-67 (top 4 panels) at 100x and TUNEL assay (bottom 4 panels) at 100x. B, treatment with dTAT/pAT2R or pTRAIL had no significant effect on proliferation of the tumor cells. C, the TUNEL positive cells were significantly increased in tumors of mice treated with dTAT/pAT2R and pTRAIL * p < 0.05 as compared to the level of PBS-treated control. ϕ, p < 0.05, as compared to the level of dTAT alone group.
Discussion
The primary objectives of this study were to examine the efficacy of dTAT as a vector, to determine whether pDNA can be distributed to lung tumor cells and cause robust expression, and to evaluate the effectiveness of dTAT NP-based delivery of AT2R or TRAIL pDNA, since AT2R or TRAIL over-expression is known to attenuate tumor cell growth (34, 36, 37). In addition, efficiency of intratracheal spray of dTAT/pDNA was evaluated utilizing luciferase, AT2R, and TRAIL pDNA. Results indicated that a bolus intratracheal spray of dTAT/pDNA caused robust gene expression, primarily in lung tumor cells. Expression of AT2R and TRAIL significantly attenuated tumor growth. Therefore, the present study introduces an effective in vivo gene delivery system using a cationic peptide, dTAT, for lung cancer therapy.
The first study indicated that dTAT NP-based transfection was comparable to PEI polyplexes (Fig. 1A). Our data indicate that under the conditions tested, dTAT did not show any practical acute cytotoxicity in vitro until nearly 5 mg/mL concentration for 24h, whereas PEI showed strong cytotoxicity at much lower concentrations (Fig. 1A). Evaluation of the cytotoxicity of dTAT alone using other cell types, such as human lung bronchial epithelial cells and human lung adenocarcinoma cells, also revealed similar low cytotoxicity (Supplemental Fig. 1). Glucose or salt solution addition also did not affect viability in these other cell types (Supplemental Fig. 2). The low cytotoxicity was also shown in vivo after intratracheal application, in which all mice receiving dTAT alone or dTAT/pDNA survived during the experimental period and did not show any histologically detectable abnormality or acute inflammatory reaction (data not shown). The low cytotoxicity of the dTAT peptide is in agreement with previous reports of TAT and other similar cell penetrating peptides (26, 39). Furthermore, our recent dTAT dose escalation toxicity study in mice with IV administration (39) suggests that this dTAT NP-based delivery system is minimally toxic. Accordingly, it is concluded that dTAT NP potentially represent an efficient and safe gene transfection vector, worthy of further in vivo studies.
The second study clarified that addition of either glucose, KCl, or NaCl to the dTAT/pDNA mixture caused equally effective DNA transfection, but addition of glucose caused the most significant attenuation of cell growth (Fig. 2). Although the first experiment clearly indicated that dTAT alone treatment was significantly less cytotoxic than PEI alone, treating cells with dTAT for two days attenuated cell viability (Fig. 2B). Growth inhibition by treatment with dTAT alone is consistent with the report that the TAT peptide itself can induce cell death (40). The present study clearly indicated that the cell growth attenuation effect of dTAT is amplified when the dTAT NP is prepared with plasmids encoding an apoptosis inducer gene such as AT2R, suggesting that dTAT NP can effectively transfect genes in tumor cells and induce tumor cell death.
Gene therapy-dependent tumor growth inhibition requires sustained and robust transgene expression in order to be effective (26). Accordingly, the intensity and duration of gene expression by dTAT NP transfection were determined by in vivo imaging after transplanting tumor cells transfected in vitro with dTAT/pLUC. Luciferase expression was detectable at days 1 and 3 after transplantation of only 100 dTAT/pLUC-transfected cells. Luciferase expression was detectable for a week after the injection of 1000 cells or more (Fig. 3). These results indicated that dTAT/pDNA caused efficient transfection both in vitro and in vivo, and the duration of the strong expression is significantly long to prompt further study.
To examine the in vivo gene transfection efficiency of dTAT NP, the expression of luciferase in the lung was monitored immunohistochemically for 14 days after administering dTAT/pLUC via intratracheal spray in LLC tumor bearing mice. A single spray of dTAT/pLUC caused robust luciferase expression, primarily in the tumor cells and bronchial epithelium, for at least 14 days (Fig. 4A). These studies proved that dTAT NP cause long-lasting, robust gene expression in vivo. Hence, the current study suggested that gene transfection using dTAT NP was an effective strategy for in vivo gene therapy and is potentially selective for rapidly dividing lung cancer cells.
In the next study, delivery of the endogenous apoptosis inducer gene AT2R was examined using LLC tumor bearing mice. pTRAIL was used as a positive control. As expected, dTAT/pTRAIL attenuated tumor growth macroscopically and microscopically by inducing apoptosis (Figs. 5 and 7), indicating that dTAT NP gene transfection was effective. In the dTAT/pAT2R treatment group, expression of AT2R was detected primarily in the tumor cells, which apparently led to the attenuation of the tumor growth. The degree of cell proliferation and apoptosis in the tumors suggested that bolus intratracheal spray of dTAT/pAT2R probably lowered tumor burden by inducing apoptosis of tumor cells rather than by attenuating cell proliferation (Fig. 7). These results are consistent with previous reports that AT2R is a strong apoptosis inducer, attenuating growth of various cell types (41-43) including human lung cancer cells (37). Accordingly, induction of AT2R over-expression is a potential treatment scheme for lung cancer.
A single intratracheal spray of dTAT alone also attenuated tumor growth significantly (Fig. 5) as compared to the PBS controls. In support of this result, it has been shown that HIV-TAT peptide can directly attenuate growth of polyamine deprived cancer cells (40). Therefore, tumor growth attenuation in the lungs of LLC graft-bearing mice by dTAT alone may partly be caused by the direct tumoricidal effect of dTAT on the tumor cells. However, all mice receiving dTAT alone survived until sacrificed and showed no abnormal clinical signs or histological abnormalities in the normal areas of the lung. These results suggest that the dTAT-dependent cell growth attenuation appears to be limited to the tumor cells. As an alternative explanation, the dTAT peptide-dependent tumor attenuation may also be caused by a secondary effect of the dTAT peptide on the tumor microenvironment. This speculation may be supported by the immunohistochemical observations that dTAT NP-dependent luciferase or AT2R expression was recognized in the alveolar epithelium (Figs. 4 and 6) and alveolar macrophages (data not shown). This observation suggests that dTAT peptides are taken up by various types of cells in the lung, although gene expression levels are weaker than those in tumor cells. Since the tumor microenvironment is an important factor for tumor growth regulation (44-46), it is possible that dTAT modulates thetumor microenvironment toward conditions less favorable to tumor growth. Although further studies are required to better understand the effect of dTAT on tumor growth, this dTAT-dependent tumor suppression may be a beneficial adjuvant property of these therapeutic nanoparticles.
In conclusion, although further studies are required to substantiate the in vivo safety of dTAT NP by formal multi-species toxicity and pharmacokinetic studies, our data indicate that dTAT NP could be a safe and effective in vivo gene transfection tool. The present study provides clear evidence that intratracheal administration of dTAT NP-based therapeutic gene delivery causes strong gene expression preferentially in tumor cells. A bolus intratracheal administration of dTAT/pAT2R or pTRAIL NP significantly attenuated the growth of fast growing Lewis lung carcinoma tumors, suggesting that dTAT NP-based gene therapy is effective and useful for lung cancer treatment. AT2R is a potentially useful gene for lung cancer therapy.
Supplementary Material
Acknowledgements
We are grateful to Ms. Marla Pyle and Garret Seiler (Department of Anatomy and Physiology, Kansas State University) for critical reading and constructive comments during the preparation of the manuscript.
Grant Support
This work was supported in part by the Kansas State University (KSU) Terry C. Johnson Center for Basic Cancer Research, KSU College of Veterinary Medicine Dean's fund, KSU Targeted Excellence Research grant, Kansas State Legislative Appropriation and NIH grants 1R21CA135599, P20 RR017686, P20 RR016475, 5P20RR015563 and Kansas Bioscience Authority collaborative cancer research grant.
Footnotes
Precis: Findings offer a preclinical validation for a non-toxic cationic peptide-based nanoparticle vector that can deliver genes via the trachea to effectively treat lung cancers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Siegel R, Ward E, Brawley O, Jamel A. Cancer Statistics, 2011.The Impact of Eliminating Socioeconomic and Racial Disparities on Premature Cancer Deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Yei S, Mittereder N, Tang K, O'Sullivan C, Trapnell BC. Adenovirus-mediated gene transfer for cystic fibrosis: quantitative evaluation of repeated in vivo vector administration to the lung. Gene Ther. 1994;1:192–200. [PubMed] [Google Scholar]
- 3.Peeters MJ, Patijn GA, Lieber A, Meuse L, Kay MA. Adenovirus-mediated hepatic gene transfer in mice: comparison of intravascular and biliary administration. Hum Gene Ther. 1996;7:1693–9. doi: 10.1089/hum.1996.7.14-1693. [DOI] [PubMed] [Google Scholar]
- 4.Felgner PL. Nonviral strategies for gene therapy. Sci Am. 1997;276:102–6. doi: 10.1038/scientificamerican0697-102. [DOI] [PubMed] [Google Scholar]
- 5.Hope MJ, Mui B, Ansell S, Ahkong QF. Cationic lipids, phosphatidylethanolamine and the intracellular delivery of polymeric, nucleic acid-based drugs (review). Mol Membr Biol. 1998;15:1–14. doi: 10.3109/09687689809027512. [DOI] [PubMed] [Google Scholar]
- 6.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–5. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 7.Thomas M, Klibanov AM. Non-viral gene therapy: polycation-mediated DNA delivery. Appl Microbiol Biotechnol. 2003;62:27–34. doi: 10.1007/s00253-003-1321-8. [DOI] [PubMed] [Google Scholar]
- 8.Benihoud K, Yeh P, Perricaudet M. Adenovirus vectors for gene delivery. Curr Opin Biotechnol. 1999;10:440–7. doi: 10.1016/s0958-1669(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 9.Simons M, Beinroth S, Gleichmann M, Liston P, Korneluk RG, MacKenzie AE, et al. Adenovirus-mediated gene transfer of inhibitors of apoptosis protein delays apoptosis in cerebellar granule neurons. J Neurochem. 1999;72:292–301. doi: 10.1046/j.1471-4159.1999.0720292.x. [DOI] [PubMed] [Google Scholar]
- 10.Bennett J. Immune response following intraocular delivery of recombinant viral vectors. Gene Ther. 2003;10:977–82. doi: 10.1038/sj.gt.3302030. [DOI] [PubMed] [Google Scholar]
- 11.Liu Q, Muruve DA. Molecular basis of the inflammatory response to adenovirus vectors. Gene Ther. 2003;10:935–40. doi: 10.1038/sj.gt.3302036. [DOI] [PubMed] [Google Scholar]
- 12.Ragusa A, Garcia I, Penades S. Nanoparticles as nonviral gene delivery vectors. IEEE Trans Nanobioscience. 2007;6:319–30. doi: 10.1109/tnb.2007.908996. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Huang L. Nonviral gene therapy: promises and challenges. Gene Ther. 2000;7:31–4. doi: 10.1038/sj.gt.3301110. [DOI] [PubMed] [Google Scholar]
- 14.Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine) and its role in gene delivery. J Control Release. 1999;60:149–60. doi: 10.1016/s0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 15.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413–7. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang MX, Redemann CT, Szoka FC., Jr In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug Chem. 1996;7:703–14. doi: 10.1021/bc9600630. [DOI] [PubMed] [Google Scholar]
- 17.Hofland HE, Nagy D, Liu JJ, Spratt K, Lee YL, Danos O, et al. In vivo gene transfer by intravenous administration of stable cationic lipid/DNA complex. Pharm Res. 1997;14:742–9. doi: 10.1023/a:1012146305040. [DOI] [PubMed] [Google Scholar]
- 18.Templeton NS, Lasic DD, Frederik PM, Strey HH, Roberts DD, Pavlakis GN. Improved DNA: liposome complexes for increased systemic delivery and gene expression. Nat Biotechnol. 1997;15:647–52. doi: 10.1038/nbt0797-647. [DOI] [PubMed] [Google Scholar]
- 19.Hortobagyi GN, Ueno NT, Xia W, Zhang S, Wolf JK, Putnam JB, et al. Cationic liposome-mediated E1A gene transfer to human breast and ovarian cancer cells and its biologic effects: a phase I clinical trial. J Clin Oncol. 2001;19:3422–33. doi: 10.1200/JCO.2001.19.14.3422. [DOI] [PubMed] [Google Scholar]
- 20.Ogris M, Wagner E. Targeting tumors with non-viral gene delivery systems. Drug Discov Today. 2002;15:479–85. doi: 10.1016/s1359-6446(02)02243-2. [DOI] [PubMed] [Google Scholar]
- 21.Hassani Z, Lemkine GF, Erbacher P, Palmier K, Alfama G, Giovannangeli C, et al. Lipid-mediated siRNA delivery down-regulates exogenous gene expression in the mouse brain at picomolar levels. J Gene Med. 2005;7:198–207. doi: 10.1002/jgm.659. [DOI] [PubMed] [Google Scholar]
- 22.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–93. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 23.Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci U S A. 1994;91:664–8. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truant R, Cullen BR. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals. Mol Cell Biol. 1999;19:1210–7. doi: 10.1128/mcb.19.2.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vivès E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–7. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 26.Baoum A, Xie SX, Fakhari A, Berkland C. “Soft” calcium crosslinks enable highly efficient gene transfection using TAT peptide. Pharm Res. 2009;12:2619–29. doi: 10.1007/s11095-009-9976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–51. [PubMed] [Google Scholar]
- 28.Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JE. Expression of AT2 receptors in the developing rat fetus. J Clin Invest. 1991;88:921–33. doi: 10.1172/JCI115395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antus B, Mucsi I, Rosivall L. Apoptosis induction and inhibition of cellular proliferation by angiotensin II: possible implication and perspectives. Acta Physiol Hung. 2000;87:5–24. doi: 10.1556/APhysiol.87.2000.1.2. [DOI] [PubMed] [Google Scholar]
- 30.Takagi T, Nakano Y, Takekoshi S, Inagami T, Tamura M. Hemizygous mice for the angiotensin II type 2 receptor gene have attenuated susceptibility to azoxymethane-induced colon tumorigenesis. Carcinogenesis. 2002;23:1235–41. doi: 10.1093/carcin/23.7.1235. [DOI] [PubMed] [Google Scholar]
- 31.Kanehira T, Tani T, Takagi T, Nakano Y, Howard EF, Tamura M. Angiotensin II type 2 receptor gene deficiency attenuates susceptibility to tobacco-specific nitrosamine-induced lung tumorigenesis: involvement of transforming growth factor-beta-dependent cell growth attenuation. Cancer Res. 2005;65:7660–5. doi: 10.1158/0008-5472.CAN-05-0275. [DOI] [PubMed] [Google Scholar]
- 32.Doi C, Egashira N, Kawabata A, Maurya DK, Ohta N, Uppalapati D, et al. Angiotensin II type 2 receptor signaling significantly attenuates growth of murine pancreatic carcinoma grafts in syngeneic mice. BMC Cancer. 2010:67. doi: 10.1186/1471-2407-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7:1001–12. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- 34.Booth NL, Sayers TJ, Brooks AD, Thomas CL, Jacobsen K, Goncharova EI, et al. A cell-based high-throughput screen to identify synergistic TRAIL sensitizers. Cancer Immunol Immunother. 2009;58:1229–44. doi: 10.1007/s00262-008-0637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Yamaguchi N, Yagita H, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in NK cell-mediated and IFN-gamma-dependent suppression of subcutaneous tumor growth. Cell Immunol. 2001;214:194–200. doi: 10.1006/cimm.2001.1896. [DOI] [PubMed] [Google Scholar]
- 36.Kedinger V, Muller S, Gronemeyer H. Targeted expression of tumor necrosis factor-related apoptosis-inducing ligand TRAIL in skin protects mice against chemical carcinogenesis. Mol Cancer. 2011;4:34. doi: 10.1186/1476-4598-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pickel L, Matsuzuka T, Doi C, Ayuzawa R, Maurya DK, Xie SX, et al. Overexpression of angiotensin II type 2 receptor gene induces cell death in lung adenocarcinoma cells. Cancer Biol Ther. 2010 doi: 10.4161/cbt.9.4.10643. In print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuzuka T, Rachakatla RS, Doi C, Maurya DK, Ohta N, Kawabata A, et al. Human umbilical cord matrix-derived stem cells expressing interferon-beta gene significantly attenuate bronchioloalveolar carcinoma xenografts in SCID mice. Lung Cancer. 2010;70:28–36. doi: 10.1016/j.lungcan.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baoum A, Ovcharenko D, Berkland C. Calcium condensed cell penetrating peptide complexes offer highly efficient, low toxicity gene silencing. Int J Pharm. 2011 doi: 10.1016/j.ijpharm.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Mani K, Sandgren S, Lilja J, Cheng F, Svensson K, Persson L, et al. HIV-Tat protein transduction domain specifically attenuates growth of polyamine deprived tumor cells. Mol Cancer Ther. 2007;6:782–8. doi: 10.1158/1535-7163.MCT-06-0370. [DOI] [PubMed] [Google Scholar]
- 41.Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest. 1995;95:651–7. doi: 10.1172/JCI117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada T, Horiuchi M, Dzau VJ. Angiotensin II type 2 receptor mediates programmed cell death. Proc Natl Acad Sci U S A. 1996;93:156–60. doi: 10.1073/pnas.93.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miura S, Karnik SS. Ligand-independent signals from angiotensin II type 2 receptor induce apoptosis. Embo J. 2000;19:4026–35. doi: 10.1093/emboj/19.15.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Roozendaal KE, Klijn JG, van Ooijen B, Claassen C, Eggermont AM, Henzen-Logmans SC, et al. Differential regulation of breast tumor cell proliferation by stromal fibroblasts of various breast tissue sources. Int J Cancer. 1996;65:120–5. doi: 10.1002/(SICI)1097-0215(19960103)65:1<120::AID-IJC20>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 45.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–60. [PubMed] [Google Scholar]
- 46.Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res. 2001;61:1320–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.