Abstract
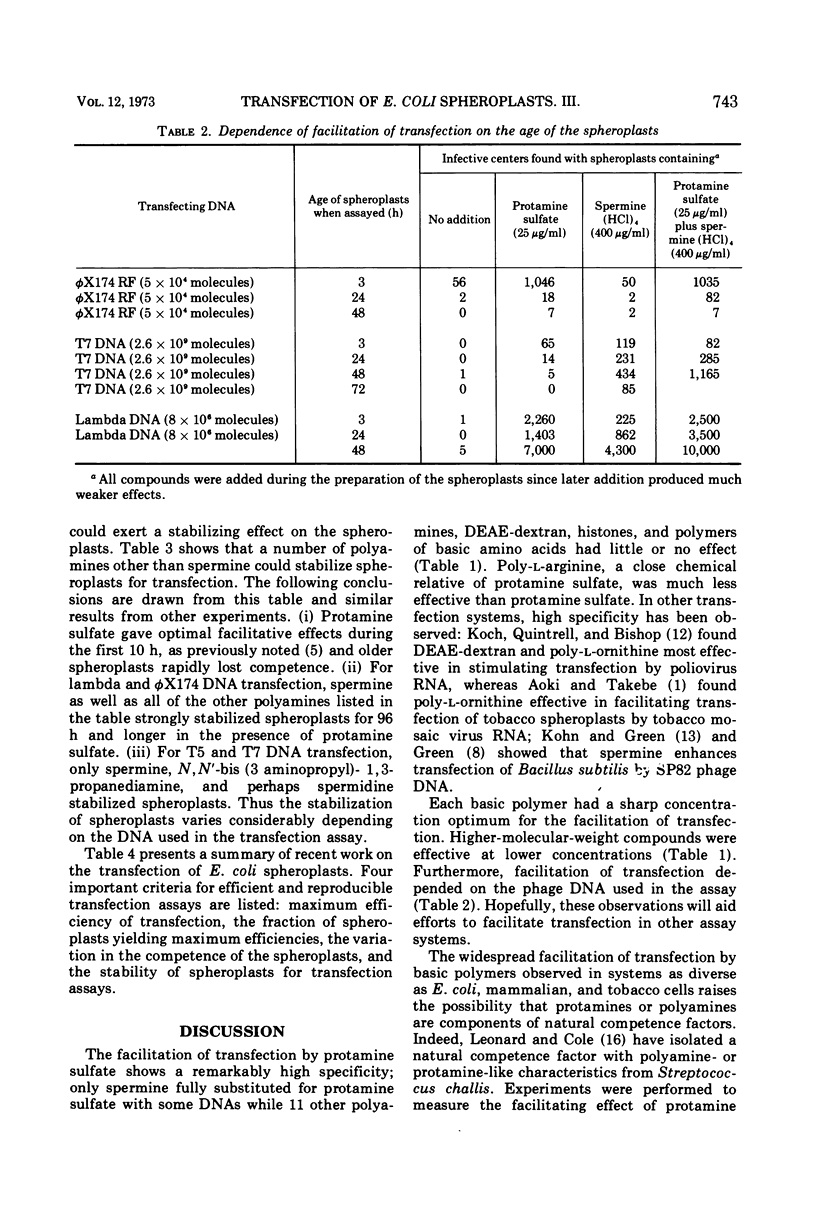
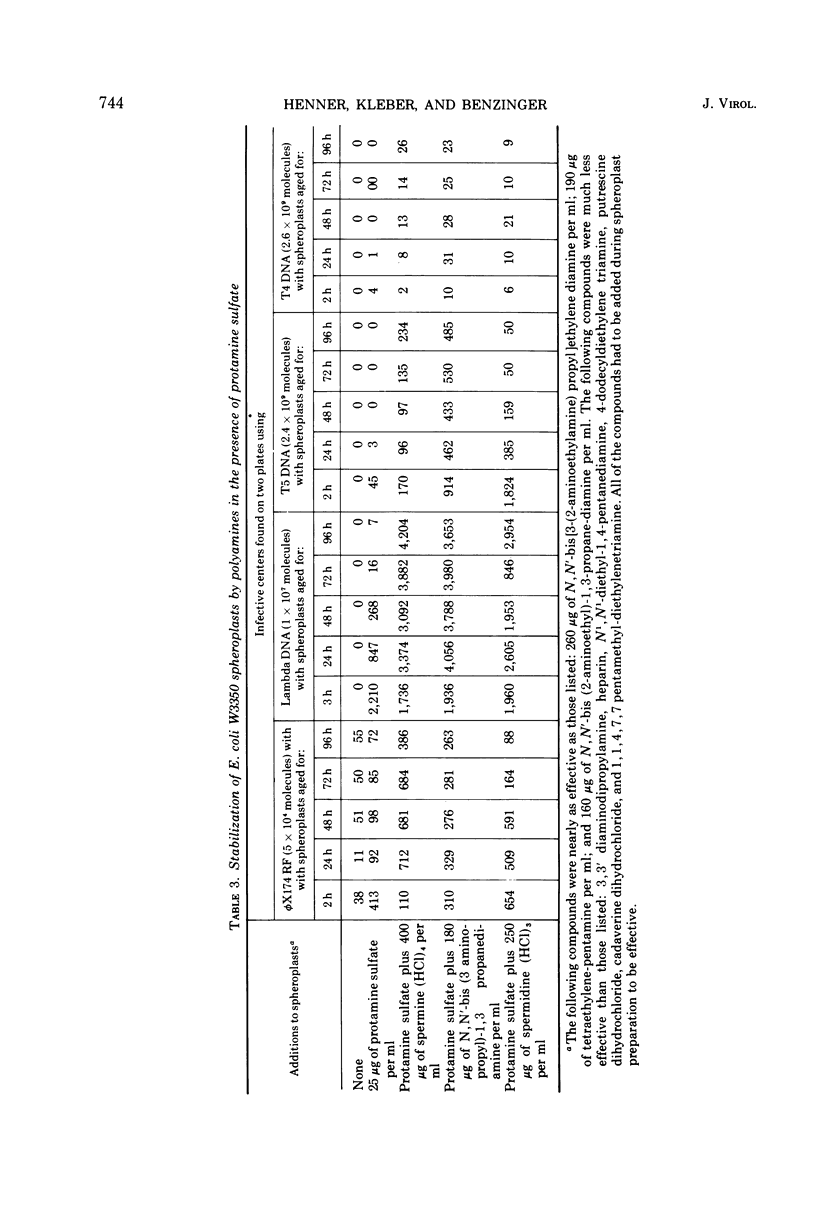
The only compound which fully replaced protamine sulfate in facilitating transfection of Escherichia coli spheroplasts by phage DNAs was spermine; poly-l-lysine, poly-l-arginine, DEAE-dextran, histones, and many other polyamines were only slightly effective. Higher-molecular-weight compounds were effective at lower concentrations, and each compound had a sharp concentration optimum. The specificity of the facilitation of transfection is discussed in light of Leonard and Cole's (1972) isolation of a polyamine- or protamine-like, natural competence factor from Streptococci. By standardizing growth conditions for spheroplast cultures, storing spheroplasts in minimal medium, and adding both protamine sulfate and polyamines to spheroplasts, reproducible competence levels were obtained. Thus, 95% of all spheroplast preparations gave efficiencies of transfection between 10−3 and 3 × 10−4 for lambda DNA; between 10−6 and 3 × 10−8 for T7 DNA; and between 3 × 10−6 and 10−7 for T5 phage DNA. The stability of the spheroplasts was extended from 10 h to between 2 and 5 days, depending on the DNA used for transfection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki S., Takebe I. Infection of tobacco mesophyll protoplasts by tobacco mosaic virus ribonucleic acid. Virology. 1969 Nov;39(3):439–448. doi: 10.1016/0042-6822(69)90092-0. [DOI] [PubMed] [Google Scholar]
- Baltz R. H. Infectious DNA of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):425–437. doi: 10.1016/0022-2836(71)90146-x. [DOI] [PubMed] [Google Scholar]
- Benzinger R., Delius H., Janenisch R., Hofschneider P. H. Infectious nucleic acids of Escherichia coli bacteriophages. 10. Preparation and properties of Escherichia coli competent for infectious DNA from bacteriophages phi X 174 and M 13 and RNA from bacteriophage M 12. Eur J Biochem. 1967 Nov;2(4):414–428. doi: 10.1111/j.1432-1033.1967.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Benzinger R., Kleber I., Huskey R. Transfection of Escherichia coli spheroplasts. I. General facilitation of double-stranded deoxyribonucleic acid infectivity by protamine sulfate. J Virol. 1971 May;7(5):646–650. doi: 10.1128/jvi.7.5.646-650.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger R., Kleber I. Transfection of Escherichia coli and Salmonella typhimurium spheroplasts: host-controlled restriction of infective bacteriophage P22 deoxyribonucleic acid. J Virol. 1971 Aug;8(2):197–202. doi: 10.1128/jvi.8.2.197-202.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Hotz G., Mauser R. Infectious DNA from coliphage T1. I. Some properties of the spheroplast assay system. Mol Gen Genet. 1969;104(2):178–194. doi: 10.1007/BF00272800. [DOI] [PubMed] [Google Scholar]
- Hua S., Mackal R. P., Werninghaus B., Evans E. A., Jr Infectious DNA preparations from T2 and T4 bacteriophages. Virology. 1971 Nov;46(2):192–199. doi: 10.1016/0042-6822(71)90022-5. [DOI] [PubMed] [Google Scholar]
- Koch G., Quintrell N., Bishop J. M. An agar cell-suspension plaque assay for isolated viral RNA. Biochem Biophys Res Commun. 1966 Aug 12;24(3):304–309. doi: 10.1016/0006-291x(66)90155-0. [DOI] [PubMed] [Google Scholar]
- Kohn K. W., Green D. M. Transforming activity of nitrogen mustard-crosslinked DNA. J Mol Biol. 1966 Aug;19(2):289–302. doi: 10.1016/s0022-2836(66)80005-0. [DOI] [PubMed] [Google Scholar]
- Kuo C. H., August J. T. Histone or bacterial basic protein required for replication of bacteriophage RNA. Nat New Biol. 1972 May 24;237(73):105–108. doi: 10.1038/newbio237105a0. [DOI] [PubMed] [Google Scholar]
- Leonard C. G., Cole R. M. Purification and properties of Streptococcal competence factor isolated from chemically defined medium. J Bacteriol. 1972 Apr;110(1):273–280. doi: 10.1128/jb.110.1.273-280.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinlay A. G., Kaiser A. D. DNA replication in head mutants of bacteriophage lambda. J Mol Biol. 1969 Feb 14;39(3):679–683. doi: 10.1016/0022-2836(69)90155-7. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Melechen N. E., Hudnik-Plevnik T. A., Pfeifer G. S. Increased stability and reproducibility of Escherichia coli spheroplasts in the transfection assay of phage lambda DNA with polyethylene glycol instead of sucrose. Virology. 1972 Mar;47(3):610–617. doi: 10.1016/0042-6822(72)90550-8. [DOI] [PubMed] [Google Scholar]
- RAZIN S., ROZANSKY R. Mechanism of the antibacterial action of spermine. Arch Biochem Biophys. 1959 Mar;81(1):36–54. doi: 10.1016/0003-9861(59)90173-0. [DOI] [PubMed] [Google Scholar]
- TABOR H., TABOR C. W. SPERMIDINE, SPERMINE, AND RELATED AMINES. Pharmacol Rev. 1964 Sep;16:245–300. [PubMed] [Google Scholar]
- Tabor C. W. STABILIZATION OF PROTOPLASTS AND SPHEROPLASTS BY SPERMINE AND OTHER POLYAMINES. J Bacteriol. 1962 May;83(5):1101–1111. doi: 10.1128/jb.83.5.1101-1111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackernagel W. An improved spheroplast assay for lambda-DNA and the influence of the bacterial genotype on the transfection rate. Virology. 1972 Apr;48(1):94–103. doi: 10.1016/0042-6822(72)90117-1. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Young F. E. The influence of temperate bacteriophage phi105 on transformation and transfection in Bacillus subtilis. Biochem Biophys Res Commun. 1972 Apr 28;47(2):365–371. doi: 10.1016/0006-291x(72)90722-x. [DOI] [PubMed] [Google Scholar]



