Abstract
Tobacco use, especially cigarette smoking, is higher than average in persons living with HIV/AIDS (PLWHA). The Public Health Service Clinical Practice Guideline for Treating Tobacco Use and Dependence states that, during every medical encounter, all smokers should be offered smoking cessation counseling, along with approved medications. The Guideline also recognizes PLWHA as a priority population, given the scarcity of research on effective cessation treatments in this group. The scant evidence suggests that conventional treatments, though worthwhile, are not as successful as might be hoped for. The reasons for this are not entirely clear, but may have to do with the complex array of medical and psychosocial factors that complicate their lives. Clinicians should consider re-treatment strategies for those patients who encounter difficulty when quitting smoking with conventional approaches, switching or augmenting treatments as needed to minimize adverse experiences, and to maximize tolerability, adherence, and cessation outcomes.
Keywords: Tobacco, Cigarette smoking, Smoking cessation, HIV/AIDS, Treatment, Adaptive treatment strategy, Bupropion, Varenicline, People living with HIV/AIDS (PLWHA), Behavioral aspects of HIV/AIDS
Introduction
Smoking is the leading cause of preventable morbidity and mortality in the U.S. [1]. Among PLWHA, smoking prevalence is up to 3 times that of the general population [2-4]. Despite high levels of interest in quitting and past quit attempts [2, 5, 6], few PLWHA are able to sustain abstinence [2, 7, 8]. Smoking creates well-known risks for conditions such as cancer, stroke, heart disease, and chronic obstructive pulmonary disease [9•]. It also poses additional threats to PLWHA, such as pulmonary-related complications (bronchitis, pneumonia, and asthma) and increased incidence of opportunistic infections [10-15]. In addition to low baseline CD4 count and older age, current smoking is a strong independent predictor of mortality in PLWHA [16]. In the Strategies for Management of Antiretroviral Therapy clinical trial [17], the population attributable risk % for current vs former and never smokers combined was 24.3 % for overall mortality, 25.3 % for major cardiovascular disease, 30.6 % for non-AIDS cancer, and 25.4 % for bacterial pneumonia. In a prospective, multi-cohort, multinational observational study (n=27,136), the adjusted incidence rate ratio of cardiovascular disease decreased from 2.32 to 1.49 after 3 years for patients who stopped smoking during follow-up, compared with those who never smoked [18].
Because smoking has an adverse impact upon physical and psychological functioning [19], smoking cessation among HIV/AIDS patients may also enhance quality of life and disease management efforts. Among PLWHA length of smoking abstinence has been significantly associated with a reduction in self-reported HIV-symptom burden [20]. Smoking itself has been associated with onset and progression of dementia [21]. Cigarette smoking is also associated with a number of mental disorders [22, 23], including other substance use and dependence. In addition to a greater prevalence of smoking, those with co-occurring mental disorders also find it more difficult to quit [24], further complicating cessation among PLWHA with co-occurring conditions.
Smoking may also interfere with the therapeutic effects of antiretroviral treatments (ART). Compared with non-smokers, women smokers on ART had lower immunological and virological response to treatment and a higher risk of viral rebound, death, and AIDS, even after adjusting for confounders [25]. Smokers are also less likely to adhere to ART regimens compared with nonsmokers [26]. Among HIV + smokers, nicotine dependence, illicit drug use, and alcohol use are associated with poor ART adherence [27].
PLWHA may face additional obstacles to quitting smoking, including co-occurring illicit drug and alcohol use, high prevalence of psychiatric comorbidity, limited socioeconomic resources, and diminished access to care [28, 29]. Race may also play a role in the type and quality of smoking cessation care received, as it does for treatment of HIV/AIDS. For example, African Americans have less access to HIV treatment and worse treatment outcomes than the overall population [30]. African American smokers are also less likely to be screened by medical providers for tobacco use, receive advice to quit, or use cessation aids during a quit attempt [31].
Given the consequences of smoking among PLWHA, and the benefits of cessation, the 2008 PHS Guideline for Treating Tobacco Use and Dependence has called for studies specifically on the effectiveness of medications, counseling, and tailored interventions among PLWHA [32]. This directive recognizes that we cannot assume that a therapy tested in the general population is safe, tolerable, and effective in this unique and vulnerable patient population. Studies of smoking cessation interventions specific to the complex psychosocial/medical needs of this vulnerable patient population are essential.
Smoking Cessation in PLWHA
Despite considerable evidence of efficacy in otherwise healthy smokers, the few studies of various medication and behavioral treatments (and their combinations) thus far appear to show mixed results in PLWHA. We now briefly review published intervention studies which were retrieved via search on PUBMED (search terms: tobacco, smoking, cessation, HIV, AIDS HIV). Study design characteristics and results are summarized in Table 1.
Table 1.
Smoking cessation intervention trials for HIV + smokers
| Author (year) | Design | Interventions | n | Follow-up | Outcome- quit |
|---|---|---|---|---|---|
| Cui (2011) | NR | Varenicline | n=36 | 3 mo (bcv) | 15/36 (42 %) |
| Elzi (2006) | NR | Counseling+NRT; No tx control | n=34 | 12 mo (sr) | 13/24 (38 %) |
| n=383 | 27/383 (7 %) | ||||
| Ingersoll (2009) | R | NRT+self-help; NRT+MOT | n=18 | 3 mo (bcv) | 9/40 (22 %) –overall, ns group difference |
| n=22 | |||||
| Lloyd-Richardson (2009) | R | NRT+MOT; NRT+Standard care | n=232 | 6 mo (bcv) | 21/232 (9 %) |
| n=212 | 21/212 (10 %) | ||||
| Moadel (2012) | R | Group treatment; Standard care | n=73 | 3 mo (bcv) | 14/73 (19.2 %) |
| n=72 | 7/72 (9.7 %) | ||||
| Pedro-Clotet (2006) | NR | Bupropion | n=21 | 12 mo (sr) | 8/21 (38 %) |
| Tornero (2009) | NR | Varenicline | n=22 | 6 mo (bcv) | 5/21 (24 %) |
| Vidrine (2011) | R | Cell phone counseling; Standard care | n=236 | 3 mo (bcv) | 21/236 (8.9 %) |
| N=238 | 7/238 (2.9 %) |
NR nonrandomized, R randomized, bcv biochemical verification, sr self-report, MOT motivational intervention.
In a non-randomized comparison control study, HIV + smokers interested in treatment (n=34) received nurse-delivered individual (30 minutes sessions once weekly during the first month and once monthly thereafter for 12 months) plus nicotine replacement therapy (NRT) [33]. Self-reported smoking abstinence at 12 months was 13/34 (38 %) in the intervention group and 27/383 (7 %) in the observational control group.
Ingersol and colleagues randomized smokers to self-guided reading plus nicotine patch (n=18) or motivational interviewing (single session) plus nicotine patch (n=22) [34]. There were no differences in 3-month cessation outcomes (rates not reported by treatment group), and overall nicotine patch use was reported to be suboptimal (37 %–63 % use on prescribed days).
Larger randomized controlled trials (RCTs) have shown modest results for behavioral treatments combined with NRT. Vidrine et al tested a cell-phone behavioral intervention plus usual care (UC; including access to NRT) vs standard care alone in 474 HIV + smokers [35]. Participants randomized to the cell phone group were given a prepaid cell phone on which a series of 11 proactive counseling sessions were conducted spanning a 3-month period. The cell phone group had a higher rate of 30-day abstinence at the 3-month follow-up compared with the standard care group (8.9 % vs 2.9 %; P<0.005), but this effect was diminished and no longer significant at 6-months.
A cohort of 145 PLWHA smokers was randomized to standard care to an 8 session group treatment program co-facilitated by a graduate student and an HIV-infected peer, and tailored to address the needs and concerns of HIV-infected smokers [36]. All were offered a 3-month supply of NRT. In an intention to treat analysis, 7-day point-prevalence abstinence at 3 months was 19.2 % for the intervention vs 9.7 % for those who received standard care (P=0.11).
In an RCT comparing behavioral treatment approaches, 444 HIV + smokers who received physician advice and the option of NRT were randomized to either a brief behavioral intervention (Standard Care; SC), or a more intensive motivational-counseling intervention (Motivational Enhancement; ME) [37]. Biochemically-verified 7-day abstinence rates at 2-, 4-, and 6-month follow-ups were 12 %, 9 %, and 9 % respectively, in the ME condition, and 13 %, 10 %, and 10 % respectively, in the SC condition. Although there were no treatment group differences at any of the follow-ups, African Americans faired particularly poorly at 6-months (5 % cessation). Overall, failure to use the nicotine patch during the study was a strong predictor of smoking at 6-month follow-up (failure to use the patch=0 % abstinence) [38]. The need to improve adherence to cessation treatment therefore is clear; even optimal treatment will have little impact if it is not used as intended.
Although promising, the safety, tolerability, and efficacy of 2 FDA-approved first line medications, bupropion and varenicline, have only been evaluated in small, uncontrolled single arm trials.
Twenty-one HIV + patients using ART were treated with bupropion, with 38 % reporting quitting for more than 1 year [39]. No clinically significant drug interactions were noted.
In a study of 18 PLWHA smokers on ART who were treated with varenicline for 2 months, 3, and 6 month abstinence rates were 28 % and 24 %, respectively. Five patients reported nausea and 6 reported sleep disturbance but none discontinued treatment [40]. In an open-label study, 36 HIV + smokers were treated with a standard 12-week course of varenicline [41]. The cotinine-verified 4-week continuous abstinence rate through weeks 9–12 was 42 %. The most frequently reported adverse events (AEs) were nausea (33 %), abnormal dreams (31 %), affective lability (19 %), and insomnia (19 %). CD4 counts increased by 69 cells/mm3 (P=0.001) at week 24. Six (17 %) patients discontinued varenicline due to AEs. Abstinence rates and the types of AEs were comparable to those observed in generally healthy HIV-negative smokers; the rates of AEs were somewhat higher, suggesting the importance of close clinical monitoring during treatment and possible overlap in AEs due to varenicline, HIV/AIDS medication treatments, and disease symptoms.
Compounding the paucity of efficacy studies in PLWHA is a failure on the part of HIV care providers to provide even the standard of care for smoking cessation [28]. In the US HIV Medical Association registry, only 22.9 % of providers had ever received formal tobacco treatment training [42]. While almost all providers agreed that smoking is an important issue in PLWHAs, they reported low levels of cessation-promoting activities. The relative lack of evidence for efficacy in PLWHA may contribute to providers’ perception that available cessation treatments do not work well or are not well tolerated and are therefore not likely to be recommended. Given the suboptimal cessation rates in PLWHAwith conventional treatments such as NRT and behavioral counseling, other cessation strategies are in need of rigorous evaluation.
Unmet Clinical and Research Needs
The paucity of cessation research in PLWHA points to a need for further evaluation of behavioral and approved pharmacotherapies, singly, in combination and with various forms of behavioral support. Suboptimal results in studies conducted so far also suggest that single courses of treatment may not adequately serve the needs of PLWHA for whom quitting is apparently exceedingly difficult and may require more time and effort. There is no firm guidance regarding choice and implementation of multiple courses of treatment for smoking cessation. In what follows we review the limited evidence for sequential treatments, and illustrate how such treatments might be implemented in the context of a sequential treatment trial design. We focus on the combined use of 2 approved, first line pharmacologic agents, bupropion and varenicline, which, although they carry potential risks, demonstrate considerable efficacy in non-medical populations of smokers. We present treatment options/sequences in the context of a trial design because this provides a formal framework for thinking about how such treatments can be implemented in a clinical context.
The PHS clinical guideline makes it clear that all smokers trying to quit should be offered medication (except when contraindicated or for specific populations for which there is insufficient evidence of effectiveness), but what follows is less clear. Approved first line medications should be chosen initially, and clinicians should consider medications shown to be more effective than the nicotine patch alone (eg, varenicline). The guideline goes on to state: “Unfortunately, there are no well-accepted algorithms to guide optimal selection among the first-line medications… Prior successful experience… suggests that the medication may be helpful to the patient in a subsequent quit attempt, especially if the patient found the medication to be tolerable and/or easy to use. It is difficult to draw firm conclusions from prior failure with a medication… Some evidence suggests that re-treating relapsed smokers with the same medication produces small or no benefit, whereas other evidence suggests that it may be of substantial benefit.” Thus, there is no firm guidance on the best course of action following a failed quit attempt.
Most smokers, particularly smokers with a host of comorbid medical and psychosocial issues, fail first line treatments [43]. For those who have failed, a different approach is required, (eg, re-treatment by augmenting or switching to a different treatment). For those who have quit, it is unclear whether continued (maintenance) treatment will help to prevent relapse. As the guideline points out, little is known about how best to maintain success after initial treatment ends, and what are optimal re-treatments following failure. Key questions, therefore, are: (1) how to maintain abstinence once it has been achieved (is continued treatment beneficial?), and (2) how to address relapse (is re-treatment beneficial?).
Maintenance Treatment (Relapse Prevention)
Risk for smoking relapse is highest in the first few weeks following a quit attempt [43, 44]. Importantly, risk does not reduce to zero, even after extended periods of abstinence. Among former smokers abstinent for at least 1 year, about 10 % relapse each year thereafter [45]. In the Swiss HIV cohort, the rate of smoking cessation over 9 years was 4.4 % per year; however, 44 % relapsed at least once during the period of follow-up [46].
Meta-analytic reviews of relapse prevention studies have considered interventions that seek to reduce relapse after an acute treatment phase, or at some time after a self-quit attempt [47, 48•]. Relapse prevention trials typically randomize smokers who have already quit, or they randomize smokers prior to quitting and provide a general smoking cessation intervention to all participants in addition to an extra component provided for those randomized to relapse prevention [47].
Relapse prevention interventions initiated prior to quitting have not been shown to be effective [47, 48•]. There is also little evidence that behavioral interventions initiated prior to or after quitting are effective, with the possible exception of self-help materials in unaided quitters [48•]. There is stronger evidence that pharmacologic interventions initiated or continued after quitting are effective maintenance strategies.
Relapse prevention with NRT improves maintenance of abstinence after smokers have quit on their own [49, 50] but not after treatment with NRT or bupropion [51, 52]. In contrast, bupropion and varenicline are effective in preventing relapse following active treatment. In an analysis of data pooled across 4 studies, maintenance treatment with bupropion was superior to placebo after initial treatment with either open-label bupropion and/or nicotine patch [48•, 51-55]. A single trial tested the efficacy of continued varenicline as a maintenance strategy. Smokers who had quit after 12 weeks of varenicline were randomized either to stay on varenicline or switch to placebo for an additional 12 weeks [56]. Maintenance with varenicline significantly increased the odds of abstinence after 6 months (OR 1.40; 95 % CI 1.12–1.76). Therefore, among approved medications, bupropion and varenicline currently offer the best options for maintenance treatment.
Re-Treatment
Remarkably little attention has been directed to ascertaining whether smokers who have failed with prior treatment: (1) are interested in trying again and, if so, how soon and with what methods; (2) will respond positively to re-treatment.
Re-treatment strategies only make sense if smokers are willing to try to quit again relatively soon after quitting. Two studies have surveyed patients who had been prescribed NRT or bupropion for smoking cessation. Among continuing smokers, 98 % were willing to make another quit attempt, 78 % within 1 month [57]. The majority preferred to try a new medication regimen. In the second study [58], two-thirds of relapsed smokers were interested in retreatment within 30 days. Of these, 91 % wanted behavioral or pharmacologic smoking cessation treatment, and 64 % wanted behavioral and pharmacologic treatment. Predictors of interest in re-treatment included black race, lower smoking level, and greater number of smoking-related medical conditions. In a longitudinal cohort study, every 6 months over 2 years, smokers were asked if they wanted to receive re-treatment with NRT or bupropion [59]. Among not quit or relapsed smokers, 46 %–65 % opted for re-treatment at some interval. Pharmacotherapy use (vs no use) was associated with the probability of quitting (OR=1.99, P=0.002), but quitting was not related to the number of previous pharmacotherapy-assisted quit attempts.
It therefore appears that interest in re-treatment is high, and when given the opportunity, most relapsed smokers will try again. Most smokers express a willingness to try to quit soon after a failed attempt, are interested in pharmacologic treatment with or without behavioral treatment, and are more likely to succeed if they are re-treated.
Re-treatment strategies are embedded in many algorithmic guidelines for smoking cessation, in which smokers are initially assigned (or matched) to treatment based upon pretreatment characteristics (eg, nicotine dependence; psychiatric comorbidity), stepped-up to more intensive treatments if they fail, or both [60-63]. Some approaches begin with a minimally intensive therapy, stepping-up to more intensive or other types of therapy as needed [64-66]. Little attention has been directed to testing whether algorithmic stepped-care treatment strategies are more efficacious compared with single courses of treatment. Few studies in particular have systematically manipulated re-treatment contingent on cessation difficulties.
In several uncontrolled studies, patients who failed to quit smoking initially using the nicotine patch were offered another course of NRT [67-69], with 6-month quit rates ranging between 0 %–20 %. In the single randomized trial, smokers who failed with NRT received either an additional 12 weeks of active or placebo patch [70]. Ninety four percent of eligible subjects agreed to participate in retreatment. At 12 weeks, 6.7 % of subjects allocated to active treatment had stopped smoking compared with 1.9 % allocated to placebo (P=0.003). Thus, smokers who fail to quit using NRT do not fare particularly well if they are re-treated with NRT, although outcomes are better than if they receive a placebo. The addition of behavioral re-treatment following initial treatment with NRT does not appear to confer an advantage [71].
Studies have also examined re-treatment with bupropion. Among prior bupropion treatment failures, 27 % re-treated with bupropion remained abstinent from weeks 4–7 vs 5 % of smokers receiving placebo (P<0.001) [72]. In another study, nicotine inhaler treatment failures that were randomized to re-treatment with bupropion fared better than retreatment with a placebo [51]. Thus, existing evidence supports re-treatment with bupropion.
Adaptive Treatment Strategies (ATS)
Standard RCTs have been criticized as being ill-suited to investigate treatments for chronic conditions (eg, HIV infection, diabetes) that require long-term monitoring and changes in treatment as the patient’s history of illness and response to previous treatment evolves [73, 74]. The result has been a call for studies of adaptive treatment strategies (also known as dynamic treatment regimes): rules that change treatment according to response vs nonresponse [74]. This is no less relevant for smoking cessation, as tobacco dependence has been characterized as a chronic relapsing condition that may require long-term treatment and/or treatment with multiple pharmacologic and behavioral aids [75, 76].
Typically, RCTs for smoking cessation test a single stage intervention against placebo or no treatment/usual care for some fixed period of time, and then follow patients after treatment ends, documenting the rate of treatment effect decay. Such RCTs are limited because they ignore the need for sustained and dynamic treatments and because they do not conform to common clinical practice [76]. Clinicians treat patients in multiple sequences, choosing the next treatment according to patient response [77]. Recent advances in design and analysis of adaptive treatment strategies (ATS) can yield optimal decision rules regarding the choice of initial and subsequent treatments to guide real-world clinical decision-making. ATS offers a way to operationalize and test the sequential, adaptive decision-making process. An ATS is a series of decision rules, 1 per stage of intervention. These rules take into account patient characteristics and treatment responses observed up to each point as input, and then recommend the next treatment course. An ATS therefore defines the treatment sequence resulting in the optimal clinical outcome: maximizing the mean long-term outcome (eg, proportion successful) observed at the end of the final stage of treatment, given prior treatment history, and other patient characteristics.
Studies of ATS are becoming more common [74, 77-79]. One strategy that lends itself well to evaluating staged sequences of treatment is the sequential multiple assignment randomization trial (SMART), [66, 80, 81, 82•] used in building and refining ATSs. In a SMART design, subjects are randomized at selected decision points, for example, to initial type of treatment, and again to subsequent treatments contingent on initial response or nonresponse [66]. SMART designs are being used to study sequential treatments in depression, cancer, diabetes, and substance abuse, but their use is not yet widespread [66, 83-88].
An ATS for smoking cessation among HIV + smokers has immediate clinical relevance given the following assumptions and constraints: (1) there is a pressing need to find the best treatments to maximize success, especially in high risk and hard-to-treat populations such as PLWHA; conventional single stage treatment RCTs are inefficient and time consuming; (2) safe and effective treatments exist, but their optimal use has not been established; (3) an ATS should optimize eventual outcomes after initial success and failure - conventional RCTs rarely make such a distinction and have not typically investigated simultaneously maintenance and re-treatment strategies; (4) results of ATS studies will have direct and immediate clinical applications, as they will help clinicians and patients decide what treatments (if any) should follow initial success or failure.
Unlike single treatment RCTs, ATS can detect positive or negative synergistic effects between successive treatments. An example of positive synergy is the delayed therapeutic effect [84], wherein treatment A may lay the foundation for an enhanced effect of certain subsequent treatments. Treatment A (eg, bupropion) may not appear best initially but may have enhanced long term effectiveness when followed by a certain maintenance treatment. Negative synergy is where treatment A may produce a higher proportion of responders (eg, varenicline) but also result in side effects that reduce the options for subsequent treatments for those who do not respond. Or the burden imposed by treatment A may be sufficiently high so that nonresponders are less likely to adhere to subsequent treatments.
Example
In this example, the efficacy of 2 sequential stages of treatment, with 2 different medications (bupropion and varenicline), are tested (see Fig. 1). Smokers are randomized initially to receive a first course of varenicline or bupropion. Relapsers are then randomized to re-treatment by augmenting with bupropion/varenicline, or switching to bupropion/varenicline. Quitters are then randomized to receive maintenance treatment with another course of varenicline/bupropion or to stop treatment.
Fig. 1.
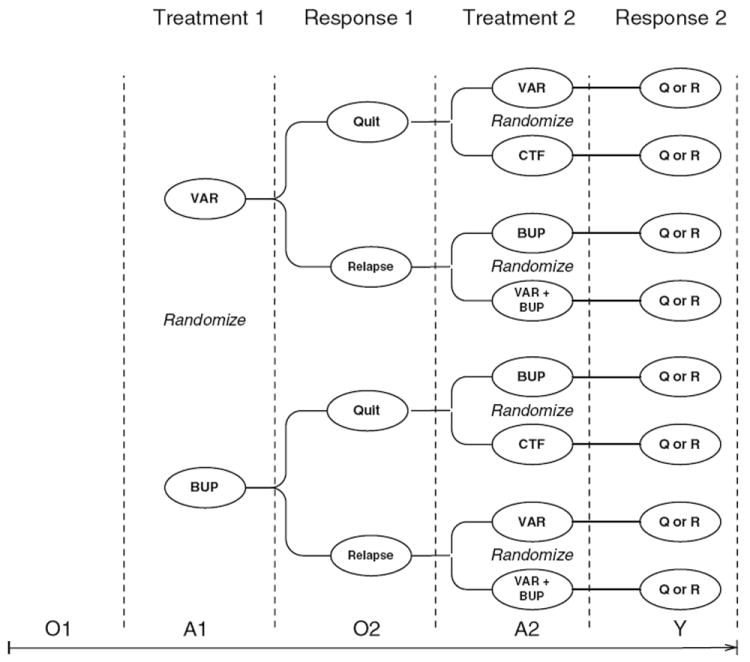
Adaptive treatment strategy example trial design. VAR = varenicline; BUP = bupropion; CTF = continue to follow; Q=quit, R=relapse; O1 denotes all of the pretreatment information at the beginning of stage 1, A1 is the treatment assigned at stage 1, O2 denotes all of the intermediate observations made on the patient prior to treatment at the beginning of stage 2, A2 is the treatment assigned at stage 2, and Y is the primary outcome
The evidence supporting the recommendations for first-line treatments is summarized in the guideline and the Cochrane Library meta-analytic reviews: The relative risk (RR) for varenicline vs placebo is 2.31 (95 % CI: 2.01–2.66; 10 trials, n=4443) [89]. When used as the sole pharmacotherapy, bupropion significantly increases long-term cessation (RR: 1.69; 95 % CI: 1.53–1.85; 36 trials, n=11,140) [90]. The RR for varenicline vs bupropion at 1 year is 1.52 (95 % CI: 1.22–1.88; 3 trials, n=1622) [89].
This example proposes to test 2 approved treatments, varenicline and bupropion, that produce superior short- and long-term outcomes compared with placebo and NRT, are familiar to clinicians, are demonstrably safe and tolerable, and could be incorporated into standard medical practice with insurance plan coverage. Their mechanisms of action and metabolism are also different and potentially complementary. Buproprion’s effects are mediated by its ability to block the reuptake of norepinephrine and dopamine in the mesolimbic system and nucleus accumbens, key brain areas for nicotine reinforcement [91]. By contrast, varenicline is a highly selective →4-β2 nicotinic acetylcholinergic receptor (nAChR) partial agonist. Thus, the effects of varenicline and bupropion can be complementary at the neural level. Varenicline is not oxidatively metabolized in the liver, but rather is primarily excreted unchanged in urine, whereas bupropion is metabolized in the liver to hydroxybupropion by CYP2B6, an isoenzyme of the cytochrome P450 system [92]. Thus, different metabolic mechanisms should decrease the potential for drug-drug adverse reactions when varenicline and bupropion are used in combination, although it should be noted that little is known about the potential for these medications to interact with medications used to treat HIV/AIDS (eg, ART). In 1 open label study in healthy smokers, 12 weeks of combined varenicline and bupropion use resulted in 58 % quit at 6-month follow-up [93]. The most common side effects were sleep disturbance (26 %) and nausea (24 %), and no patients discontinued treatment. Bupropion and varenicline also have similar, twice-a-day dosing regimens. This should make adherence easier during augmentation or a switch as there is no change in dosing schedule.
If, after the first treatment sequence (typically 8–12 weeks), patients are abstinent, they can be followed without medication, or they can be maintained for another 8–12 weeks on the same medication. This choice should be dictated, in part, by the difficulty the patient has experienced thus far. The same treatment can be reinstated if the patient has enjoyed success but relapses after the medication is withdrawn. For those who have failed to quit during the initial round of treatment, reasons for failure should be ascertained, (eg, AEs, lack of compliance, lack of efficacy). This should help guide the choice of augmenting treatment with another medication, or switching to another medication altogether. For patients whose lack of compliance is not related to inability to tolerate the medication, it may be worth considering behavioral treatments designed to enhance compliance.
Different medications, behavioral treatments, or combinations thereof, can be tested via the SMART design. In addition to varying treatment components, treatments of different duration can be tested. Different (interim) endpoints could be incorporated, such as (non) adherence to treatment or AEs, which prompt a switch to a different treatment that might better tolerated. Importantly, patient characteristics that might influence response to treatment (eg, nicotine dependence, psychiatric comorbidity) could be tested as treatment moderators, suggesting different decision rules regarding selection of initial treatments and subsequent treatment adaptations.
Conclusions
Treatment of tobacco dependence in PLWHA remains a priority and a challenge. Conventional treatments appear to offer some limited benefit, but properly controlled research studies in this area are lacking. Clinicians interested in learning about strategies to improve tobacco screening and smoking cessation that can be integrated into care in a variety of clinical settings are encouraged to consult the PHS Clinical Guideline [32] and especially the accompanying clinician and systems decision makers guides (both are available at: http://www.ahrq.gov/path/tobacco.htm). One challenge is to figure out how best to configure existing treatments that will meet the needs of this patient population in terms of acceptability, tolerability, safety, and adherence. Common clinical practice suggests that failed treatments be followed with re-treatment, but, with smoking, this remains largely unexplored territory. Clinicians are encouraged to explore sequential treatment strategies and to develop treatment and re-treatment algorithms that can then be subjected to empirical scrutiny.
Footnotes
Disclosure No potential conflicts of interest relevant to this article were reported.
Contributor Information
Raymond Niaura, Email: rniaura@legacyforhealth.org, Schroeder Institute for Tobacco Research and Policy Studies, Legacy, 1724 Massachusetts Avenue NW, Washington, DC 20036, USA.
Geetanjali Chander, Email: Gchande1@jhmi.edu, Johns Hopkins Medical Institutions, Johns Hopkins University, 1830 E. Monument Street, Office 8060, Baltimore, MD 21287, USA.
Heidi Hutton, Email: hhutton@jhmi.edu, Johns Hopkins Medical Institutions, Johns Hopkins University, 1830 E. Monument Street, Office 8060, Baltimore, MD 21287, USA.
Cassandra Stanton, Email: cas337@georgetown.edu, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, USA.
References
Papers of particular interest, published recently, have been highlighted as:
Of importance
- 1.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 2.Burkhalter JE, Springer CM, Chhabra R, Ostroff JS, Rapkin BD. Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine Tob Res. 2005;7:511–22. doi: 10.1080/14622200500186064. [DOI] [PubMed] [Google Scholar]
- 3.Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking among HIV positive New Yorkers: prevalence, frequency, and opportunities for cessation. AIDS Behav. 2010;14:824–35. doi: 10.1007/s10461-008-9449-2. [DOI] [PubMed] [Google Scholar]
- 4.Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clin Infect Dis. 2000;31:808–12. doi: 10.1086/314048. [DOI] [PubMed] [Google Scholar]
- 5.Benard A, Bonnet F, Tessier JF, Fossoux H, Dupon M, Mercie P, et al. Tobacco addiction and HIV infection: toward the implementation of cessation programs. ANRS CO3 Aquitaine cohort. AIDS Patient Care STDS. 2007;21:458–68. doi: 10.1089/apc.2006.0142. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Richardson EE, Stanton CA, Papandonatos GD, Betancourt RM, Stein M, Tashima K, et al. HIV-positive smokers considering quitting: differences by race/ethnicity. Am J Health Behav. 2008;32:3. doi: 10.5555/ajhb.2008.32.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahmanian S, Wewers ME, Koletar S, Reynolds N, Ferketich A, Diaz P. Cigarette smoking in the HIV-infected population. Proc Am Thorac Soc. 2011;8:313–9. doi: 10.1513/pats.201009-058WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gritz ER, Vidrine DJ, Lazev AB, Amick BCI, Arduino RC. Smoking behavior in a low-income multiethnic HIV/AIDS population. Nicotine Tob Res. 2004;6:71–7. doi: 10.1080/14622200310001656885. [DOI] [PubMed] [Google Scholar]
- 9•.Lifson AR, Lando HA. Smoking and HIV: prevalence, health risks, and cessation strategies. Curr HIV/AIDS Rep. 2012;9(3):223–30. doi: 10.1007/s11904-012-0121-0. A comprehensive review of the health consequences of smoking in HIV-infected patients. [DOI] [PubMed] [Google Scholar]
- 10.Kohli R, Lo Y, Homel P, Flanigan TP, Gardner LI, Howard AA, et al. Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (HER) study. Clin Infect Dis. 2006;43(1):90–8. doi: 10.1086/504871. [DOI] [PubMed] [Google Scholar]
- 11.Patel N, Talwar A, Reichert VC, Brady T, Jain M, Kaplan MH. Tobacco and HIV. Clin Occup Environ Med. 2006;5:193. doi: 10.1016/j.coem.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 13.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC Veterans Aging Cohort 5 Project Team. Increased COPD among HIV-positive compared with HIV-negative veterans. Chest. 2006;130:1326–33. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 14.Crothers K, Griffith TA, McGinnis KA, Rodriguez-Barradas MC, Leaf DA, Weissman S, et al. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J Gen Intern Med. 2005;20:1142–5. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crothers K, Tindle HA. Prevention of bacterial pneumonia in HIV infection: focus on smoking cessation. Expert Rev Anti Infect Ther. 2011;9:759–62. doi: 10.1586/eri.11.67. [DOI] [PubMed] [Google Scholar]
- 16.Cockerham L, Scherzer R, Zolopa A, Rimland D, Lewis CE, Bacchetti P, et al. Association of HIV infection, demographic and cardiovascular risk factors with all-cause mortality in the recent HAART era. J Acquir Immune Defic Syndr. 2010;53:102–6. doi: 10.1097/QAI.0b013e3181b79d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lifson AR, Neuhaus J, Arribas JR, van den Berg-Wolf M, Labriola AM, Read TR. Smoking-related health risks among persons with HIV in the strategies for management of antiretroviral therapy clinical trial. Am J Public Health. 2010;100(10):1896–903. doi: 10.2105/AJPH.2009.188664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petoumenos K, Worm S, Reiss P, de Wit S, d’ ArminioMonforte A, Sabin C, et al. Rates of cardiovascular disease following smoking cessation in patients with HIV infection: results from the D:A:D study. HIV Med. 2011;12(7):412–21. doi: 10.1111/j.1468-1293.2010.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piper ME, Kenford S, Fiore MC, Baker TB. Smoking cessation and quality of life: changes in life satisfaction over 3 years following a quit attempt. Ann Behav Med. 2011;43(2):262–70. doi: 10.1007/s12160-011-9329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidrine DJ, Arduino RC, Gritz ER. The effects of smoking abstinence on symptom burden and quality of life among persons living with HIV/AIDS. AIDS Patient Care STDS. 2007;21:659–66. doi: 10.1089/apc.2007.0022. [DOI] [PubMed] [Google Scholar]
- 21.Flicker L. Modifiable lifestyle risk factors for Alzheimer’s disease. J Alzheimers Dis. 2010;20:803–11. doi: 10.3233/JAD-2010-091624. [DOI] [PubMed] [Google Scholar]
- 22.Lasser K, Boyd J, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 23.Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health Report. Nicotine Tob Res. 2008;10:1691–715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- 24.McClave AK, McKnight-Eily LR, Davis SP, Dube SR. Smoking characteristics of adults with selected lifetime mental illnesses: results from the 2007 National Health Interview Survey. Am J Public Health. 2010;100:2464–72. doi: 10.2105/AJPH.2009.188136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldman JG, Minkoff H, Schneider MF, Gange SJ, Cohen M, Watts H, et al. Association of cigarette smoking with HIV prognosis among women in the HAART era: a report from the Women’s Interagency HIV Study. Am J Public Health. 2006;96:1060–5. doi: 10.2105/AJPH.2005.062745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shuter J, Bernstein SL. Cigarette smoking is an independent predictor of nonadherence in HIV-infected individuals receiving highly active antiretroviral therapy. Nicotine Tob Res. 2008;10:731–6. doi: 10.1080/14622200801908190. [DOI] [PubMed] [Google Scholar]
- 27.Marks King R, Vidrine DJ, Danysh HE, Fletcher FE, McCurdy S, Arduino RC, et al. Factors associated with nonadherence to anti-retroviral therapy in HIV-positive smokers. AIDS Patient Care STDS. 2012;26(8):479–85. doi: 10.1089/apc.2012.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds NR. Cigarette smoking and HIV: more evidence for action. AIDS Educ Prev. 2009;21(3 Suppl):106–21. doi: 10.1521/aeap.2009.21.3_supp.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humfleet GL, Delucchi K, Kelley K, Hall SM, Dilley J, Harrison G. Characteristics of HIV-positive cigarette smokers: a sample of smokers facing multiple challenges. AIDS Educ Prev. 2009;21(Suppl 3):54–64. doi: 10.1521/aeap.2009.21.3_supp.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cargill VA, Stone VE. HIV/AIDS: a minority health issue. Med Clin North Am. 2005;89:895–912. doi: 10.1016/j.mcna.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Cox LS, Okuyemi K, Choi WS, Ahluwalia JS. A review of tobacco use treatments in U.S. ethnic minority populations. Am J Health Promot. 2011;25(5 Suppl):S11–30. doi: 10.4278/ajhp.100610-LIT-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U S Public Health Service Report. Am J Prev Med. 2008;35:158–76. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elzi L, Spoerl D, Voggensperger J, Nicca D, Simcock M, Bucher HC, et al. A smoking cessation program in HIV-infected individuals: a pilot study. Antivir Ther. 2006;11:787–95. [PubMed] [Google Scholar]
- 34.Ingersoll KS, Cropsey KL, Heckman CJ. A test of motivational plus nicotine replacement interventions for HIV positive smokers. AIDS Behav. 2009;13:545–54. doi: 10.1007/s10461-007-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidrine DJ, Marks RM, Arduino RC, Gritz ER. Efficacy of cell phone-delivered smoking cessation counseling for persons living with HIV/AIDS: 3-month outcomes. Nicotine Tob Res. 2011;14:106–10. doi: 10.1093/ntr/ntr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moadel AB, Bernstein SL, Mermelstein RJ, Arnsten JH, Dolce EH, Shuter J. A randomized controlled trial of a tailored group smoking cessation intervention for HIV-infected smokers. J Acquir Immune Defic Syndr. 2012 doi: 10.1097/QAI.0b013e3182645679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lloyd-Richardson EE, Stanton CA, Papandonatos GD, Shadel WG, Stein M, Tashima K, et al. Motivation and patch treatment for HIV + smokers: a randomized controlled trial. Addiction. 2009;104:1891–900. doi: 10.1111/j.1360-0443.2009.02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanton CA, Lloyd-Richardson EE, Papandonatos GD, de Dios MA, Niaura R. Mediators of the relationship between nicotine replacement therapy and smoking abstinence among people living with HIV/AIDS. AIDS Educ Prev. 2009;21(3 Suppl):65–80. doi: 10.1521/aeap.2009.21.3_supp.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedrol-Clotet E, Deig-Comerma E, Ribell-Bachs M, Vidal-Castell I, García-Rodríguez P, Soler A. Bupropion use for smoking cessation in hiv-infected patients receiving antiretroviral therapy. Enferm Infecc Microbiol Clin. 2006;24:509–11. doi: 10.1157/13092468. [DOI] [PubMed] [Google Scholar]
- 40.Tornero C, Mafé C. Varenicline and antiretroviral therapy in patients with HIV. J Acquir Immune Defic Syndr. 2009;52:656. doi: 10.1097/QAI.0b013e3181ba1beb. [DOI] [PubMed] [Google Scholar]
- 41.Cui Q, Robinson L, Elston D, Smaill F, Cohen J, Quan C, et al. Safety and tolerability of varenicline tartrate (champix(®)/chantix (®)) for smoking cessation in HIV-infected subjects: a pilot open-label study. AIDS Patient Care STDS. 2011;26(1):12–19. doi: 10.1089/apc.2011.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shuter J, Salmo LN, Shuter AD, Nivasch EC, Fazzari M, Moadel AB. Provider beliefs and practices relating to tobacco use in patients living with HIV/AIDS: a national survey. AIDS Behav. 2011;16(2):288–94. doi: 10.1007/s10461-011-9891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirshenbaum AP, Olsen DM, Bickel WK. A quantitative review of the ubiquitous relapse curve. J Subst Abuse Treat. 2009;36:8–17. doi: 10.1016/j.jsat.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 45.Hughes JR, Peters EN, Naud S. Relapse to smoking after 1 year of abstinence: a meta-analysis. Addict Behav. 2008;33:1516–20. doi: 10.1016/j.addbeh.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huber M, Ledergerber B, Jaccard R, Elzi L, Furrer H, Hirschel B, et al. Smoking prevalence, cessation rates and relapse rates in the Swiss HIV cohort study (SHCS) J Int AIDS Soc. 2010;13:1–2. [Google Scholar]
- 47.Hajek P, Stead LF, West R, Jarvis M, Lancaster T. Relapse prevention interventions for smoking cessation. Status and date: new search for studies and content updated (conclusions changed), Published in 2009. Cochrane Database Syst Rev. 2009;21(1) CD003999. [Google Scholar]
- 48•.Agboola S, McNeill A, Coleman T, Leonardi Bee J. A systematic review of the effectiveness of smoking relapse prevention interventions for abstinent smokers. Addiction. 2010;105:1362–80. doi: 10.1111/j.1360-0443.2010.02996.x. A comprehensive review of behavioral and pharmacologic smoking relapse prevention treatments, along with meta-analyses. [DOI] [PubMed] [Google Scholar]
- 49.Killen JD, Fortmann SP, Newman B, Varady A. Evaluation of a treatment approach combining nicotine gum with self-guided behavioral treatments for smoking relapse prevention. J Consult Clin Psychol. 1990;58:85–92. doi: 10.1037//0022-006x.58.1.85. [DOI] [PubMed] [Google Scholar]
- 50.Fortmann SP, Killen JD. Nicotine gum and self-help behavioral treatment for smoking relapse prevention: results from a trial using population-based recruitment. J Consult Clin Psychol. 1995;63:460–8. doi: 10.1037//0022-006x.63.3.460. [DOI] [PubMed] [Google Scholar]
- 51.Croghan IT, Hurt RD, Dakhil SR, Croghan GA, Sloan JA, Novotny PJ, et al. Randomized comparison of a nicotine inhaler and bupropion for smoking cessation and relapse prevention. Mayo Clin Proc. 2007;82:186–95. doi: 10.4065/82.2.186. [DOI] [PubMed] [Google Scholar]
- 52.Covey LS, Glassman AH, Jiang H, Fried J, Masmela J, LoDuca C, et al. A randomized trial of bupropion and/or nicotine gum as maintenance treatment for preventing smoking relapse. Addiction. 2007;102:1292–302. doi: 10.1111/j.1360-0443.2007.01887.x. [DOI] [PubMed] [Google Scholar]
- 53.Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. A randomized, controlled trial. Ann Intern Med. 2001;135:423–33. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- 54.Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. J Clin Oncol. 2003;21:914. doi: 10.1200/JCO.2003.08.160. [DOI] [PubMed] [Google Scholar]
- 55.Killen JD, Fortmann SP, Murphy GM, Hayward C, Arredondo C, Cromp D, et al. Extended treatment with bupropion SR for cigarette smoking cessation. J Consult Clin Psychol. 2006;74:286–94. doi: 10.1037/0022-006X.74.2.286. [DOI] [PubMed] [Google Scholar]
- 56.Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR Varenicline Phase 3 Study Group. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 57.Joseph AM, Rice K, An LC, Mohiuddin A, Lando H. Recent quitters’ interest in recycling and harm reduction. Nicotine Tob Res. 2004;6:1075. doi: 10.1080/14622200412331324893. [DOI] [PubMed] [Google Scholar]
- 58.Fu SS, Partin MR, Snyder A, An LC, Nelson DB, Clothier B, et al. Promoting repeat tobacco dependence treatment: are relapsed smokers interested? Am J Manag Care. 2006;12:235–43. [PubMed] [Google Scholar]
- 59.Cupertino AP, Wick JA, Richter KP, Mussulman L, Nazir N, Ellerbeck EF. The impact of repeated cycles of pharmacotherapy on smoking cessation: a longitudinal cohort study. Arch Intern Med. 2009;169:1928–30. doi: 10.1001/archinternmed.2009.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hughes J. An algorithm for choosing among smoking cessation treatments. J Subst Abuse Treat. 2008;34:426–32. doi: 10.1016/j.jsat.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz N, Curry SJ, et al. Clinical Practice Guideline Executive Summary. Rockville MD: US Department of Health and Human Services Public Health Service; May, 2008. Treating tobacco use and dependence: 2008 update. [Google Scholar]
- 62.Niaura R, Abrams DB. Smoking cessation: progress, priorities, and prospectus. J Consult Clin Psychol. 2002;70:494. doi: 10.1037//0022-006x.70.3.494. [DOI] [PubMed] [Google Scholar]
- 63.Abrams DB, Orleans CT, Niaura RS, Goldstein MG, Prochaska JO, Velicer W. Integrating individual and public health perspectives for treatment of tobacco dependence under managed health care: a combined stepped-care and matching model. Ann Behav Med. 1996;18:290–304. doi: 10.1007/BF02895291. [DOI] [PubMed] [Google Scholar]
- 64.Sobell MB, Sobell LC. Stepped care as a heuristic approach to the treatment of alcohol problems. J Consult Clin Psychol. 2000;68:573. [PubMed] [Google Scholar]
- 65.Breslin FC, Sobell MB, Sobell LC, Cunningham JA, Sdao-Jarvie K, Borsoi D. Problem drinkers: evaluation of a stepped-care approach. J Subst Abuse. 1998;10:217–32. doi: 10.1016/s0899-3289(99)00008-5. [DOI] [PubMed] [Google Scholar]
- 66.Murphy SA, Lynch KG, Oslin D, McKay JR, TenHave T. Developing adaptive treatment strategies in substance abuse research. Drug Alcohol Depend. 2007;88(Suppl 2):S24–30. doi: 10.1016/j.drugalcdep.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hughes JR, Grass JA, Pillitteri JL. Treatment resistant smokers: a pilot study of nicotine nasal spray and inhaler. J Addict Dis. 2000;19:95–100. doi: 10.1300/J069v19n01_08. [DOI] [PubMed] [Google Scholar]
- 68.Tønnesen P, Nørregaard J, Säwe U, Simonsen K. Recycling with nicotine patches in smoking cessation. Addiction. 1993;88:533–9. doi: 10.1111/j.1360-0443.1993.tb02060.x. [DOI] [PubMed] [Google Scholar]
- 69.Tonnesen P, Mikkelsen K, Norregaard J, Jorgensen S. Recycling of hard-core smokers with nicotine nasal spray. Eur Respir J. 1996;9:1619. doi: 10.1183/09031936.96.09081619. [DOI] [PubMed] [Google Scholar]
- 70.Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers. BMJ. 1995;311:363–6. doi: 10.1136/bmj.311.7001.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith SS, Jorenby DE, Fiore MC, Anderson JE, Mielke MM, Beach KE, et al. Strike while the iron is hot: can stepped-care treatments resurrect relapsing smokers? J Consult Clin Psychol. 2001;69:429. doi: 10.1037//0022-006x.69.3.429. [DOI] [PubMed] [Google Scholar]
- 72.Gonzalez DH, Nides M, Ferry LH, Segall N, Herrero LA, Krishen A, et al. Bupropion SR as an aid to smoking cessation in smokers treated previously with bupropion: a randomized placebo-controlled study. Clin Pharmacol Ther. 2001;69:438–44. doi: 10.1067/mcp.2001.115750. [DOI] [PubMed] [Google Scholar]
- 73.Luce BR, Kramer JM, Goodman SN, Connor JT, Tunis S, Whicher D, Schwartz JS. Rethinking randomized clinical trials for comparative effectiveness research: the need for transformational change. Ann Intern Med. 2009;151:206. doi: 10.7326/0003-4819-151-3-200908040-00126. [DOI] [PubMed] [Google Scholar]
- 74.Lavori PW, Dawson R. Adaptive treatment strategies in chronic disease. Annu Rev Med. 2008;59:443–53. doi: 10.1146/annurev.med.59.062606.122232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinberg MB, Schmelzer AC, Richardson DL, Foulds J. The case for treating tobacco dependence as a chronic disease. Ann Intern Med. 2008;148:554–6. doi: 10.7326/0003-4819-148-7-200804010-00012. [DOI] [PubMed] [Google Scholar]
- 76.Joseph AM, Fu SS, Lindgren B, Rothman AJ, Kodl M, Lando H, et al. Chronic disease management for tobacco dependence: a randomized, controlled trial. Arch Intern Med. 2011;171:1894–900. doi: 10.1001/archinternmed.2011.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chakraborty B. Dynamic treatment regimes for managing chronic health conditions: a statistical perspective. Am J Public Health. 2011;101:40–5. doi: 10.2105/AJPH.2010.198937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lavori PW, Dawson R. Dynamic treatment regimes: practical design considerations. Clin Trials. 2004;1:9. doi: 10.1191/1740774s04cn002oa. [DOI] [PubMed] [Google Scholar]
- 79.Chakraborty B, Murphy S, Strecher V. Inference for non-regular parameters in optimal dynamic treatment regimes. Stat Methods Med Res. 2010;19:317–43. doi: 10.1177/0962280209105013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murphy SA, Van Der Laan MJ, Robins JM. Marginal mean models for dynamic regimes. J Am Stat Assoc. 2001;96:1410–23. doi: 10.1198/016214501753382327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murphy SA. An experimental design for the development of adaptive treatment strategies. Stat Med. 2005;24:1455–81. doi: 10.1002/sim.2022. [DOI] [PubMed] [Google Scholar]
- 82•.Lei H, Nahum-Shani I, Lynch K, Oslin D, Murphy SA. A “SMART” design for building individualized treatment sequences. Annu Rev Clin Psychol. 2012;8:21–48. doi: 10.1146/annurev-clinpsy-032511-143152. A good review of how to conceive of, construct, and test adaptive treatment strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pineau J, Bellemare MG, Rush AJ, Ghizaru A, Murphy SA. Constructing evidence-based treatment strategies using methods from computer science. Drug Alcohol Depend. 2007;88(Suppl 2):S52–60. doi: 10.1016/j.drugalcdep.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thall PF, Logothetis C, Pagliaro LC, Wen S, Brown MA, Williams D, et al. Adaptive therapy for androgen-independent prostate cancer: a randomized selection trial of 4 regimens. J Natl Cancer Inst. 2007;99:1613–22. doi: 10.1093/jnci/djm189. [DOI] [PubMed] [Google Scholar]
- 85.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent health interventions. Am J Prev Med. 2007;32(5 Suppl):S112–8. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dawson R, Green AI, Drake RE, McGlashan TH, Schanzer B, Lavori PW. Developing and testing adaptive treatment strategies using substance-induced psychosis as an example. Psychopharmacol Bull. 2008;41:51–67. [PMC free article] [PubMed] [Google Scholar]
- 87.Buyze J, Van Rompaye B, Goetghebeur E. Designing a sequentially randomized study with adherence enhancing interventions for diabetes patients. Stat Med. 2010;29:1114–26. doi: 10.1002/sim.3856. [DOI] [PubMed] [Google Scholar]
- 88.Brown CH, Ten Have TR, Jo B, Dagne G, Wyman PA, Muthén B, et al. Adaptive designs for randomized trials in public health. Annu Rev Public Health. 2009;30:1–25. doi: 10.1146/annurev.publhealth.031308.100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2011;2 doi: 10.1002/14651858.CD006103.pub2. CD006103. [DOI] [PubMed] [Google Scholar]
- 90.Hughes J, Stead L, Lancaster T, Rev CDS. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;1 doi: 10.1002/14651858.CD000031.pub3. CD000031. [DOI] [PubMed] [Google Scholar]
- 91.Carrozzi L, Pistelli F, Viegi G. Pharmacotherapy for smoking cessation. Ther Adv Respir Dis. 2008;2:301–17. doi: 10.1177/1753465808096136. [DOI] [PubMed] [Google Scholar]
- 92.Faessel HM, Obach RS, Rollema H, Ravva P, Williams KE, Burstein AH. A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clin Pharmacokinet. 2010;49:799–816. doi: 10.2165/11537850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 93.Ebbert JO, Croghan IT, Sood A, Schroeder DR, Hays JT, Hurt RD. Varenicline and bupropion sustained-release combination therapy for smoking cessation. Nicotine Tob Res. 2009;11:234–9. doi: 10.1093/ntr/ntn031. [DOI] [PubMed] [Google Scholar]


