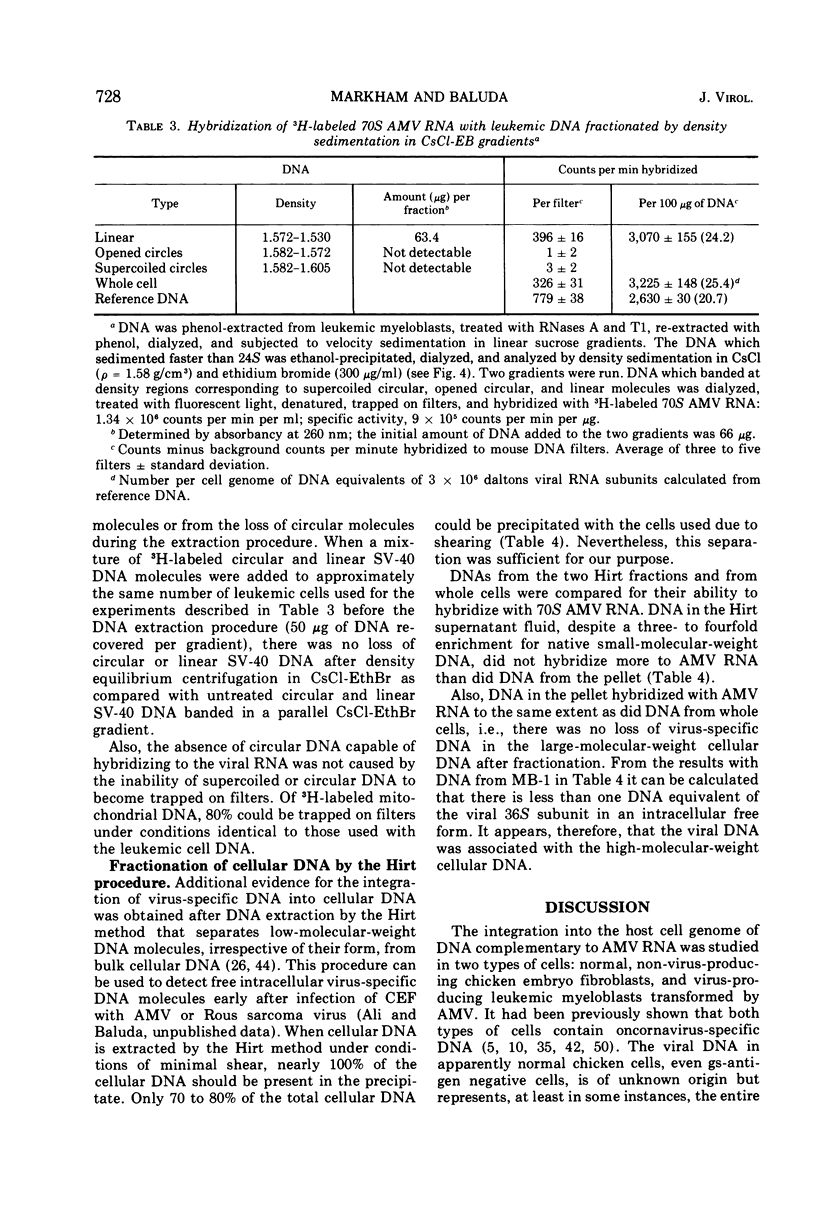
Abstract
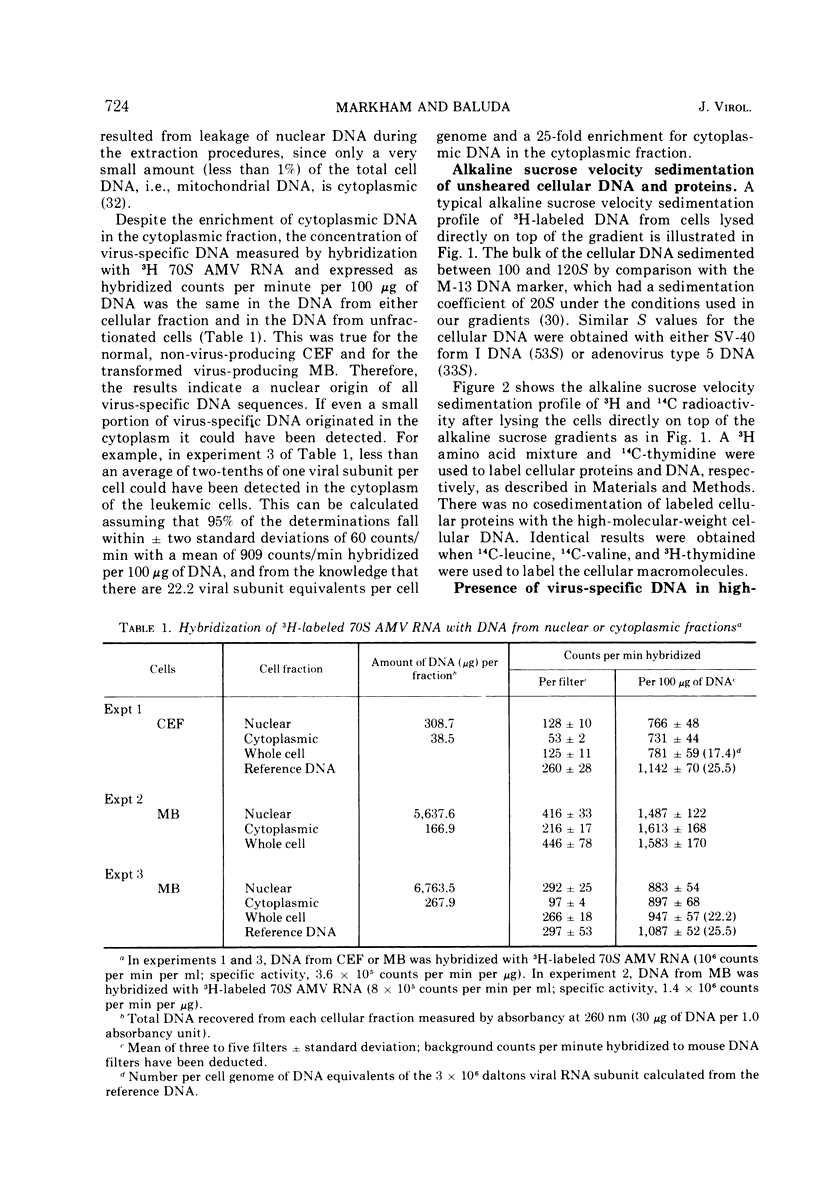
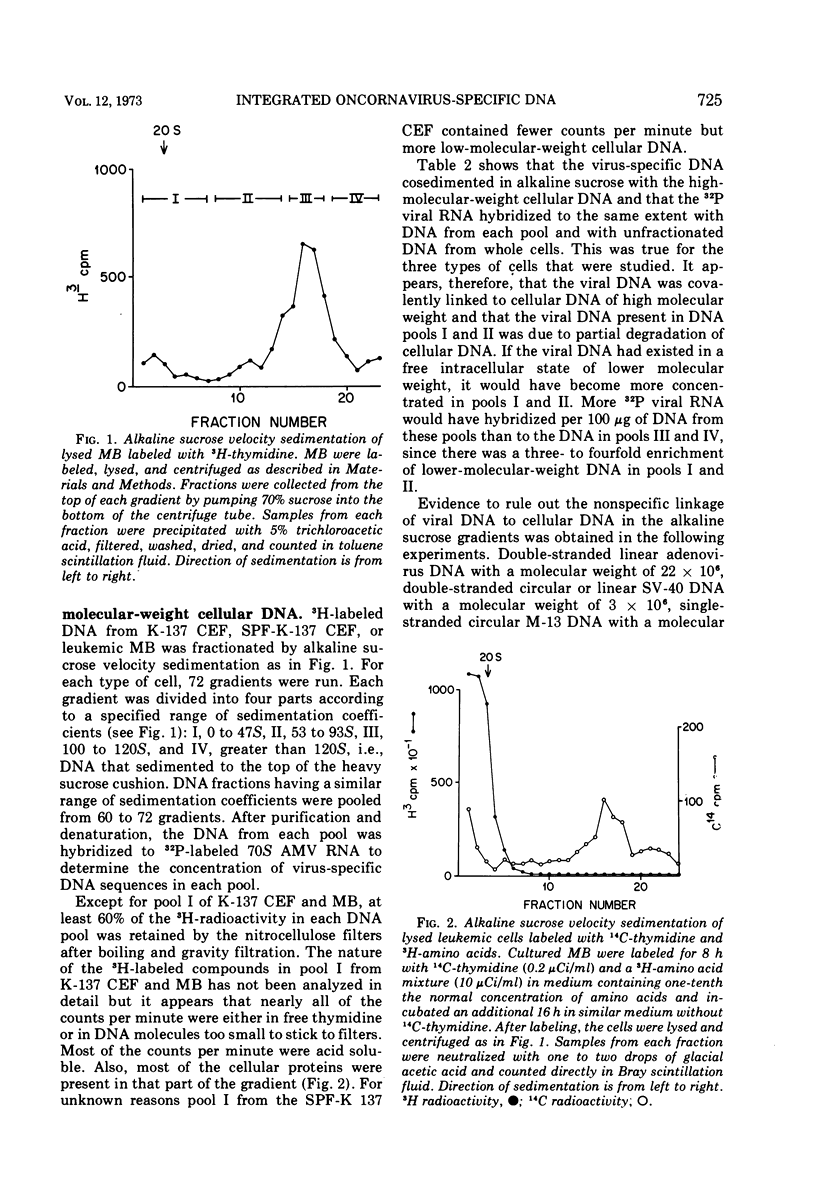
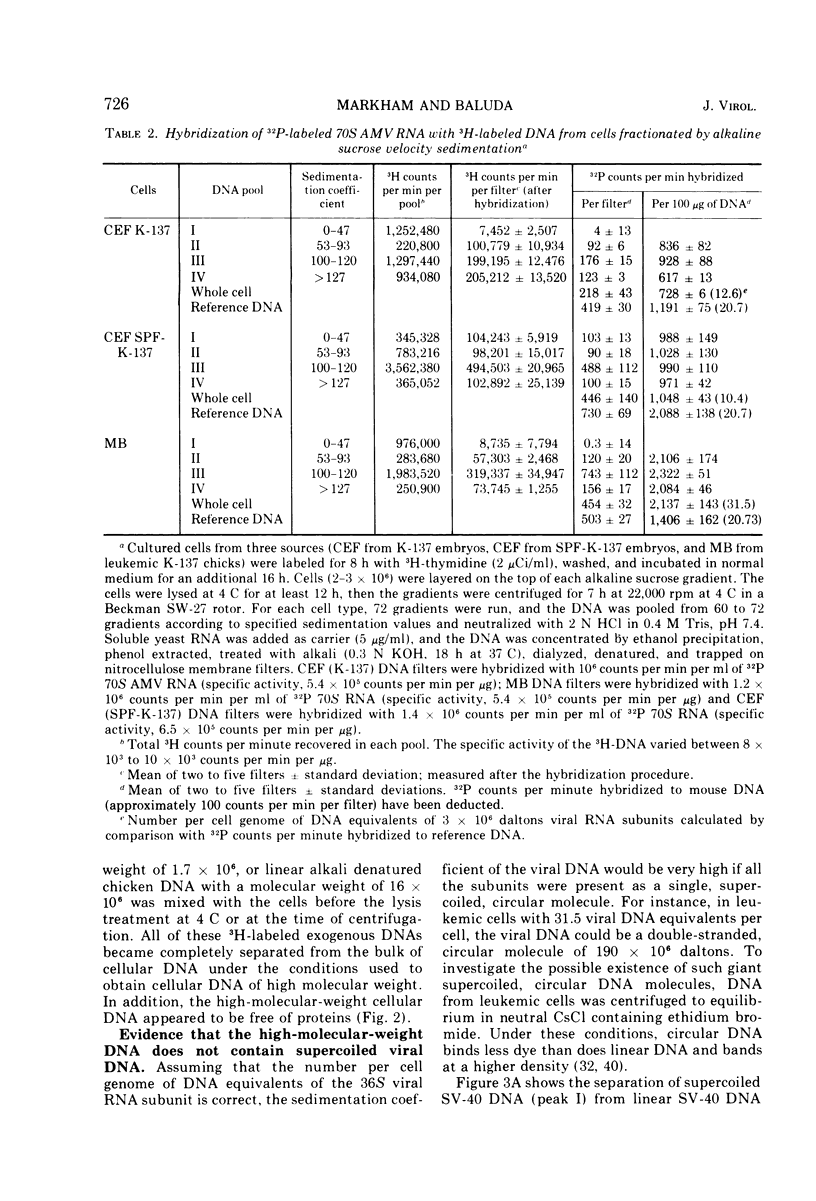
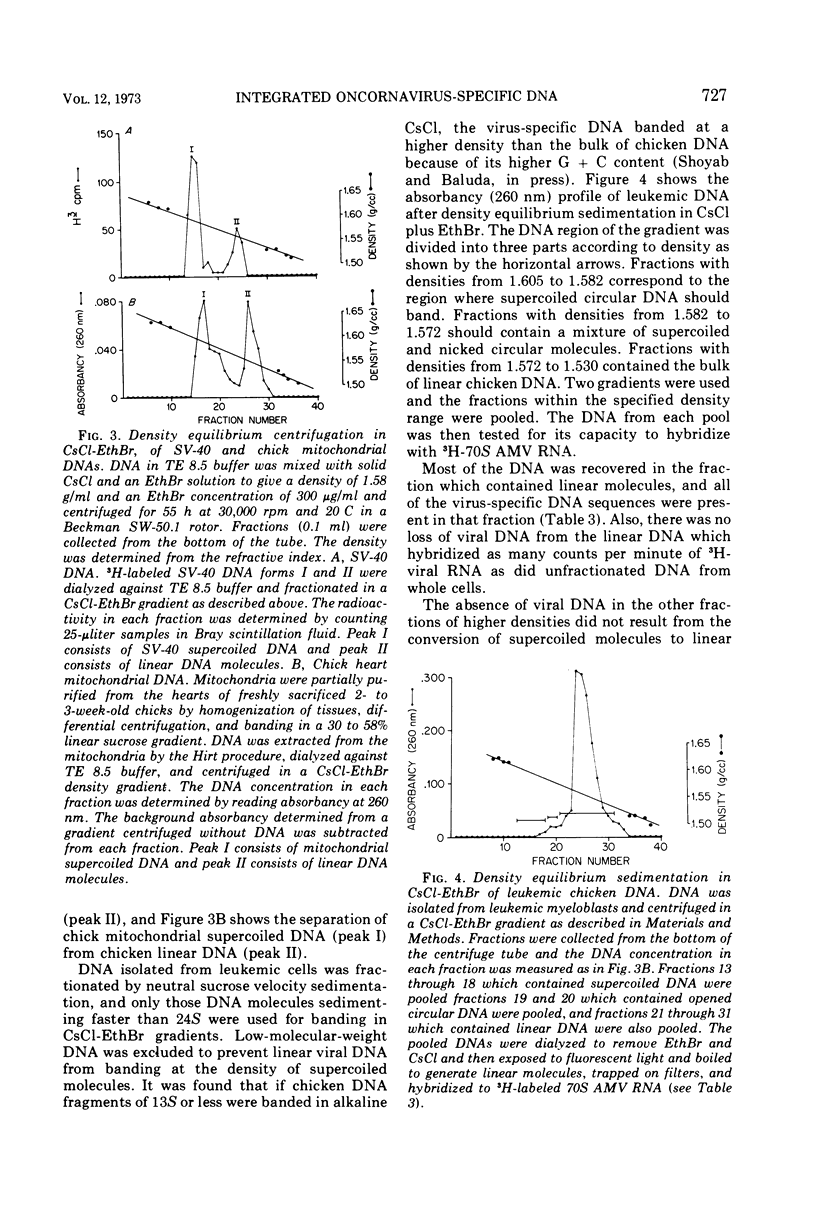
The covalent linkage of oncornavirus-specific DNA to chicken DNA was investigated in normal chicken embryo fibroblasts (CEF) and in virus-producing leukemic cells transformed by avian myeloblastosis virus (AMV). The virus-specific sequences present in cellular DNA fractionated by different methods were detected by DNA-RNA hybridization by using 70S AMV RNA as a probe. In CEF and in leukemic cells, the viral DNA appeared to be present only in the nucleus. After cesium chloride-ethidium bromide density equilibrium sedimentation, the viral DNA was present as linear, double-stranded molecules not separable from linear chicken DNA. After extraction by the Hirt procedure, the viral DNA precipitated with the high-molecular-weight DNA. After alkaline sucrose velocity sedimentation, the viral DNA cosedimented with the high-molecular-weight cellular DNA. The results indicate that in both types of cells studied, the oncornavirus-specific DNA sequences were linked by alkali stable bonds to nuclear cellular DNA of high molecular weight and did not appear to be present in free form of any size.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALUDA M. A., GOETZ I. E. Morphological conversion of cell cultures by avian myeloblastosis virus. Virology. 1961 Oct;15:185–199. doi: 10.1016/0042-6822(61)90234-3. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Hudson J. B. 'Integration' of polyoma virus DNA into mammalian genomes. Biochem Biophys Res Commun. 1972 Apr 14;47(1):111–118. doi: 10.1016/s0006-291x(72)80017-2. [DOI] [PubMed] [Google Scholar]
- Bader J. P. Temperature-dependent transformation of cells infected with a mutant of Bryan Rous sarcoma virus. J Virol. 1972 Aug;10(2):267–276. doi: 10.1128/jvi.10.2.267-276.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A., Drohan W. N. Distribution of deoxyribonucleic acid complementary to the ribonucleic acid of avian myeloblastosis virus in tissues of normal and tumor-bearing chickens. J Virol. 1972 Nov;10(5):1002–1009. doi: 10.1128/jvi.10.5.1002-1009.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A., Markham P. D. Nucleotide composition of RNA hybridized to homologous DNA from cells transformed by avian tumour viruses. Nat New Biol. 1971 May 19;231(20):90–91. doi: 10.1038/newbio231090a0. [DOI] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. DNA complementary to viral RNA in leukemic cells induced by avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):329–336. doi: 10.1073/pnas.66.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. Incorporation of precursors into ribonucleic acid, protein, glycoprotein, and lipoprotein of avian myeloblastosis virions. J Virol. 1969 Nov;4(5):554–566. doi: 10.1128/jvi.4.5.554-566.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc Natl Acad Sci U S A. 1972 Mar;69(3):576–580. doi: 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentvelzen P., Daams J. H., Hageman P., Calafat J. Genetic transmission of viruses that incite mammary tumor in mice. Proc Natl Acad Sci U S A. 1970 Sep;67(1):377–384. doi: 10.1073/pnas.67.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek E., Ryan A. Lysogenic induction. Prog Nucleic Acid Res Mol Biol. 1973;13:249–300. doi: 10.1016/s0079-6603(08)60105-1. [DOI] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W. Three size-classes of intracellular adenovirus deoxyribonucleic acid. J Virol. 1971 Jun;7(6):707–719. doi: 10.1128/jvi.7.6.707-719.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Melnick J. L. Recent advances in molecular pathology. The state of the viral genome in cells transformed by simian virus 40: a review. Exp Mol Pathol. 1972 Aug;17(1):103–119. doi: 10.1016/0014-4800(72)90061-5. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Rescue of rous sarcoma virus from rous sarcoma virus-transformed mammalian cells. J Virol. 1972 Jul;10(1):153–156. doi: 10.1128/jvi.10.1.153-156.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty R. M., Di Stefano H. S., Roth F. K. Virus particles and viral antigens in chicken tissues free of infectious avian leukosis virus. Proc Natl Acad Sci U S A. 1967 Sep;58(3):808–817. doi: 10.1073/pnas.58.3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H. Lysogeny: viral repression and site-specific recombination. Annu Rev Biochem. 1971;40:827–854. doi: 10.1146/annurev.bi.40.070171.004143. [DOI] [PubMed] [Google Scholar]
- Fogel M. Induction of virus synthesis in polyoma-transformed cells by DNA antimetabolites and by irradiation after pretreatment with 5-bromodeoxyuridine. Virology. 1972 Jul;49(1):12–22. doi: 10.1016/s0042-6822(72)80003-5. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B., Cassai E., Nahmias A. A DNA fragment of Herpes simplex 2 and its transcription in human cervical cancer tissue. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3784–3789. doi: 10.1073/pnas.69.12.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H. Isolation of leukosis-type virus from pheasant embryo cells: possible presence of viral genes in cells. Virology. 1973 Jan;51(1):247–251. doi: 10.1016/0042-6822(73)90388-7. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Detection of avian tumor virus RNA in uninfected chicken embryo cells. J Virol. 1973 Feb;11(2):157–167. doi: 10.1128/jvi.11.2.157-167.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M., Hillova J. Recovery of the temperature-sensitive mutant of Rous sarcoma virus from chicken cells exposed to DNA extracted from hamster cells transformed by the mutant. Virology. 1972 Jul;49(1):309–313. doi: 10.1016/s0042-6822(72)80034-5. [DOI] [PubMed] [Google Scholar]
- Hirai K., Defendi V. Integration of simian virus 40 deoxyribonucleic acid into the deoxyribonucleic acid of permissive monkey kidney cells. J Virol. 1972 Apr;9(4):705–707. doi: 10.1128/jvi.9.4.705-707.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tomizawa J. Prophage P1, and extrachromosomal replication unit. Cold Spring Harb Symp Quant Biol. 1968;33:791–798. doi: 10.1101/sqb.1968.033.01.091. [DOI] [PubMed] [Google Scholar]
- Lavi S., Winocour E. Acquisition of sequences homologous to host deoxyribonucleic acid by closed circular simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):309–316. doi: 10.1128/jvi.9.2.309-316.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M., Kodama M., Wells R. T. DNA ligase and exonuclease activities in virions of rous sarcoma virus. Nat New Biol. 1971 Apr 21;230(16):232–235. doi: 10.1038/newbio230232a0. [DOI] [PubMed] [Google Scholar]
- Nass M. M. Mitochondrial DNA. I. Intramitochondrial distribution and structural relations of single- and double-length circular DNA. J Mol Biol. 1969 Jun 28;42(3):521–528. doi: 10.1016/0022-2836(69)90240-x. [DOI] [PubMed] [Google Scholar]
- Nass M. M. Mitochondrial DNA. II. Structure and physicochemical properties of isolated DNA. J Mol Biol. 1969 Jun 28;42(3):529–545. doi: 10.1016/0022-2836(69)90241-1. [DOI] [PubMed] [Google Scholar]
- Nass M. M. Mitochondrial DNA: Advances, Problems, and Goals. Science. 1969 Jul 4;165(3888):25–35. doi: 10.1126/science.165.3888.25. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Rous sarcoma virus nucleotide sequences in cellular DNA: measurement by RNA-DNA hybridization. Science. 1972 Nov 17;178(4062):750–753. doi: 10.1126/science.178.4062.750. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Homology between Epstein-Barr virus DNA and viral DNA from Burkitt's lymphoma and nasopharyngeal carcinoma determined by DNA-DNA reassociation kinetics. Nature. 1973 Mar 2;242(5392):44–47. doi: 10.1038/242044a0. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972 Aug 9;238(84):169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- Payne L. N., Chubb R. C. Studies on the nature and genetic control of an antigen in normal chick embryos which reacts in the COFAL test. J Gen Virol. 1968 Dec;3(3):379–391. doi: 10.1099/0022-1317-3-3-379. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Baluda M. A. The nucleic acid from avian myeloblastosis virus compared with the RNA from the Bryan strain of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1686–1692. doi: 10.1073/pnas.54.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal P. N., Robinson H. L., Robinson W. S., Hanafusa T., Hanafusa H. DNA in uninfected and virus-infected cells complementary to avian tumor virus RNA. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2336–2340. doi: 10.1073/pnas.68.10.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Lowy D. R., Teich N., Hartley J. W. Some implications of the activation of murine leukemia virus by halogenated pyrimidines. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1033–1035. doi: 10.1073/pnas.69.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Westphal H., Srinivasan P. R., Dulbecco R. The integrated state of viral DNA in SV40-transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1288–1295. doi: 10.1073/pnas.60.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Carcinogenesis by avian sarcoma viruses. Cancer Res. 1968 Sep;28(9):1835–1838. [PubMed] [Google Scholar]
- Temin H. M. Mechanism of cell transformation by RNA tumor viruses. Annu Rev Microbiol. 1971;25:609–648. doi: 10.1146/annurev.mi.25.100171.003141. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Possible implications for medicine of RNA-directed DNA synthesis. Triangle. 1972;11(2):37–42. [PubMed] [Google Scholar]
- Varmus H. E., Weiss R. A., Friis R. R., Levinson W., Bishop J. M. Detection of avian tumor virus-specific nucleotide sequences in avian cell DNAs (reassociation kinetics-RNA tumor viruses-gas antigen-Rous sarcoma virus, chick cells). Proc Natl Acad Sci U S A. 1972 Jan;69(1):20–24. doi: 10.1073/pnas.69.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Friis R. R. An avian leukosis virus related to RSV(O): properties and evidence for helper activity. Virology. 1971 Jan;43(1):223–234. doi: 10.1016/0042-6822(71)90240-6. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Payne L. N. The heritable nature of the factor in chicken cells which acts as a helper virus for Rous sarcoma virus. Virology. 1971 Aug;45(2):508–515. doi: 10.1016/0042-6822(71)90351-5. [DOI] [PubMed] [Google Scholar]


