Abstract
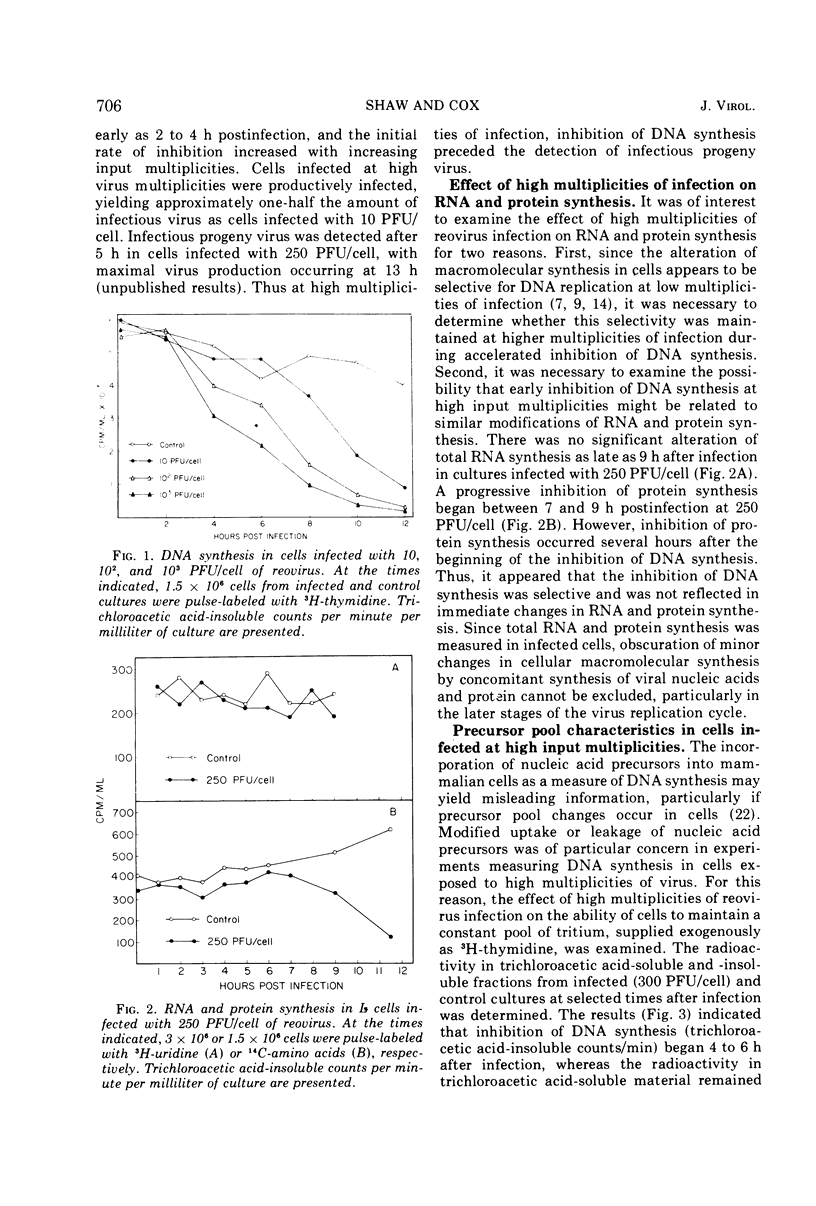
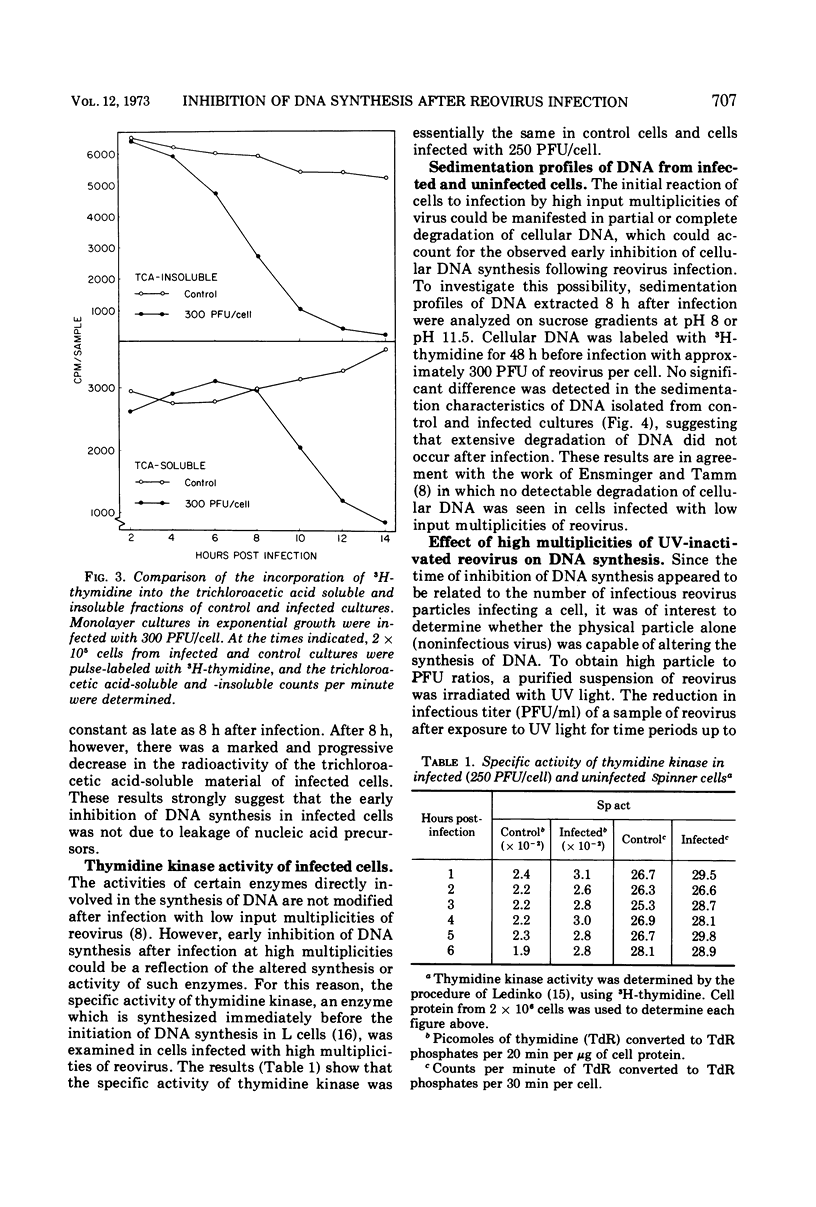
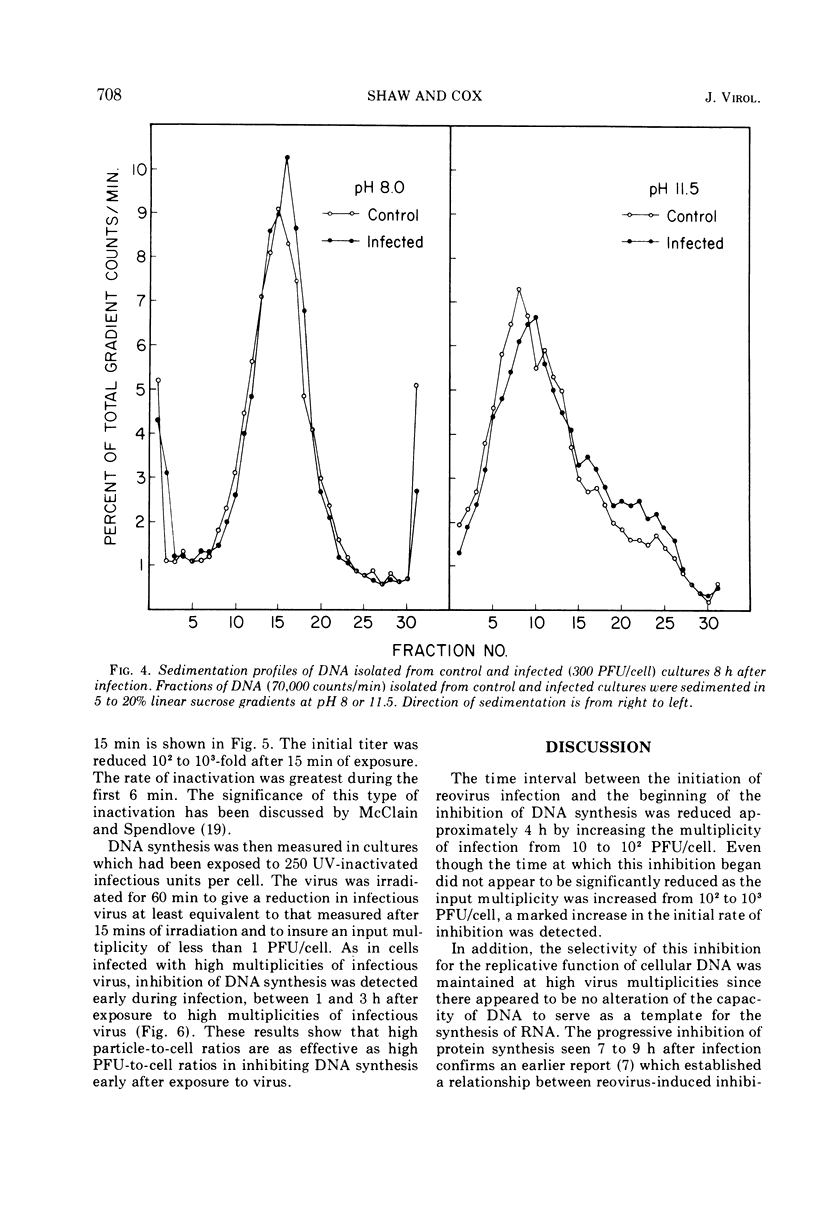
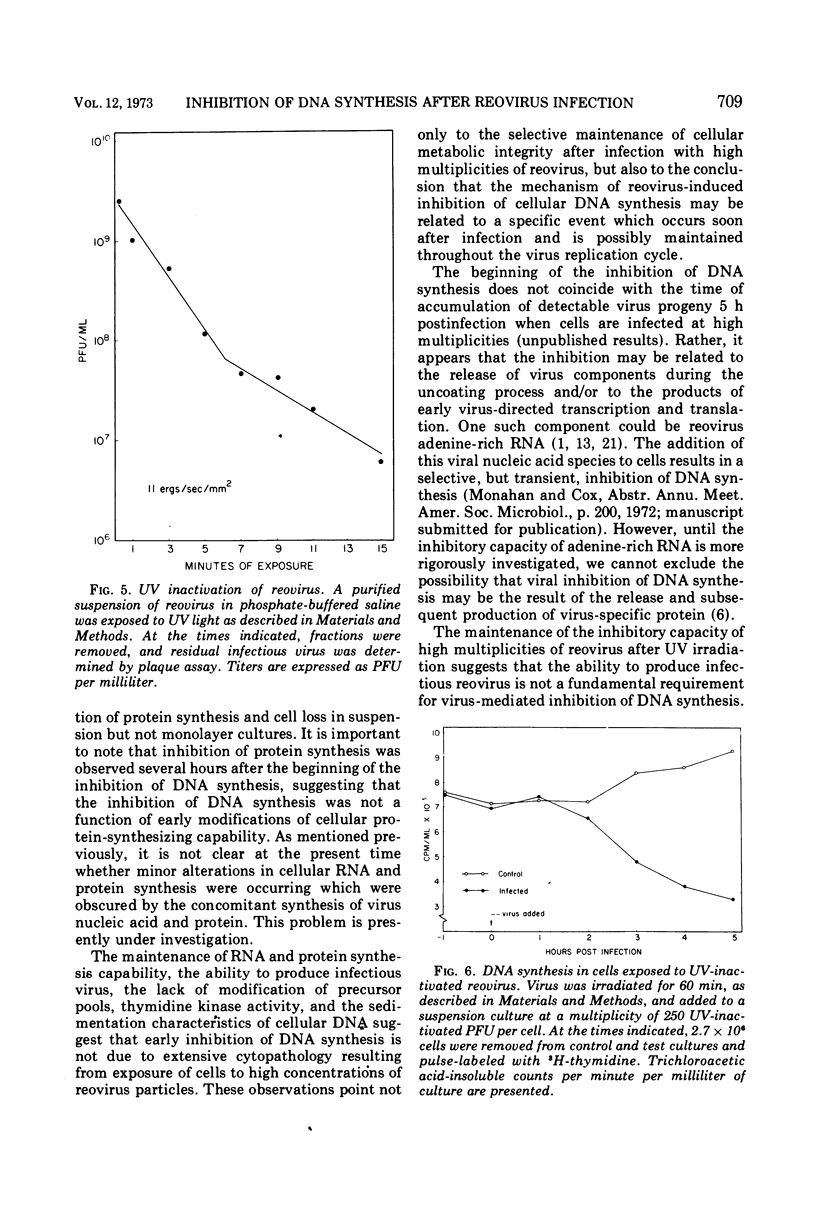
Inhibition of cellular DNA synthesis began 6 to 8 h after reovirus infection at a multiplicity of infection of 10 PFU per cell. However, as the multiplicity of infection was increased to a maximum of 103 PFU/cell, inhibition of DNA synthesis began earlier after infection (2-4 h postinfection), and the initial rate of inhibition increased. The enhanced inhibition of DNA replication at high virus multiplicities appeared to be selective since RNA synthesis was not detectably altered as late as 9 h postinfection and inhibition of protein synthesis did not begin until 7 to 9 h after infection. Early inhibition of DNA synthesis did not appear to be related to changes in thymidine pool characteristics, thymidine kinase activity, or detectable degradation of cellular DNA. Even though the particle-to-PFU ratio was increased by ultraviolet light inactivation of virus, the ability to induce early inhibition of DNA synthesis was not diminished.
Full text
PDF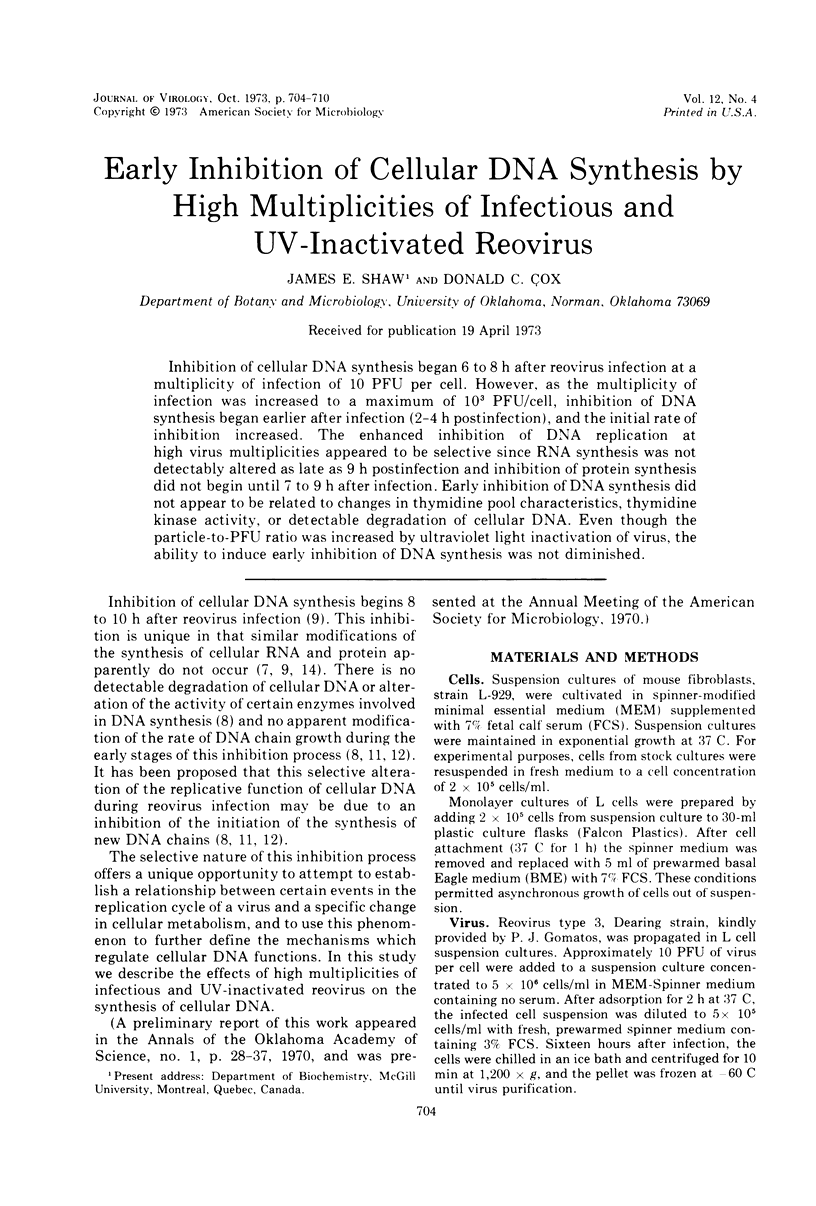


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellamy A. R., Joklik W. K. Studies on the A-rich RNA of reovirus. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1389–1395. doi: 10.1073/pnas.58.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy A. R., Shapiro L., August J. T., Joklik W. K. Studies on reovirus RNA. I. Characterization of reovirus genome RNA. J Mol Biol. 1967 Oct 14;29(1):1–17. doi: 10.1016/0022-2836(67)90177-5. [DOI] [PubMed] [Google Scholar]
- Borsa J., Graham A. F. Reovirus: RNA polymerase activity in purified virions. Biochem Biophys Res Commun. 1968 Dec 30;33(6):895–901. doi: 10.1016/0006-291x(68)90396-3. [DOI] [PubMed] [Google Scholar]
- COLTER J. S., BROWN R. A., ELLEM K. A. Observations on the use of phenol for the isolation of deoxyribonucleic acid. Biochim Biophys Acta. 1962 Jan 22;55:31–39. doi: 10.1016/0006-3002(62)90928-9. [DOI] [PubMed] [Google Scholar]
- Ensminger W. D., Tamm I. Cellular DNA and protein synthesis in reovirus-infected L cells. Virology. 1969 Oct;39(2):357–360. doi: 10.1016/0042-6822(69)90062-2. [DOI] [PubMed] [Google Scholar]
- Ensminger W. D., Tamm I. The step in cellular DNA synthesis blocked by reovirus infection. Virology. 1969 Dec;39(4):935–938. doi: 10.1016/0042-6822(69)90032-4. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., TAMM I., DALES S., FRANKLIN R. M. Reovirus type 3: physical characteristics and interaction with L cells. Virology. 1962 Jul;17:441–454. doi: 10.1016/0042-6822(62)90139-3. [DOI] [PubMed] [Google Scholar]
- GOMATOS P. J., TAMM I. MACROMOLECULAR SYNTHESIS IN REOVIRUS-INFECTED L CELLS. Biochim Biophys Acta. 1963 Aug 20;72:651–653. [PubMed] [Google Scholar]
- Hand R., Ensminger W. D., Tamm I. Cellular DNA replication in infections with cytocidal RNA viruses. Virology. 1971 Jun;44(3):527–536. doi: 10.1016/0042-6822(71)90366-7. [DOI] [PubMed] [Google Scholar]
- Hand R., Tamm I. Rate of DNA chain growth in mammalian cells infected with cytocidal RNA viruses. Virology. 1972 Feb;47(2):331–337. doi: 10.1016/0042-6822(72)90268-1. [DOI] [PubMed] [Google Scholar]
- Koide F., Suzuka I., Sekiguchi K. Some properties of an adenine-rich polynucleotide fragment from the avian reovirus. Biochem Biophys Res Commun. 1968 Jan 11;30(1):95–99. doi: 10.1016/0006-291x(68)90718-3. [DOI] [PubMed] [Google Scholar]
- Kudo H., Graham A. F. Synthesis of reovirus ribonucleic acid in L cells. J Bacteriol. 1965 Oct;90(4):936–945. doi: 10.1128/jb.90.4.936-945.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ledinko N. Stimulation of DNA synthesis and thymidine kinase activity in human embryonic kidney cells infected by Adenovirus 2 or 12. Cancer Res. 1967 Aug;27(8):1459–1469. [PubMed] [Google Scholar]
- Littlefield J. W. The periodic synthesis of thymidine kinase in mouse fibroblasts. Biochim Biophys Acta. 1966 Feb 21;114(2):398–403. doi: 10.1016/0005-2787(66)90319-4. [DOI] [PubMed] [Google Scholar]
- Loh P. C., Oie H. K. Growth characteristics of reovirus type 2: ultraviolet light inactivated virion preparations and cell death. Arch Gesamte Virusforsch. 1969;26(3):197–208. doi: 10.1007/BF01242372. [DOI] [PubMed] [Google Scholar]
- McClain M. E., Spendlove R. S. Multiplicity reactivation of reovirus particles after exposure to ultraviolet light. J Bacteriol. 1966 Nov;92(5):1422–1429. doi: 10.1128/jb.92.5.1422-1429.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. RNA polymerase activity in purified reoviruses. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1462–1469. doi: 10.1073/pnas.61.4.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. Single-stranded, adenine-rich RNA from purified reoviruses. Proc Natl Acad Sci U S A. 1968 Jan;59(1):246–253. doi: 10.1073/pnas.59.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets L. A. Discrepancies between precursor uptake and DNA synthesis in mammalian cells. J Cell Physiol. 1969 Aug;74(1):63–66. doi: 10.1002/jcp.1040740109. [DOI] [PubMed] [Google Scholar]


