Abstract
The mouse cell line, BALB/c 3T3, and its derivatives transformed either spontaneously or by treatment with a variety of external agents, were analyzed for cytoplasmic RNA complementary to DNA products prepared from the Kirsten strain of murine sarcoma-leukemia virus, and from an endogenous type C virus of BALB/c 3T3. Although none of these cell lines spontaneously releases complete type C virions, they all contain RNA which is partially homologous to a portion of the 35S RNA isolated from these viruses. The parental cell line, BALB/c 3T3, contains a low level of viral-related RNA, and there is an increased amount of this RNA in some of the transformed cells. The RNA detected represents only a fraction of the viral RNA found in virus-producing cells. The formation of RNA:DNA hybrids was detected by equilibrium centrifugation in Cs2SO4 density gradients and by analysis with a single-strand-specific nuclease from Aspergillus oryzae. Viral DNA products prepared either from an endogenous reaction with whole virus in the presence of actinomycin D or from purified 70S viral RNA as template using avian myeloblastosis virus DNA polymerase yield comparable data. In addition, all of the BALB/c lines examined produce detectable levels of murine type C virus group-specific antigen.
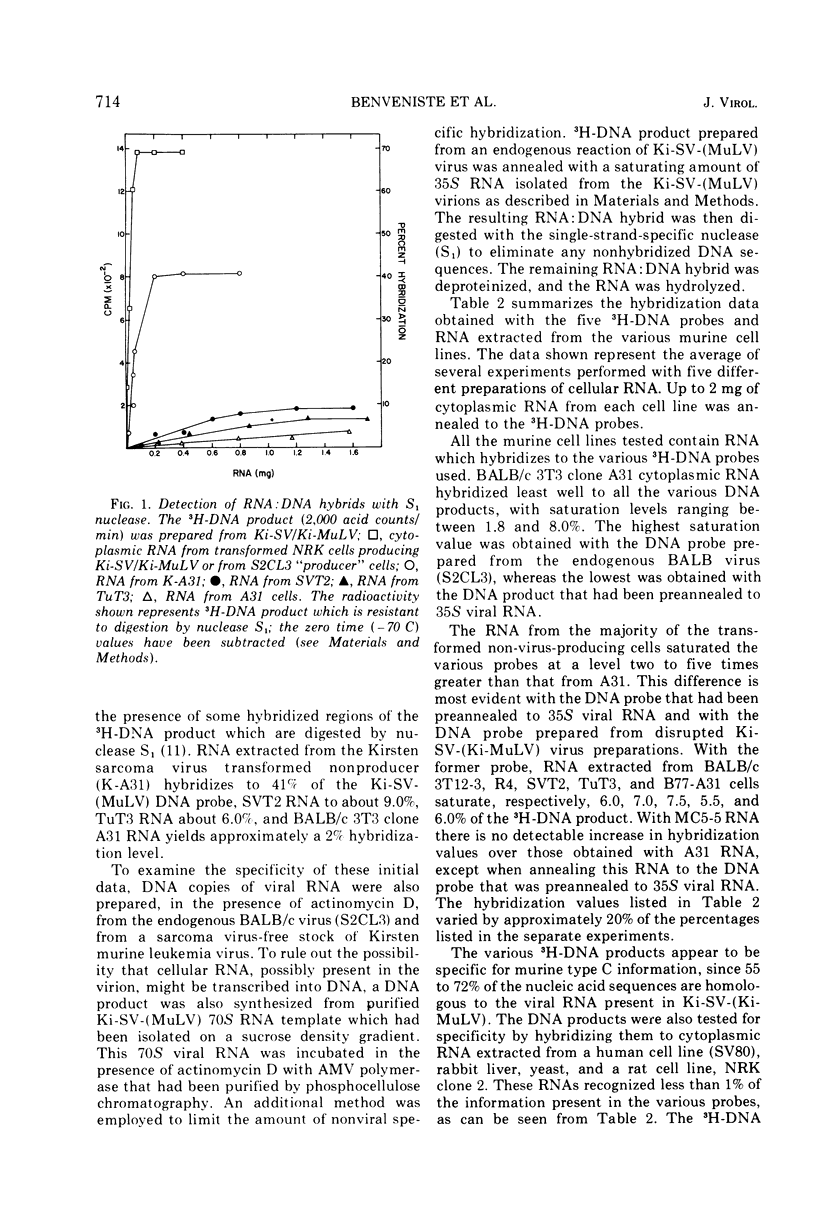
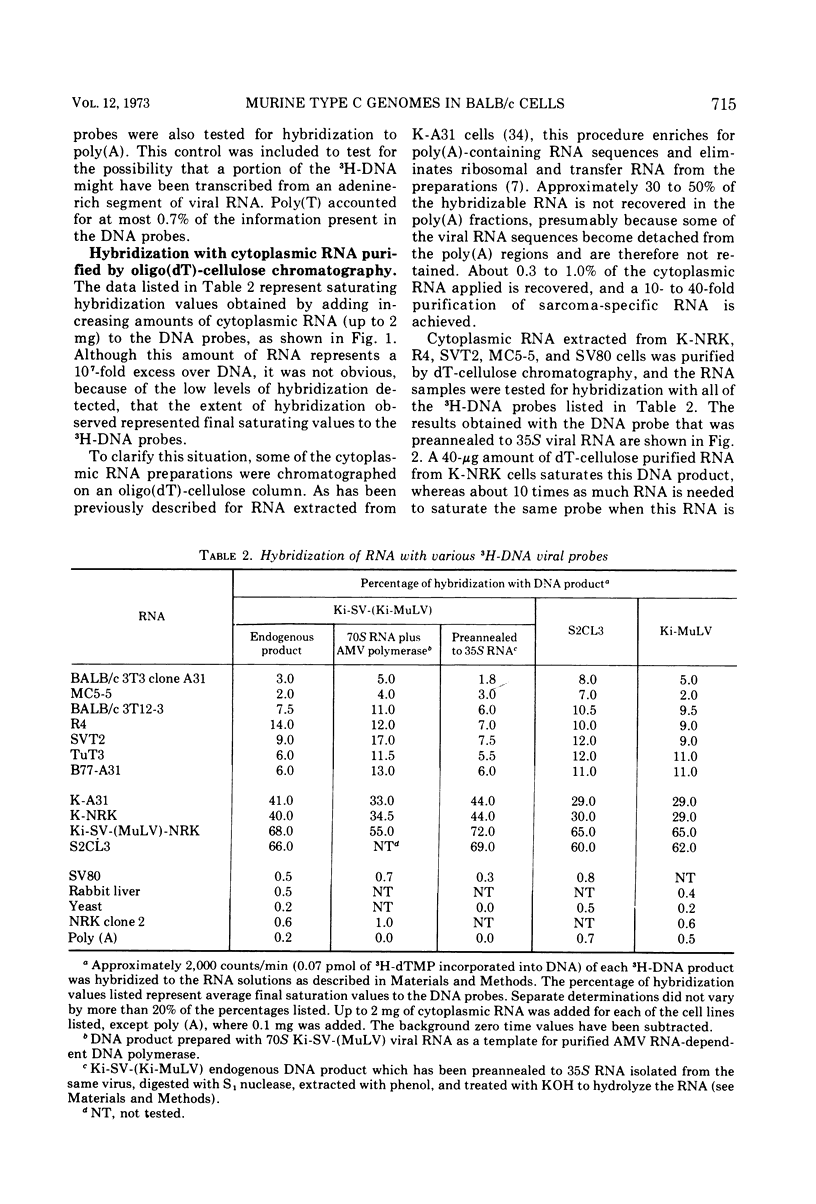
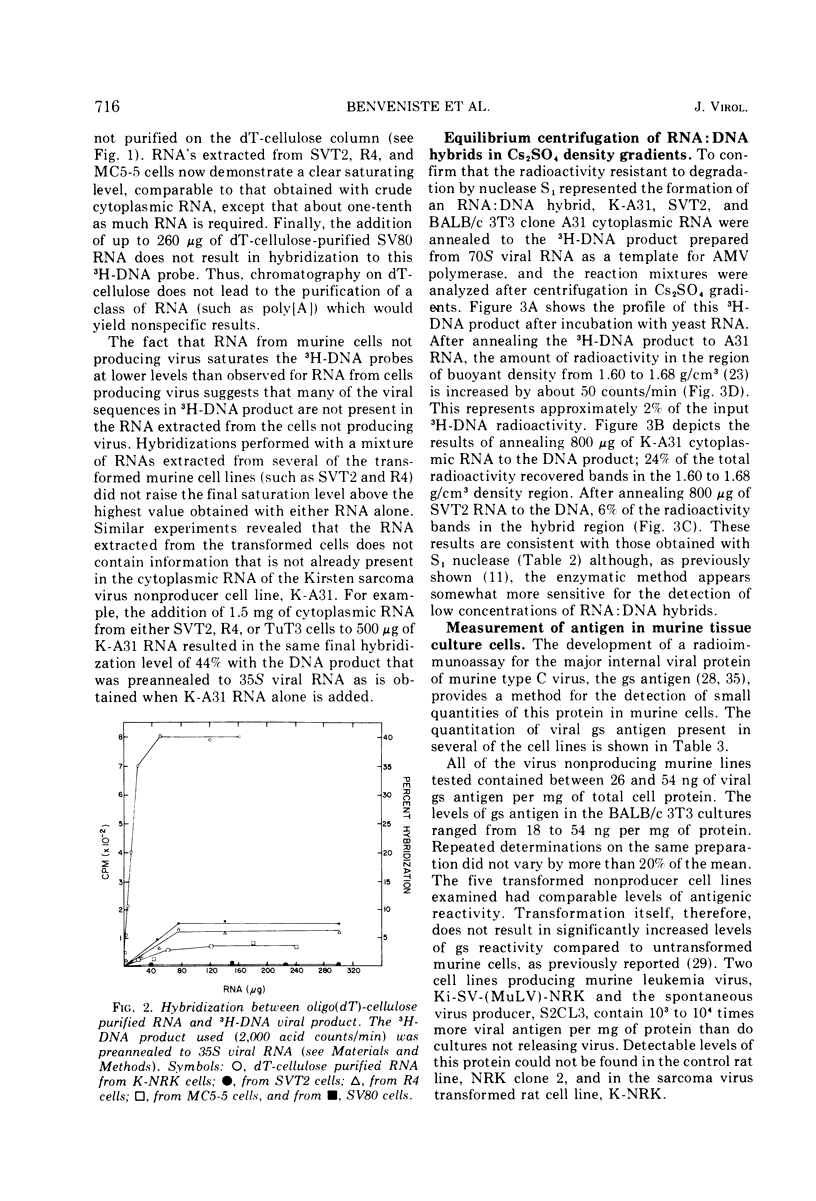
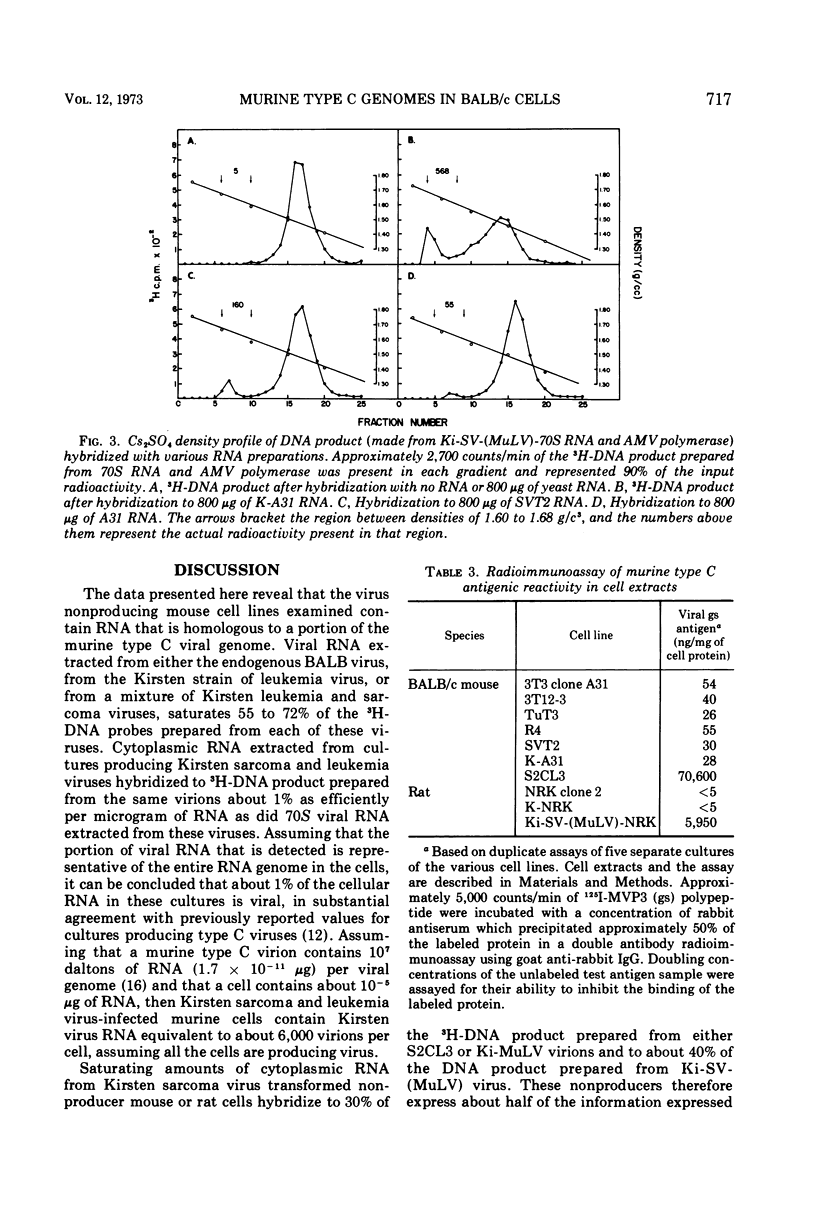
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Hartley J. W., Todaro G. J. Mouse leukemia virus: "spontaneous" release by mouse embryo cells after long-term in vitro cultivation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):87–94. doi: 10.1073/pnas.64.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J., Scolnick E. M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science. 1971 Oct 8;174(4005):157–159. doi: 10.1126/science.174.4005.157. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Weaver C. A. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971 Nov;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Ardelt W., Laskowski M., Sr Mung bean nuclease I. IV. An improved method of preparation. Biochem Biophys Res Commun. 1971 Sep;44(5):1205–1211. doi: 10.1016/s0006-291x(71)80214-0. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Schlom J., Spiegelman S. Evidence for translation of viral-specific RNA in cells of a mouse mammary carcinoma. Proc Natl Acad Sci U S A. 1972 Mar;69(3):535–538. doi: 10.1073/pnas.69.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc Natl Acad Sci U S A. 1972 Mar;69(3):576–580. doi: 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Temin H. M. Hybridization of Rous sarcoma virus deoxyribonucleic acid polymerase product and ribonucleic acids from chicken and rat cells infected with Rous sarcoma virus. J Virol. 1972 May;9(5):766–775. doi: 10.1128/jvi.9.5.766-775.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Canaani E. Complementarity between Rous sarcoma virus (RSV) RNA and the in vitro-synthesized DNA of the virus-associated DNA polymerase. Virology. 1970 Nov;42(3):783–788. doi: 10.1016/0042-6822(70)90325-9. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. On the structure of RNA tumor viruses. Curr Top Microbiol Immunol. 1970;51:78–104. [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapin A. C., Varmus H. E., Faras A. J., Levinson W. E., Bishop J. M. RNA-directed DNA synthesis by virions of Rous sarcoma virus: further characterization of the templates and the extent of their transcription. Virology. 1973 Mar;52(1):264–274. doi: 10.1016/0042-6822(73)90414-5. [DOI] [PubMed] [Google Scholar]
- Green M., Rokutanda H., Rokutanda M. Virus specific RNA in cells transformed by RNA tumour viruses. Nat New Biol. 1971 Apr 21;230(16):229–232. doi: 10.1038/newbio230229a0. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Detection of avian tumor virus RNA in uninfected chicken embryo cells. J Virol. 1973 Feb;11(2):157–167. doi: 10.1128/jvi.11.2.157-167.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehlmann R., Kufe D., Spiegelman S. RNA in human leukemic cells related to the RNA of a mouse leukemia virus (leukocytes-RNA-DNA hybridization-rauscher virus-polysomal RNA). Proc Natl Acad Sci U S A. 1972 Feb;69(2):435–439. doi: 10.1073/pnas.69.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement V., Nicolson M. O., Huebner R. J. Rescue of the genome of focus forming virus from rat non-productive lines by 5'-bromodeoxyruidine. Nat New Biol. 1971 Nov 3;234(44):12–14. doi: 10.1038/newbio234012a0. [DOI] [PubMed] [Google Scholar]
- LINN S., LEHMAN I. R. AN ENDONUCLEASE FROM NEUROSPORA CRASSA SPECIFIC FOR POLYNUCLEOTIDES LACKING AN ORDERED STRUCTURE. II. STUDIES OF ENZYME SPECIFICITY. J Biol Chem. 1965 Mar;240:1294–1304. [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Huebner R. J., Gilden R. V. Species-specific and interspecific antigenic determinants associated with the structural protein of feline C-type virus. Proc Natl Acad Sci U S A. 1971 May;68(5):901–904. doi: 10.1073/pnas.68.5.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Livingston D. M., Todaro G. J., Benveniste R. E., Scolnick E. M. Radioimmunoassay of mammalian type C viral proteins. 3. Detection of viral antigen in normal murine cells and tissues. J Exp Med. 1973 Mar 1;137(3):622–635. doi: 10.1084/jem.137.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock E. J., Aaronson S. A., Todaro G. J. X-irradiation of Balb-3T3. Sarcoma-forming ability and virus induction. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(1):97–100. doi: 10.1080/09553007014550111. [DOI] [PubMed] [Google Scholar]
- Ross J., Scolnick E. M., Todaro G. J., Aaronson S. A. Separation of murine cellular and murine leukaemia virus DNA polymerases. Nat New Biol. 1971 Jun 9;231(23):163–167. doi: 10.1038/newbio231163a0. [DOI] [PubMed] [Google Scholar]
- Ross J., Tronick S. R., Scolnick E. M. Polyadenylate rich RNA in the 70 S RNA of murine leukemia-sarcoma virus. Virology. 1972 Jul;49(1):230–235. doi: 10.1016/s0042-6822(72)80025-4. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Lander M. R., Pugh W. E., Teich N. Noninfectious AKR mouse embryo cell lines in which each cell has the capacity to be activated to produce infectious murine leukemia virus. Virology. 1971 Dec;46(3):866–876. doi: 10.1016/0042-6822(71)90087-0. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Benveniste R., Parks W. P. Purification by oligo(dT)-cellulose of viral-specific RNA from sarcoma virus-transformed mammalian nonproducer cells. J Virol. 1973 Apr;11(4):600–602. doi: 10.1128/jvi.11.4.600-602.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P., Livingston D. M. Radioimmunoassay of mammalian type C viral proteins. I. Species specific reactions of murine and feline viruses. J Immunol. 1972 Sep;109(3):570–577. [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., WOLMAN S. R., GREEN H. RAPID TRANSFORMATION OF HUMAN FIBROBLASTS WITH LOW GROWTH POTENTIAL INTO ESTABLISHED CELL LINES BY SV40. J Cell Physiol. 1963 Dec;62:257–265. doi: 10.1002/jcp.1030620305. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Todaro G. J. Spontaneous release of type C viruses from clonal lines of spontaneously transformed Blab-3T3 cells. Nat New Biol. 1972 Nov 29;240(100):157–160. doi: 10.1038/newbio240157a0. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Robin M. S., Green M. Viral RNA subunits in cells transformed by RNA tumor viruses. Science. 1972 Jun 30;176(4042):1418–1420. doi: 10.1126/science.176.4042.1418. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Levinson W. E., Bishop J. M. Extent of transcription by the RNA-dependent DNA polymerase of Rous sarcoma virus. Nat New Biol. 1971 Sep 1;233(35):19–21. doi: 10.1038/newbio233019a0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Weiss R. A., Friis R. R., Levinson W., Bishop J. M. Detection of avian tumor virus-specific nucleotide sequences in avian cell DNAs (reassociation kinetics-RNA tumor viruses-gas antigen-Rous sarcoma virus, chick cells). Proc Natl Acad Sci U S A. 1972 Jan;69(1):20–24. doi: 10.1073/pnas.69.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]



