Abstract
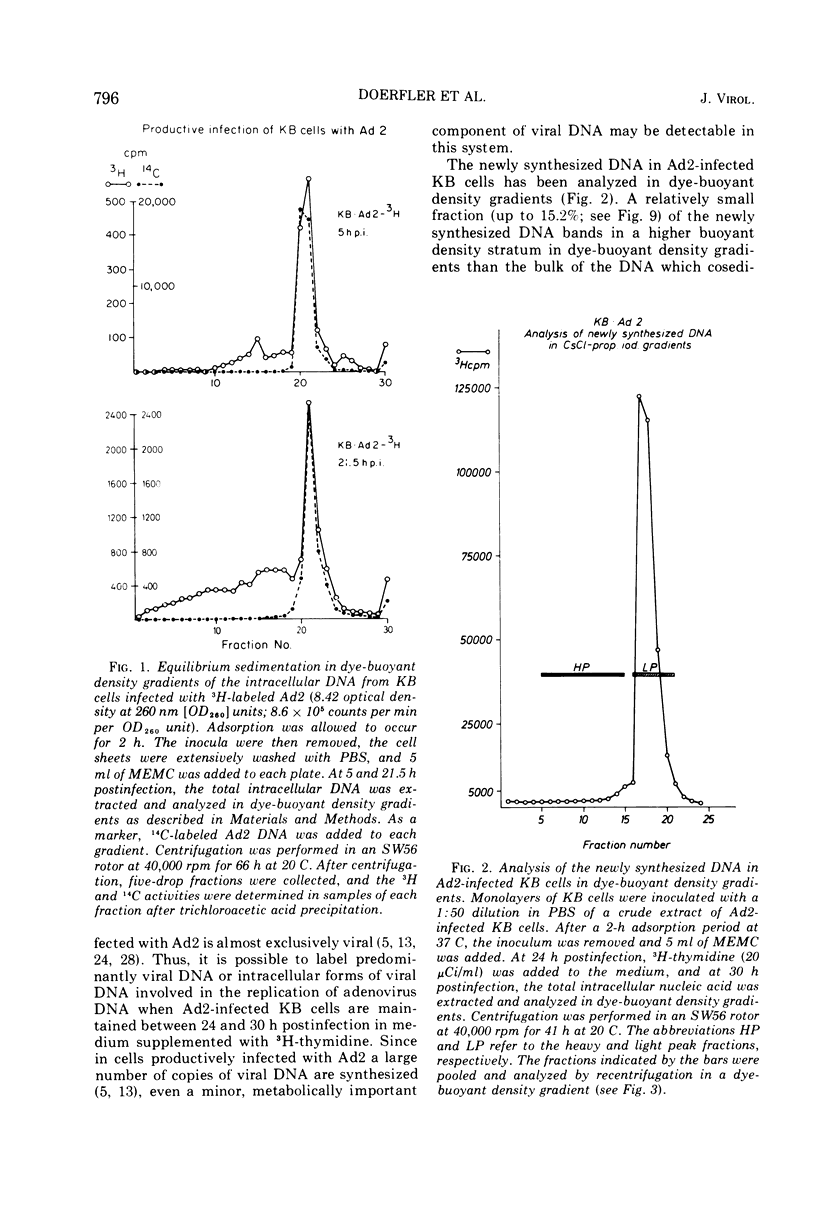
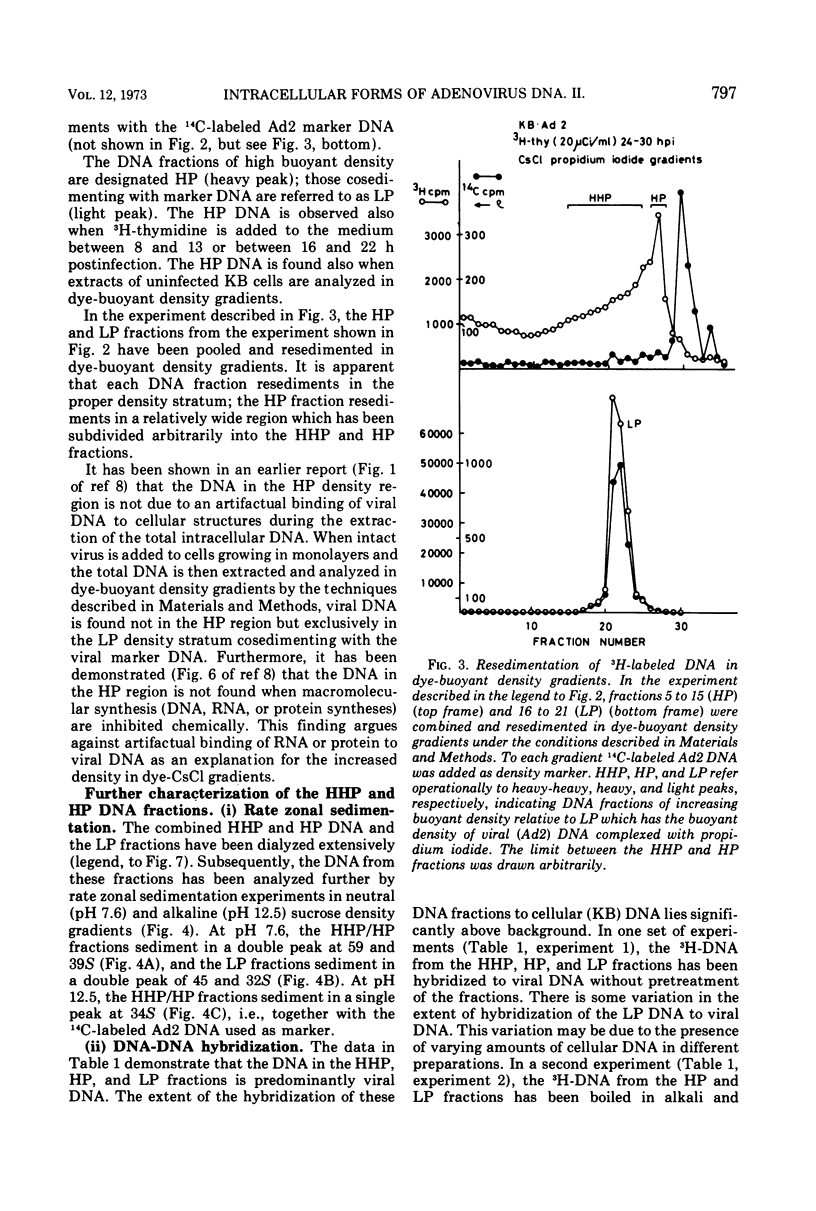
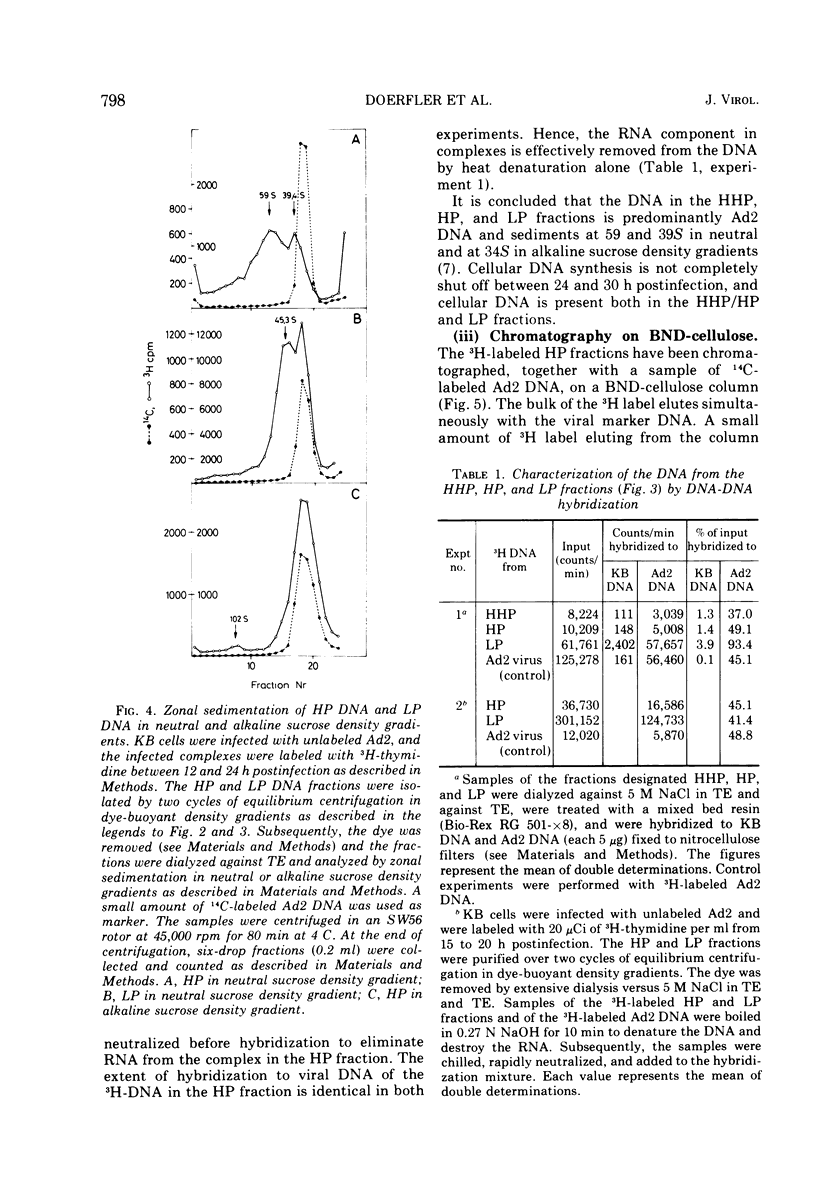
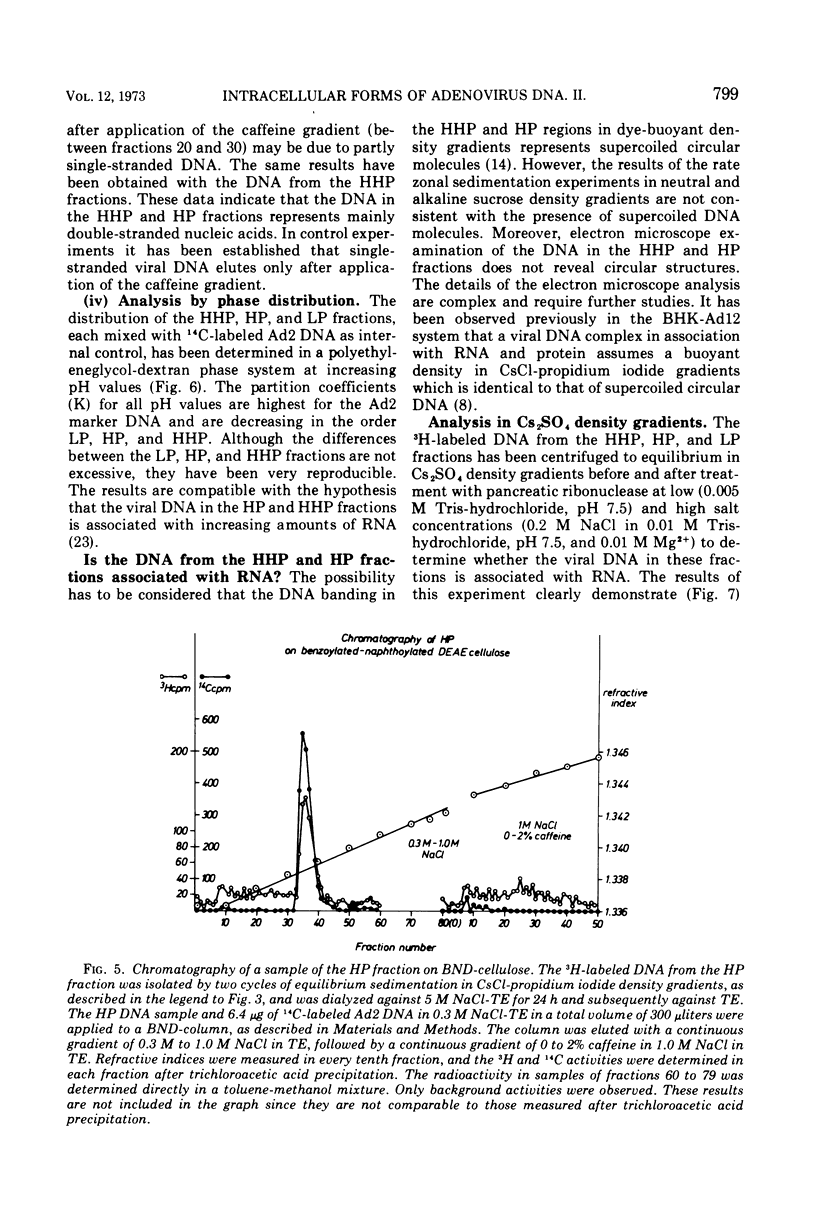
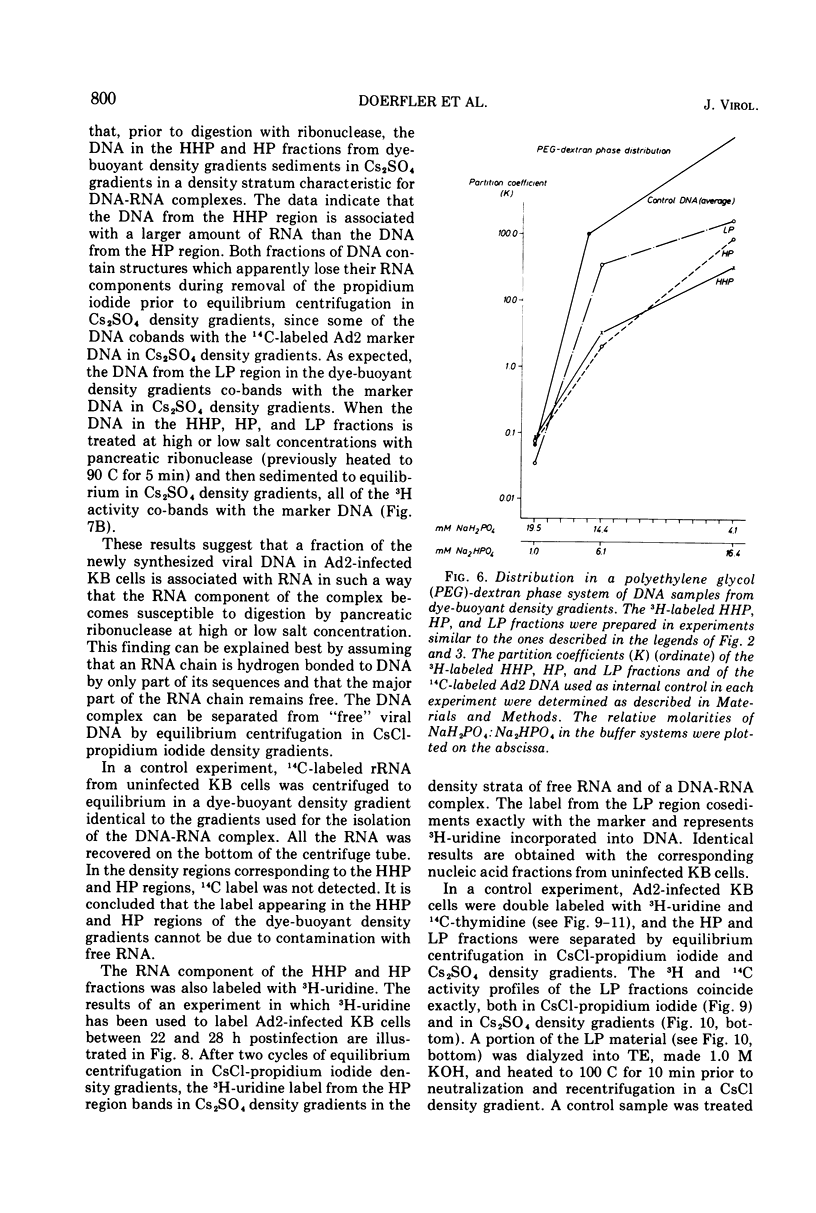
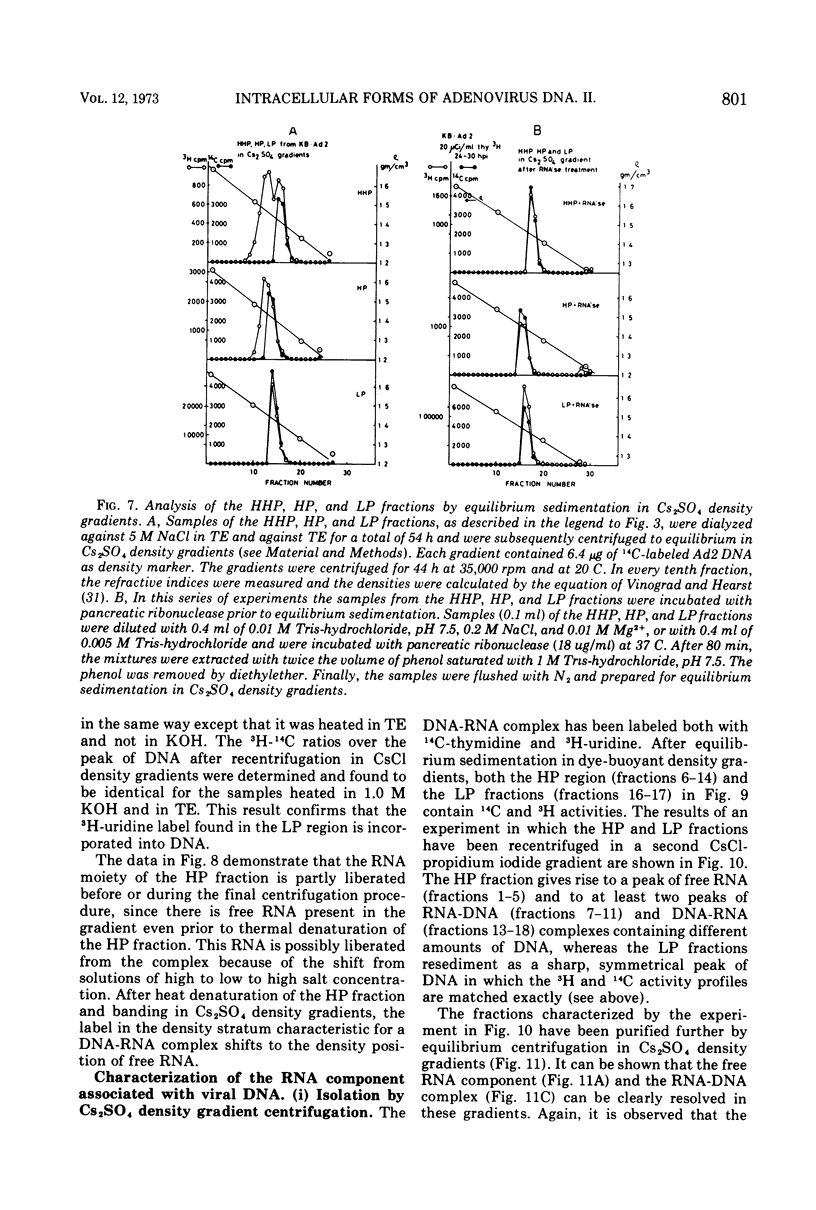
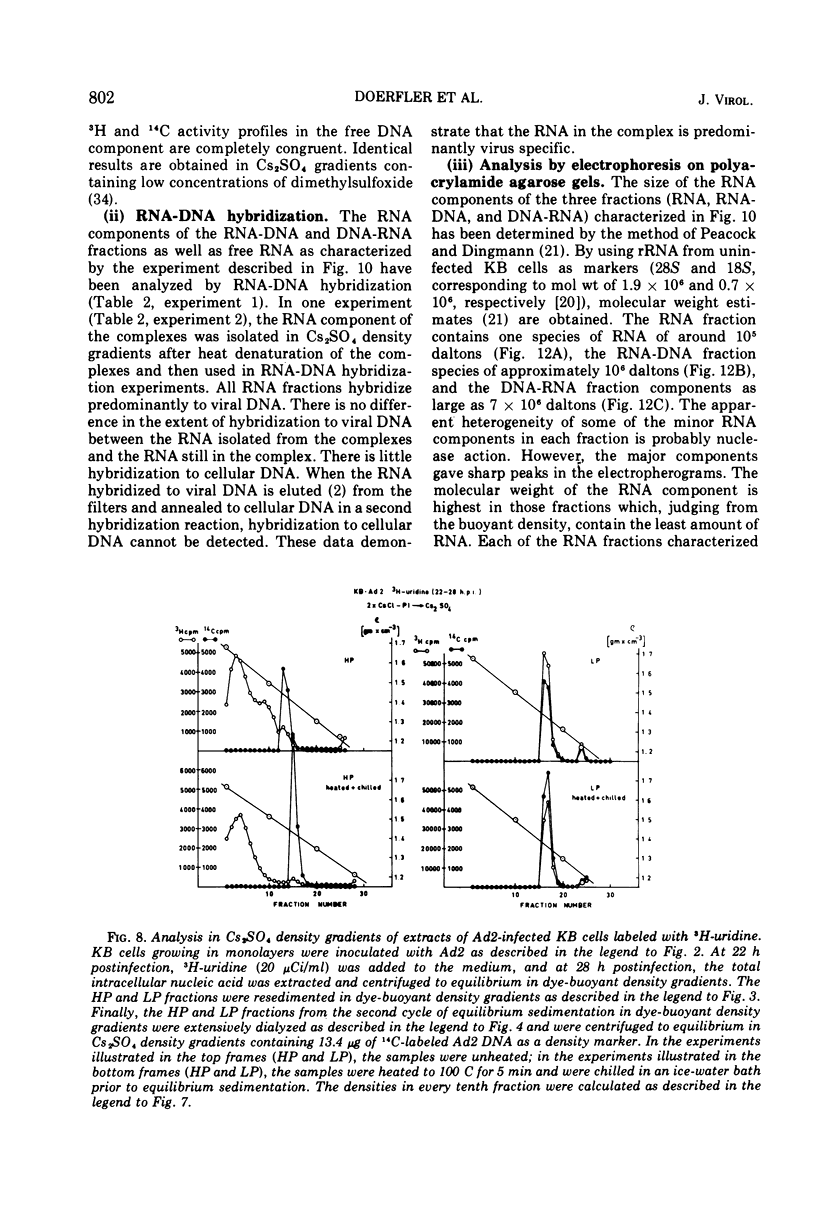
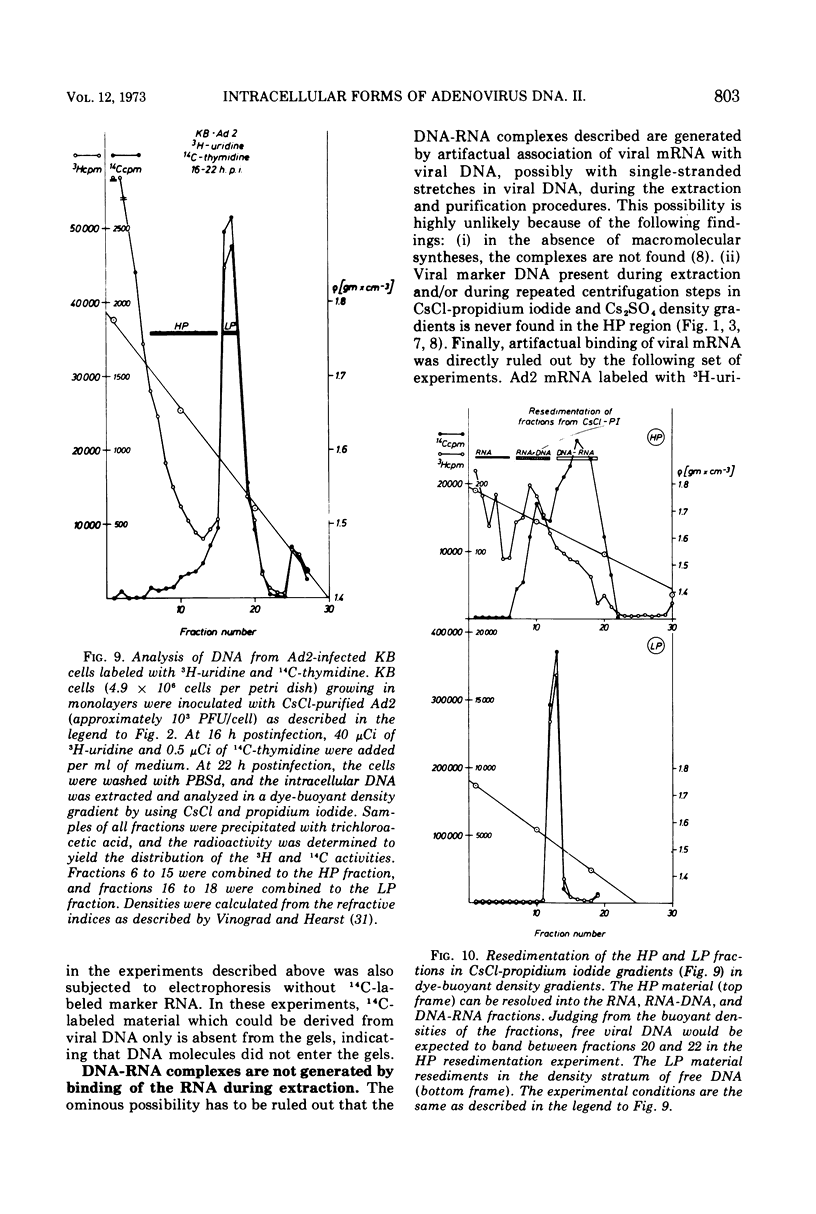
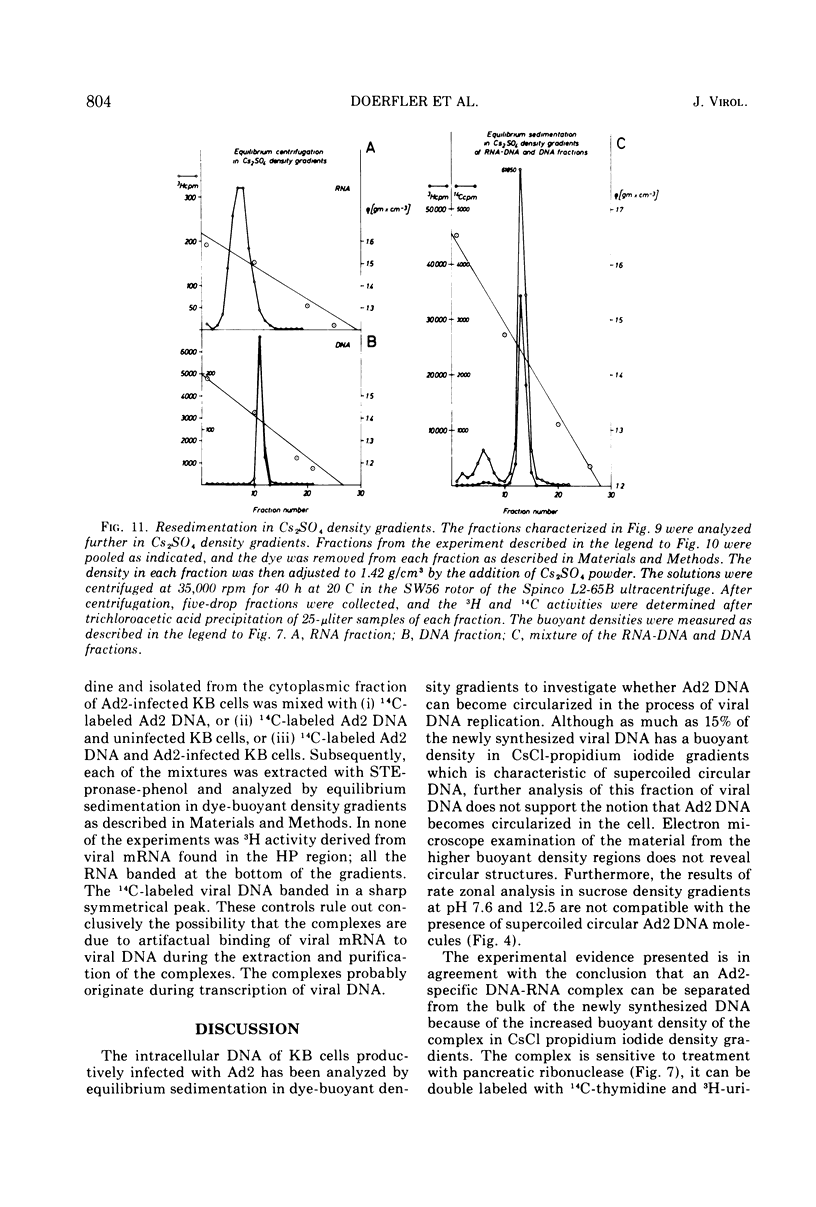
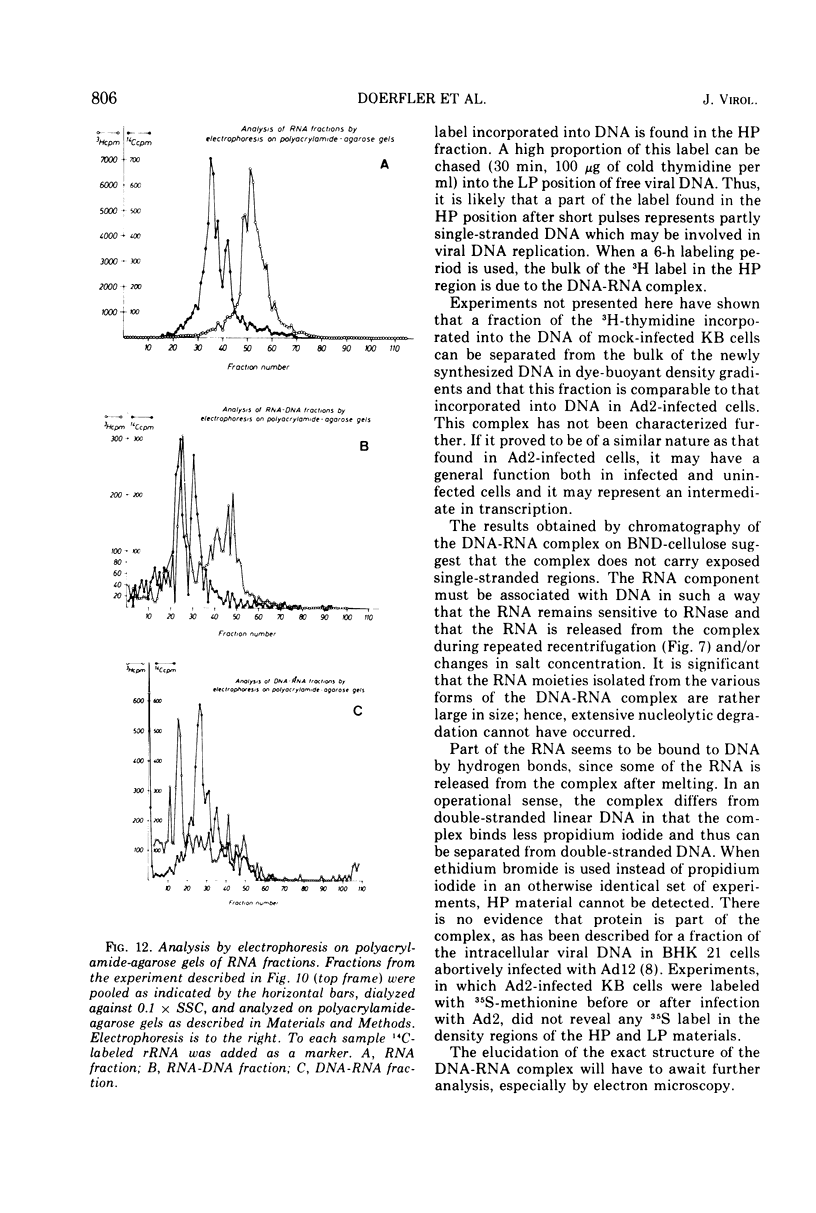
DNA-RNA complexes with different DNA/RNA ratios have been isolated from KB cells productively infected with human adenovirus type 2 by the dye-buoyant density procedure by using propidium iodide. Both the DNA and RNA components of these complexes are virus specific, and parental as well as newly synthesized viral DNA can be recovered from these complexes. The RNA component is susceptible to digestion with pancreatic ribonuclease at low and high salt concentrations. The RNA is in part liberated from the complex during purification over several cycles of equilibrium centrifugation in dye-buoyant density and Cs2SO4 gradients. Analysis in the latter gradients reveals at least four classes of virus-specific nucleic acid: (i) RNA loosely bound and released during purification, (ii) and (iii) two distinct classes of RNA-DNA complexes with different DNA/RNA ratios, and (iv) DNA apparently not associated with RNA. The size of the RNA molecules is large but varying in the different classes of complexes. The biological function of these complexes is still uncertain, but they may be transcription complexes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burlingham B. T., Doerfler W. Three size-classes of intracellular adenovirus deoxyribonucleic acid. J Virol. 1971 Jun;7(6):707–719. doi: 10.1128/jvi.7.6.707-719.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Integration of the deoxyribonucleic acid of adenovirus type 12 into the deoxyribonucleic acid of baby hamster kidney cells. J Virol. 1970 Nov;6(5):652–666. doi: 10.1128/jvi.6.5.652-666.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Kleinschmidt A. K. Denaturation pattern of the DNA of adenovirus type 2 as determined by electron microscopy. J Mol Biol. 1970 Jun 28;50(3):579–593. doi: 10.1016/0022-2836(70)90086-0. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Hirsch-Kauffmann M. Intracellular forms of adenovirus deoxyribonucleic acid. I. Evidence for a deoxyribonucleic acid-protein complex in baby hamster kidney cells infected with adenovirus type 12. J Virol. 1972 Feb;9(2):297–308. doi: 10.1128/jvi.9.2.297-308.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Propagation in a fluid medium of a human epidermoid carcinoma, strain KB. Proc Soc Exp Biol Med. 1955 Jul;89(3):362–364. doi: 10.3181/00379727-89-21811. [DOI] [PubMed] [Google Scholar]
- GREEN M. Studies on the biosynthesis of viral DNA. Cold Spring Harb Symp Quant Biol. 1962;27:219–235. doi: 10.1101/sqb.1962.027.001.022. [DOI] [PubMed] [Google Scholar]
- Gillespie S., Gillespie D. Ribonucleic acid-deoxyribonucleic acid hybridization in aqueous solutions and in solutions containing formamide. Biochem J. 1971 Nov;125(2):481–487. doi: 10.1042/bj1250481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger J. A., Jr, Sinsheimer R. L. Vegetative lambda DNA. IV. Fractionation of replicating lambda DNA on benzoylated-naphthoylated DEAE cellulose. J Mol Biol. 1969 Mar 28;40(3):467–490. doi: 10.1016/0022-2836(69)90166-1. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm U., Doerfler W. Temperature-sensitive mutants of human adenovirus type 12. Virology. 1971 Sep;45(3):827–829. doi: 10.1016/0042-6822(71)90206-6. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Heterogeneity in deoxyribonucleic acids. I. Dependence on composition of the configurational stability of deoxyribonucleic acids. Nature. 1959 May 23;183(4673):1427–1429. doi: 10.1038/1831427a0. [DOI] [PubMed] [Google Scholar]
- McConkey E. H., Hopkins J. W. Molecular weights of some HeLa ribosomal RNA's. J Mol Biol. 1969 Feb 14;39(3):545–550. doi: 10.1016/0022-2836(69)90144-2. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Pearson G. D., Hanawalt P. C. Isolation of DNA replication complexes from uninfected and adenovirus-infected HeLa cells. J Mol Biol. 1971 Nov 28;62(1):65–80. doi: 10.1016/0022-2836(71)90131-8. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. A study o DNA, partially denatured DNA and protein-DNA complexes in the polyethyleneglycol-dextran phase system. Eur J Biochem. 1967 Dec;3(1):25–32. doi: 10.1111/j.1432-1033.1967.tb19495.x. [DOI] [PubMed] [Google Scholar]
- Piña M., Green M. Biochemical studies on adenovirus multiplication. XIV. Macromolecule and enzyme synthesis in cells replicating oncogenic and nononcogenic human adenovirus. Virology. 1969 Aug;38(4):573–586. doi: 10.1016/0042-6822(69)90178-0. [DOI] [PubMed] [Google Scholar]
- Sugino A., Hirose S., Okazaki R. RNA-linked nascent DNA fragments in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1863–1867. doi: 10.1073/pnas.69.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C., Ellens D. J., Jansz H. S. Linear intermediates in the replication of adenovirus DNA. Nat New Biol. 1972 Sep 13;239(89):47–49. [PubMed] [Google Scholar]
- Takahashi M., Ogino T., Baba K., Onaka M. Synthesis of deoxyribonucleic acid in human and hamster kidney cells infected with human adenovirus types 5 and 12. Virology. 1969 Apr;37(4):513–520. doi: 10.1016/0042-6822(69)90269-4. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]
- Wallace R. D., Kates J. State of adenovirus 2 deoxyribonucleic acid in the nucleus and its mode of transcription: studies with isolated viral deoxyribonucleic acid-protein complexes and isolated nuclei. J Virol. 1972 Apr;9(4):627–635. doi: 10.1128/jvi.9.4.627-635.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. E., Vinograd J. The buoyant behavior of RNA and DNA in cesium sulfate solutions containing dimethylsulfoxide. Biochim Biophys Acta. 1971 Jan 28;228(2):423–439. doi: 10.1016/0005-2787(71)90048-7. [DOI] [PubMed] [Google Scholar]
- van der Eb A. J. Intermediates in type 5 adenovirus DNA replication. Virology. 1973 Jan;51(1):11–23. doi: 10.1016/0042-6822(73)90361-9. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Sussenbach J. S. The mechanism of adenovirus-DNA synthesis in isolated nuclei. Eur J Biochem. 1972 Nov 7;30(3):584–592. doi: 10.1111/j.1432-1033.1972.tb02130.x. [DOI] [PubMed] [Google Scholar]



