Abstract
The transcription factor Twist1 induces Epithelial-Mesenchymal Transition and extracellular matrix degradation to promote tumor metastasis. Although Twist1 also plays a role in embryonic vascular development and tumor angiogenesis, the molecular mechanisms that underlie these processes are not as well understood. Here, we report a novel function for Twist1 in modifying the tumor microenvironment to promote progression. We found that expression of Twist1 in human mammary epithelial cells potently promoted angiogenesis. Surprisingly, Twist1 expression did not increase the secretion of the common pro-angiogenic factors VEGF and bFGF, but rather induced expression of the macrophage chemoattractant CCL2. Attenuation of endogenous Twist1 in vivo blocked macrophage recruitment and angiogenesis, whereas exogenous CCL2 rescued the ability of tumor cells lacking Twist1 to attract macrophages and promote angiogenesis. Macrophage recruitment also was essential for the ability of Twist1-expressing cells to elicit a strong angiogenic response. Together, our findings show how Twist1 recruits stromal macrophages through CCL2 induction to promote angiogenesis and tumor progression. Since Twist1 expression has been associated with poor survival in many human cancers, this finding suggests that anti-CCL2 therapy may offer a rational strategy to treat Twist1-positive metastatic cancers.
Keywords: Twist1, angiogenesis, CCL2, macrophage, tumor microenvironment
INTRODUCTION
Increased expression of the Twist1 transcription factor has been correlated with metastasis and poor survival in many human cancers including breast cancer (1–3). To date, the prominent role of Twist1 in tumor progression has been to induce Epithelial-Mesenchymal Transition (EMT) and extracellular matrix degradation (4–6). During EMT, Twist1 promotes stationary epithelial cells to lose cell-cell junctions and gain migratory and invasive capacities. Interestingly, Twist1 is also indicated in vascularization both during development and in tumor models. In Xenopus, Twist1 is required for proper embryonic vascular development (7). During human embryoid body formation, Twist1 is co-upregulated with several genes regulating vascular development (8). Under hypoxia, Twist1 expression in tumor cells is directly induced by HIF-1a and -2a transcription factors, molecules known to regulate pro-angiogenic genes such as VEGF (9, 10). Finally, Twist1 expression promotes vascularization in tumor xenograft models (11, 12). Together, these observations suggest that Twist1 is involved in tumor angiogenesis.
The ability of tumor cells to promote angiogenesis, or blood vessel growth, is one of the critical contributing factors to metastatic spread. Tumor-associated neovasculature exhibits increased permeability due to poorly aligned endothelial cells with wide fenestrations and sparse pericyte coverage (13). Besides supporting tumor growth by increasing oxygen and nutrient influx, angiogenesis gives malignant tumor cells access into the systemic circulation for metastasis.
Tumor angiogenesis may be modulated by the presence of myeloid cells such as macrophages and neutrophils, important agents within the tumor microenvironment (14–18). In particular, several lines of evidence support the role of macrophages in tumor angiogenesis and metastasis. In invasive human breast cancers, the presence of macrophages correlates with microvessel density, VEGF content, tumor grade, lymph node metastasis, and poor overall survival (19, 20). In the PyMT mammary tumor model, macrophage depletion delays the onset of angiogenesis, tumor formation, and metastasis, while addition of exogenous macrophages shortens tumor latency (18). In various tumor xenograft models, co-injection with macrophages increases VEGF abundance and neovascularization (21). Importantly, macrophages have been shown to contribute to later steps of the metastatic cascade such as extravasation and metastatic seeding (22–24). Together, these studies demonstrate the importance of tumor-associated macrophages in regulating tumor angiogenesis and promoting metastasis.
Currently, it is unknown whether Twist1 can modulate the tumor microenvironment to promote angiogenesis. In this study, we have used various direct and quantitative assays to uncover a novel function of Twist1 in recruiting macrophages to facilitate angiogenesis.
MATERIALS AND METHODS
Cell Lines and Cell Culture
Human mammary epithelial cells (HMLE), human breast cancer cell line SUM1315, and mouse mammary tumor cell lines 168FARN and 4T1 were obtained from Dr. Robert Weinberg and cultured as described (4). The MDA-MB-468 line was obtained from the American Type Culture Collection (ATCC). All cell lines were reauthenticated by microsatellite profiling immediately before manuscript submission. Primary bone marrow-derived macrophages (BMDM) were harvested and differentiated as described (25).
DNA Constructs
The Twist1, Twist1-ER, and shTwist1 vectors were previously described (4, 26). Control shRNA pLKO.1 was obtained from the Sabatini lab (Addgene) and two Mission shRNAs against CCL2 in pLKO.1 (Sigma) contain the following targeting sequences:
shCCL2.1: GCTCGCGAGCTATAGAAGAAT
shCCL2.4: GATGTGAAACATTATGCCTTA
Chick Chorioallantoic Membrane (CAM) Angiogenesis Assay
The shell-less chick CAM angiogenesis assay was performed as previously described (27). Test cells were suspended in neutralized collagen and pipetted onto two layers of nylon mesh (Sefar America) at a final density of 75K cells per 30 µL “onplant.” Angiogenesis was scored in the onplants of the live embryos 72–96 hrs later using a dissecting microscope.
Enzyme-Linked Immunosorbant Assay (ELISA) and Cytokine Array
ELISAs for VEGF, bFGF (Peprotech), and CCL2 and the human cytokine arrays (Raybiotech) were performed according to the manufacturers’ protocols using 48hr cell-conditioned media that were controlled for cell number and media volume, and were supplemented with 10 µg/mL heparin to promote cytokine release (28).
Quantitative Polymerase Chain Reaction (QPCR)
Total RNA was purified using the RNeasy Mini Kits with DNase treatment (Qiagen). 2µg of RNA were reverse transcribed using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Resulting cDNAs were analyzed in triplicate using SYBR-Green Master PCR mix (Applied Biosystems). Relative mRNA levels were determined by 2−Ct−Cc where Ct and Cc are the mean threshold cycle differences after normalizing to GAPDH values. Primers used for QPCR are as follows:
hCCL2: CAGCCAGATGCAATCAATGCC, TGGAATCCTGAACCCACTTCT
hGAPDH: GAGAGACCCTCACTGCTG, GATGGTACATGACAAGGTGC
mCcl2: TTAAAAACCTGGATCGGAACCAA, GCATTAGCTTCAGATTTACGGGT
mGapdh: GACCCCTTCATTGACCTCAAC, CTTCTCCATGGTGGTGAAGA
Macrophage Migration Assay
On day 10 of differentiation, BMDM were scraped and resuspended in RPMI-1640, 0.5% heat inactivated FBS, 20 ng/mL M-CSF (Shenandoah Biotechnology). Cell suspensions (200K cells/100 uL) were added to the upper chamber of 8 um pore Transwell inserts (Corning) for 2 hrs at 37°C to attach to the membrane. Transwells were then moved to 24-well plates containing 0.6 mL cell-conditioned media and incubated at 37°C for 2–4 hr. Cells were fixed in 4% paraformaldehyde and stained with 0.1% crystal violet. Cells in the upper chamber were removed with a cotton swab and migrated cells in the lower chamber were quantified using 12–15 random fields. 2–3 independent experiments were performed for each assay.
Matrigel Plug Injection
All animal care and experiments were approved by the Institutional Animal Care and Use Committee of University of California, San Diego. Adult BALB/c mice were given 2–3 s.c. injections of 200 µL of a 3:1 mixture of growth factor reduced Matrigel (BD Biosciences) and 2×106 cells in DMEM. After 5 days, plugs were harvested, imaged, and fixed in 10% zinc formalin followed by paraffin sectioning. For macrophage depletion, 200 uL of liposome suspension was injected via tail vein 2 days before plug injections or 50 µL of liposome suspension was co-injected with the plug. Cl2MDP (or clodronate) was a gift of Roche Diagnostics GmbH, Mannheim, Germany. Clodronate liposomes and control liposomes containing PBS were prepared as described previously (29). Where indicated, Matrigel plugs were supplemented with recombinant hCCL2 (10 ng/ml, R&D).
Immunohistochemistry
Following antigen retrieval in citrate buffer and peroxidase blocking in H2O2, tissues were blocked with 2% goat serum and 2% BSA in PBS. Serial sections were stained using rabbit anti-CD31 (1:50, Abcam) or rat anti-F4/80 (1:50, #CI:AI3-1 Santa Cruz Biotechnology) antibodies, species-specific biotinylated secondary antibodies, followed by ABC reagent, developed with DAB substrate and counter-stained using Hematoxylin QS (Vector labs).
For blood vessel quantification, CD31+ vessels were counted in five random fields (20×) in vascular hot spots and averaged for each section. A vessel was defined as a positively stained circle, a branch from a multi-branched structure, or a solitary branch. For macrophage quantification, five random fields (20×) in F4/80 positive hot spots were scored on a scale of 0–6 for staining intensity and distribution within a field: 0, undetectable; 1, faint, discrete patches; 2 faint, all over; 3 medium, discrete patches; 4 medium, all over; 5 intense, discrete patches; 6 intense, all over.
Statistics
Statistical analysis was performed using GraphPad Prism.
RESULTS
Twist1 promotes angiogenesis in a chicken chorioallantoic membrane angiogenesis assay
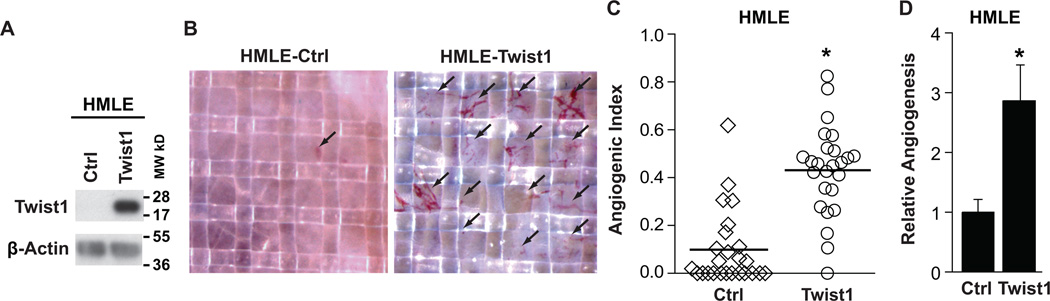
To directly and quantitatively test whether Twist1 can promote angiogenesis, we stably expressed Twist1 in immortalized human mammary epithelial cells (HMLE) (Fig. 1A, Supplementary Fig. S1) and examined their angiogenic potential using a quantitative chicken chorioallantoic membrane (CAM) angiogenesis assay (27). For each cell type tested, cells were suspended in collagen that solidified over two nylon meshes to form an “onplant.” Thirty-six onplants were evenly distributed onto the CAMs of approximately six chicken embryos at 10 days post-fertilization, a time when the developing vasculature is able to sprout in response to additional angiogenic stimuli. Between 72–96 hours after grafting, nascent vessels had grown vertically into the onplants and were visible within the grids of the upper mesh (Fig. 1B). Frequently, nucleated red blood cells could be seen moving within the vessels, indicating that these vessels were functional. As shown in Figure 1C of one representative experiment, we quantified Angiogenic Index, defined as the percentage of grids that contained nascent vessels, in at least 25 onplants per condition and found that onplants containing HMLE-Twist1 cells presented a significantly higher Angiogenic Index compared to onplants with control HMLE cells where new vessels were nearly absent (0.43 vs. 0.10). Consistently, results from 11 independent experiments showed that HMLE-Twist1 cells exhibited a robust and repeatable average 2.9-fold increase in angiogenesis (Fig. 1D). These results demonstrate that expression of Twist1 in human mammary epithelial cells is sufficient to promote angiogenesis.
Figure 1. Twist1 promotes angiogenesis in a chicken chorioallantoic membrane angiogenesis assay.
A) Lysates from HMLE-Ctrl and HMLE-Twist1 cells were analyzed by SDS-PAGE and probed for Twist1 and β-actin (full-length blot, Supplementary Fig. S1). B) Cells were suspended in collagen and resulting onplants were grafted onto the CAMs of 10-day embryos. Live angiogenic scoring was performed 72 hrs later using a dissection microscope. Shown are representative images of onplants with new vessel growth. C) Angiogenesis quantification for one representative experiment. Angiogenic index is the percentage of vessel positive grids in each onplant. Each data point represents the angiogenic index in one of approximately 25 onplants distributed across 4–6 embryos per cell type, * p<0.05 by Student’s T-test. D) Quantification of average angiogenesis for 11 independent experiments. Error bars are SEM, *p<0.05 by Student’s T-test.
Twist1 is necessary and sufficient to induce CCL2 expression
To investigate the mechanism underlying Twist1-induced angiogenesis, we examined factors secreted from Twist1-expressing cells that could attract or stimulate proliferation of endothelial cells. Previous studies suggested that Twist1 could regulate VEGF mRNA or protein expression (11, 12). To explore how Twist1 promotes angiogenesis in our system, we compared the levels of 174 cytokines in conditioned media from HMLE cells using a cytokine array. Interestingly, HMLE-Twist1 cells did not significantly increase the expression of two major pro-angiogenic molecules bFGF and VEGF (Supplementary Fig. S2A). This result was further validated by quantitative measurement of secreted bFGF and VEGF in conditioned media by ELISA (Supplementary Fig. S2B, S2C).
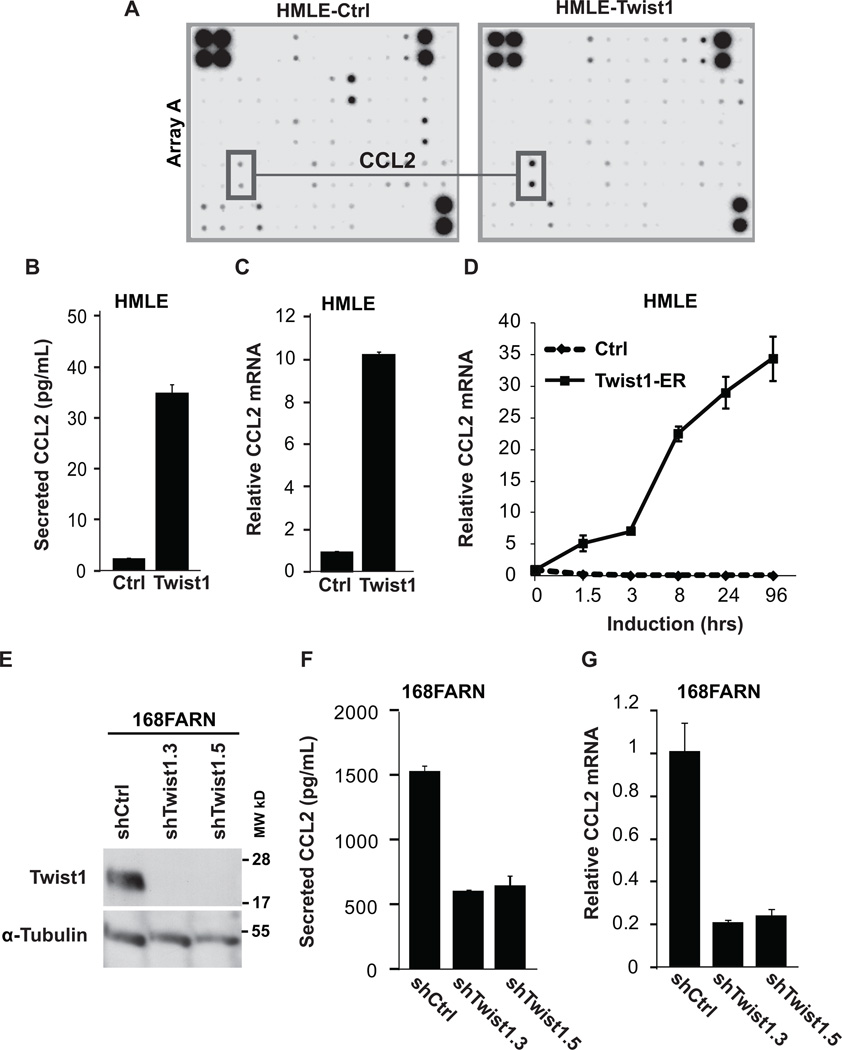
Interestingly, the cytokine array detected increased levels of CCL2 (Monocyte Chemotactic Protein-1 or MCP-1) in conditioned media from HMLE-Twist1 cells (Fig. 2A). CCL2 is a major chemoattractant for macrophages, cells that have been demonstrated to have pro-angiogenic functions in physiological and disease states (30, 31). Based on this knowledge, we focused our studies on examining the role of CCL2 in Twist1-induced angiogenesis. ELISA analysis confirmed that Twist1 expression increased secreted CCL2 from HMLE cells by 15.0-fold (Fig. 2B). Furthermore, quantitative PCR (QPCR) analysis revealed that CCL2 mRNA in HMLE-Twist1 cells was 10.2-fold higher than control cells (Fig. 2C). Using HMLE cells that express an inducible Twist1-ER construct, we found that CCL2 mRNA increased by 5.2-fold within 1.5 hours of Twist1 activation and continued to increase up to 4 days later (Fig. 2D), which is consistent with a previous study showing that Twist1 directly regulates CCL2 in white adipose tissue (32). It is worth noting that the immediate induction of CCL2 in response to Twist1-ER activation is higher than the CCL2 level in HMLE cells stably expressing Twist1. We found that this difference is largely due to reduced Twist1 transgene expression upon long-term culture of HMLE-Twist1 cells (data not shown). Importantly, direct induction of CCL2 transcription was specific to Twist1 since another EMT-inducing transcription factor Snail only induced CCL2 by 1.6-fold 4 days after activation (Supplementary Fig. 2G). To extend our studies to breast tumor cells, we expressed Twist1 in MDA-468 breast cancer cells (Supplementary Fig. S2D). Consistent with results from HMLE cells, Twist1 expression increased secreted CCL2 protein by 5.1-fold and CCL2 mRNA by 2.9-fold in MDA-468 cells (Supplementary Fig. S2E, S2F). Collectively, these results suggest that Twist1 expression is sufficient to induce CCL2 in normal and cancer mammary epithelial cells.
Figure 2. Twist1 is necessary and sufficient to induce CCL2 expression.
A) Analysis of secreted cytokines by HMLE-Ctrl and HMLE-Twist1 cells by cytokine expression array. Conditioned media were collected for 48 hr and incubated with array membranes to measure 174 human cytokines. CCL2 levels are highlighted on one set of membranes. B) Levels of CCL2 protein were measured by ELISA in conditioned media from indicated HMLE cells. C) Levels of CCL2 mRNA were measured by QPCR analysis inindicated HMLE cells. D) Levels of CCL2 mRNA were measured by QPCR analysis in HMLE-Twist1-ER cells (solid line) or HMLE cells (dashed line) treated with 20 nM 4-hydroxy-tamoxifen. E) Lysates from 168FARN cells expressing two shRNAs against Twist1 or a control shRNA were analyzed by SDS-PAGE and probed for Twist1 and α-Tubulin (full-length blots Supplementary Fig. S2D). F) The levels of CCL2 were measured by ELISA in conditioned media from indicated 168FARN cells. G) Levels of CCL2 mRNA as measured by QPCR analysis in indicated 168FARN cells.
To determine whether Twist1 is important for CCL2 expression in breast tumor cells, we knocked down the high level of endogenous Twist1 in multiple cell lines. In 168FARN mouse mammary tumor cells, we used two independent shRNA constructs against Twist1 (Fig. 2E, Supplementary Fig. S2D) (4) and found that knocking down Twist1 reduced the level of secreted CCL2 protein by approximately 65% as measured by ELISA (Fig. 2F). Similarly, using QPCR we found Ccl2 mRNA was reduced by approximately 80% upon knocking down Twist1 in 168FARN cells (Fig. 2G), further indicating an essential role of Twist1 in CCL2 induction. Using the more aggressive 4T1 mouse mammary tumor cell line and the SUM1315 human mammary cancer cell line, which both also express high levels of endogenous Twist1 (4, 5), knockdown of Twist1 also reduced CCL2 mRNA (Supplemental Fig. S2H, S2I). Together, results from these complementary sets of over-expression and knockdown experiments demonstrate that Twist1 is both necessary and sufficient to induce CCL2 expression in mammary epithelial cells.
CCL2 mediates Twist1-induced angiogenesis in the CAM assay
To test whether induction of CCL2 is important to mediate Twist1-induced angiogenesis, we stably knocked down CCL2 in HMLE-Twist1 cells and tested the effect on angiogenesis using the CAM assay. Two independent shRNA constructs efficiently repressed CCL2 mRNA and protein levels in HMLE-Twist1 cells, close to the baseline levels of control HMLE cells (Fig.3A, 3B). Knocking down CCL2 did not affect Twist1 expression (Fig. 3C). Likewise, knockdown of CCL2 did not alter the EMT phenotype induced by Twist1, since expression of the epithelial marker E-cadherin and the mesenchymal marker fibronectin were unchanged and the cells remained elongated and scattered, similar to parental HMLE-Twist1 cells and HMLE-Twist1 cells expressing a control shRNA (Fig. 3C, Supplementary Fig. S3A, S3B). Importantly, in the CAM assay, we found that knocking down CCL2 in HMLE-Twist1 cells reduced the Angiogenic Index by approximately 50% from 0.35 to 0.16 and 0.18, respectively (Fig. 3D). These results indicate that Twist1 induces CCL2 to promote angiogenesis, and this function of Twist1 in angiogenesis is independent of its ability to induce EMT.
Figure 3. CCL2 mediates Twist1-induced angiogenesis in the CAM assay.
A) QPCR analysis of CCL2 mRNA levels in HMLE-Ctrl and HMLE-Twist1 cells with indicated shRNAs. B) The levels of CCL2 protein were measured by ELISA in conditioned media from indicated HMLE cells. C) Lysates from indicated HMLE cells were analyzed by SDS-PAGE and probed for Twist1, E-cadherin, fibronectin, and α-tubulin (full-length blots, SFig.3A). D) Quantification of angiogenesis from three independent CAM angiogenesis experiments. For each experiment, 5–8 onplants were evenly distributed across 4–6 ten-day-old embryos per cell type tested, *p<0.05 versus HMLE-Ctrl, #p<0.05 versus HMLE-Twist1 shCtrl by ANOVA followed by Tukey Test.
Twist1 expression in mammary epithelial cells promotes macrophage attraction in a CCL2-dependent manner
We next set out to determine how CCL2 mediates Twist1-induced angiogenesis. Because CCL2 is a chemoattractant for macrophages (30) and macrophages have been demonstrated to promote angiogenesis (31), we asked whether induction of CCL2 by Twist1 promotes macrophage attraction in culture. As the literature regarding the function of avian cytokines and macrophage regulation is limited (33, 34), we used mouse models to address this question. We isolated bone marrow from mice and differentiated the monocytes into macrophages in vitro. Flow cytometry analysis revealed strong surface expression of the CCL2 receptor CCR2 in these bone marrow-derived macrophages (BMDM) (Fig. 4A). We seeded isolated BMDM on the top of a Boyden chamber to determine their ability to migrate toward various conditioned media. As shown in Figure 4B, significantly more macrophages were attracted to the conditioned media from HMLE-Twist1 cells compared to HMLE control cells. Alternatively, when we knocked down Twist1 in 168FARN cells, BMDM migration was significantly reduced compared to 168FARN control knockdown conditioned media (Fig. 4C). Importantly, we were able to rescue this migration defect by supplementing conditioned media from 168FARN-shTwist1 cells with recombinant CCL2 (rCCL2) (Fig. 4D). Finally, macrophage migration was significantly reduced towards conditioned media from HMLE-Twist1 cells expressing CCL2 shRNAs when compared to HMLE-Twist1 cells expressing a control shRNA (Fig. 4E). Collectively, these results demonstrate that induction of CCL2 is important for the ability of Twist1 to promote macrophage attraction.
Figure 4. Twist1 expression in mammary epithelial cells promotes macrophage attraction in a CCL2-dependent manner.
A) Cell surface expression of CCR2 on isolated mouse BMDM as measured by flow cytometry analysis using anti-CCR2 (open) versus Control IgG (shaded) antibody staining. B) BMDM were subjected to Boyden Chamber migration assays toward conditioned media from HMLE-Ctrl or HMLE-Twist1 cells. Representative images of migrated macrophages stained with crystal violet (top) and quantification of relative migration (bottom) for one representative experiment, *p<0.05 by Student’s T-test. C) Macrophage migration toward conditioned media from 168FARN cells with indicated shRNAs for one representative experiment, *p<0.05 versus shCtrl by ANOVA followed by Tukey Test. D) Macrophage migration toward conditioned media from 168FARN cells with shRNAs against Twist1, coincubated with either PBS or rCCL2 (10 ng/mL) for one representative experiment, *p<0.05 by Student’s T-test. E) Macrophage migration toward conditioned media from HMLE-Twist1 cells expressing indicated shRNAs for one representative experiment, *p<0.05 versus shCtrl by ANOVA followed by Tukey Test.
Twist1 expression promotes angiogenesis and macrophage infiltration in the Matrigel plug assay
We next aimed to determine whether Twist1 expression in mammary tumor cells leads to macrophage recruitment in vivo. To do this, we performed Matrigel plug assays in mice using 168FARN cells in which CCL2 expression depends on endogenous Twist1 (Fig. 2E–G). We harvested the plugs five days after implantation for analysis. Macroscopic examination revealed that Matrigel plugs containing 168FARN cells expressing a control shRNA appeared to contain more blood than plugs with 168FARN cells expressing Twist1 shRNAs (Fig. 5A, top row). Using CD31 as a marker for endothelial cells, we found that knocking down Twist1 significantly reduced the number of blood vessels in Matrigel plug tissue sections by approximately 50%, further supporting our conclusion that Twist1 promotes angiogenesis in vivo (Fig. 5A, middle row, Fig. 5B). Importantly, we measured macrophage content in serial sections by staining for the mature macrophage marker F4/80 and found that Twist1 knockdown also significantly decreased macrophage content by approximately 50% (Fig. 5A, bottom row, Fig. 5C). We conclude that Twist1 expression in tumor cells is required for macrophage recruitment and angiogenesis in vivo.
Figure 5. Twist1 expression promotes angiogenesis and macrophage infiltration in the Matrigel plug assay.
A) A mixture of Matrigel and 168FARN mouse mammary tumor cells was subcutaneously injected into 4–5 mice/group. Resulting plugs were harvested 5 days later and processed for immunohistochemical staining. Top row, macroscopic images of plugs; middle row, images of tissue sections stained for blood vessel marker CD31(brown); bottom row, images of tissue sections stained for mature macrophage marker F4/80 (brown). Nuclei were stained with hematoxylin (blue). Insets show magnification for staining detail, scale bar = 2 mm. B and C) Quantification of stains in (A), 5 random high-powered fields per section were counted for number of CD31 stained vessels or were scored for F4/80 staining intensity and distribution; 7–10 plugs/group and 1–2 sections/plug were analyzed. Scale bar = 50 µm, error bars are SEM, *p<0.05 versus shCtrl by ANOVA followed by Tukey Test.
CCL2 and macrophage recruitment are essential for Twist1-induced angiogenesis
We next set out to understand whether macrophages and CCL2 are important for Twist1 to promote angiogenesis. First, we depleted macrophages using clodronate liposomes in the Matrigel plug assay. In vivo, macrophages recognize liposomes as foreign bodies. Upon phagocytosis and release of the encapsulated clodronate, macrophages undergo apoptosis and are not replenished during the experimental period (29). Indeed, clodronate liposome injection compared to control liposome injection, effectively reduced the presence of F4/80 positive macrophages to nearly undetectable levels in Matrigel plugs containing 168FARN cells (Fig. 6A, bottom row, Fig. 6C). As expected, control liposome injection did not affect the strong angiogenic response induced by 168FARN cells as observed both macroscopically and as measured by CD31 staining (Fig. 6A, top and middle rows, Fig. 6B). In contrast, macrophage depletion by clodronate liposome treatment significantly reduced Twist1-induced angiogenesis by 74.4% (Fig. 6A, top and middle rows, Fig. 6B). This data indicate that macrophage recruitment is essential for the ability of Twist1 to promote angiogenesis.
Figure 6. CCL2 and macrophage recruitment are essential for Twist1-induced angiogenesis.
A) A mixture of Matrigel and 168FARN cells was subcutaneously injected mice either previously depleted of macrophages by clodronate-encapsulated liposomes (9 mice) or treated with Control liposomes (6 mice). Resulting plugs were harvested 5 days later and processed for immunohistochemical staining. Top row, macroscopic images of plugs; middle row, images of tissue sections stained for blood vessel marker CD31(brown); bottom row, images of tissue sections stained for mature macrophage marker F4/80 (brown). Nuclei were stained with hematoxylin (blue). Scale bar = 2 mm. B and C) Quantification of stains in (A), 5 random high-powered fields per section were counted for number of CD31 stained vessels or were scored for F4/80 staining intensity and distribution; 10–15 plugs/group and 1–2 sections/plug were analyzed. Scale bar = 50 µm, error bars are SEM. *p<0.05 by Student’s T-test. D) Matrigel plug assays were performed using 168FARN mouse mammary tumor cells and supplemented with recombinant CCL2 (10 ng/mL final concentration) or PBS using at least 3 mice/group. Scale bar = 2 mm. E and F) Quantification of stains in (D), with 6–8 plugs/group and 1–2 sections/plug analyzed. Scale bar = 50 µm, error bars are SEM, *p<0.05 by Student’s T-test.
Second, we asked whether addition of exogenous CCL2 could rescue the ability of 168FARN tumor cells with Twist1 knockdown to attract macrophages and promote angiogenesis. To test this, we added rCCL2 to Matrigel plugs with 168FARN cells expressing two independent Twist1 shRNA constructs. Significantly, addition of rCCL2 increased macrophage influx by approximately 1.9-fold and 2.3-fold compared to PBS-added Matrigel plugs (Fig. 6D, bottom row, Fig. 6F). Importantly, rCCL2 addition increased the number of CD31 positive vessels by 1.9-fold in both groups compared to Matrigel plugs supplemented with PBS (Fig. 6D, top and middle rows, Fig. 6E). Together, results from macrophage depletion and CCL2 rescue experiments indicate that CCL2-dependent macrophage recruitment is essential for the ability of Twist1 to promote angiogenesis in vivo.
DISCUSSION
This study supports and expands upon the observations that Twist1 facilitates multiple important steps in metastasis including angiogenesis. Importantly, we have demonstrated the ability of Twist1-expressing cells to promote angiogenesis via a non-cell autonomous mechanism involving macrophage recruitment. We show that Twist1 induces robust CCL2 expression of CCL2 and knockdown of CCL2 leads to a decrease in Twist1-induced angiogenesis in a quantitative CAM angiogenesis assay. We demonstrate that isolated macrophages, which express high levels of CCR2, can migrate toward conditioned medium from Twist1-expressing cells in a CCL2-dependent manner. Using mouse Matrigel plug assays, we show that expression of Twist1 promotes both angiogenesis and macrophage infiltration and both CCL2 and macrophage influx are important for Twist1-induced neovascularization. Based on these results, we propose a model in which upregulation of Twist1 in carcinoma cells activates transcription of CCL2 mRNA; secretion of CCL2 protein from Twist1-expressing cells creates a chemotactic gradient to promote macrophage infiltration and subsequent angiogenic stimulation (Fig. 7).
Figure 7. Proposed model for Twist1-induced angiogenesis through CCL2 induction and macrophage recruitment.
Carcinoma cells expressing Twist1 upregulate CCL2 transcript leading to increased CCL2 protein in the tumor microenvironment. The CCL2 gradient attracts macrophages, which then promote angiogenesis.
To date, the predominant role of Twist1 in tumor progression is thought to be cell autonomous through the induction of Epithelial Mesenchymal Transition (EMT) (4). Our current study uncovers a novel function of Twist1 to generate tumor-stromal cell communication by recruiting macrophages, a cell type previously shown to facilitate angiogenesis and metastasis in a mammary tumor model (18). Interestingly, ovarian tumor cells that have undergone EMT are shown to promote monocyte differentiation to a pro-angiogenic phenotype (35). Importantly, we show that the ability of Twist1 to promote angiogenesis is independent of its role in EMT since CCL2 suppression specifically hindered the angiogenic response without affecting the Twist1-induced EMT phenotype.
We show that knocking down Twist1 in 168FARN tumor cells caused 70% reduction of CCL2. Such CCL2 reduction resulted in over 50% suppression of macrophage infiltration in vivo, but less than 30% reduction in macrophage migration in vitro. One explanation for this difference is that mouse macrophages are highly sensitive to mouse CCL2 (36), and the conditioned media used for the in vitro macrophage migration assay contains CCL2 at concentrations well above the level required for macrophage sensitivity. Another possibility is that Twist1 regulates other molecules to attract additional stromal cell types to indirectly recruit macrophages, which could also explain why addition of CCL2 alone did not completely rescue Twist1-dependent angiogenesis and macrophage recruitment.
This study focuses mainly on the role of Twist1-induced CCL2 in angiogenesis. Angiogenesis gives invading tumor cells access to blood circulation for systemic dissemination; therefore this function of Twist1 likely aids its role in metastasis. Because macrophages have been shown to contribute to many steps of tumor progression, Twist1-induced CCL2 production and macrophage recruitment likely play critical roles in additional steps of tumor development, which merits future studies. First, macrophages are known to secrete proteases such as MMPs, which can degrade extracellular matrix and release matrix-bound growth factors (37). Functionally, macrophage-mediated matrix degradation could allow endothelial cells to invade tumor tissues during angiogenesis (38) and also could facilitate tumor cell invasion. Of note, a previous study showed that co-culture of tumor cells and macrophages promoted collagen degradation ability, highlighting a symbiotic relationship between them (39). Twist1, in addition to inducing EMT and invadopodia formation (4–6), may engage macrophages to promote tumor invasion.
Second, several new studies emphasize important roles that CCL2 and macrophages play not only at the primary tumor site, but also at the site of metastasis. The Pollard group found that the onset of metastasis was delayed when mice lacking macrophages were crossed to the PyMT mammary tumor model (40). Interestingly, they found that metastatic tumor cells secrete CCL2 to engage macrophages, thus increasing endothelial permeability and facilitating extravasation and metastatic seeding (22, 23). In parallel, another study found that tumor cell-derived CCL2 engaged two CCR2-positive cell types derived from the monocyte lineage to facilitate metastasis: macrophages for lung metastasis and osteoclasts for osteolytic bone metastasis (24). Our study identified Twist1 as a potent inducer of CCL2 in metastatic tumor cells. Potentially, induction of CCL2 by Twist1 could likewise function to attract macrophages to promote extravasation and metastatic seeding in distant organs.
In summary, we demonstrate that Twist1 has important non-cell autonomous functions to aid in metastasis. Twist1 expression in tumor cells allows interactions with macrophages through upregulation of CCL2. As macrophages have important roles in many steps of tumor progression, our study has extended the depth at which Twist1 may impact the metastatic cascade and suggests the rationale for anti-CCL2 therapy in treating Twist1-positive metastatic tumors.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Elena Deryugina, Tracy Handel, Catherina Salanga, Michael Lam, Yumiko Tanaka, and Etienne Danis for reagents and invaluable technical expertise. We thank the members of the Yang lab for helpful discussions and critically reading the manuscript.
Grant Support:
This work was supported by the UCSD Genetics Graduate Training Program from NIH (T32 GM08666) and California Breast Cancer Research Program Dissertation Grant (14GB-0144) to J.M.L, by NIH (R01 CA105412 and R01 CA129484) to J.P.Q, and by NIH (DP2 OD002420-01), American Cancer Society (RSG-09-282-01-CSM), Sidney Kimmel Foundation for Cancer Research, California Breast Cancer Program, and Mary Kay Ash Charitable Foundation to J.Y.
Footnotes
Disclosure of Potential Conflict of interest:
No potential conflict of interest is disclosed.
REFERENCES
- 1.Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–496. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Gomez I, Pena C, Herrera M, Munoz C, Larriba MJ, Garcia V, et al. TWIST1 is expressed in colorectal carcinomas and predicts patient survival. PLoS One. 2011;6:e18023. doi: 10.1371/journal.pone.0018023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie F, Li K, Ouyang X. Twist, an independent prognostic marker for predicting distant metastasis and survival rates of esophageal squamous cell carcinoma patients. Clin Exp Metastasis. 2009;26:1025–1032. doi: 10.1007/s10585-009-9292-5. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Casas E, Kim J, Bendesky A, Ohno-Machado L, Wolfe CJ, Yang J. Snail2 is an essential mediator of Twist1-induced epithelial mesenchymal transition and metastasis. Cancer Res. 2011;71:245–254. doi: 10.1158/0008-5472.CAN-10-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues CO, Nerlick ST, White EL, Cleveland JL, King ML. A Myc-Slug (Snail2)/Twist regulatory circuit directs vascular development. Development. 2008;135:1903–1911. doi: 10.1242/dev.011296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerecht-Nir S, Dazard JE, Golan-Mashiach M, Osenberg S, Botvinnik A, Amariglio N, et al. Vascular gene expression and phenotypic correlation during differentiation of human embryonic stem cells. Dev Dyn. 2005;232:487–497. doi: 10.1002/dvdy.20247. [DOI] [PubMed] [Google Scholar]
- 9.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al. Direct Regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 10.Gort EH, van Haaften G, Verlaan I, Groot AJ, Plasterk RH, Shvarts A, et al. The TWIST1 oncogene is a direct target of hypoxia-inducible factor-2alpha. Oncogene. 2008;27:1501–1510. doi: 10.1038/sj.onc.1210795. [DOI] [PubMed] [Google Scholar]
- 11.Mironchik Y, Winnard PT, Jr, Vesuna F, Kato Y, Wildes F, Pathak AP, et al. Twist overexpression induces in vivo angiogenesis and correlates with chromosomal instability in breast cancer. Cancer Res. 2005;65:10801–10809. doi: 10.1158/0008-5472.CAN-05-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu L, Roth JM, Brooks P, Ibrahim S, Karpatkin S. Twist Is Required for Thrombin-Induced Tumor Angiogenesis and Growth. Cancer Res. 2008;68:4296–4302. doi: 10.1158/0008-5472.CAN-08-0067. [DOI] [PubMed] [Google Scholar]
- 13.Ribatti D, Nico B, Crivellato E, Vacca A. The structure of the vascular network of tumors. Cancer Lett. 2007;248:18–23. doi: 10.1016/j.canlet.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 15.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, et al. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011;179:1455–1470. doi: 10.1016/j.ajpath.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 19.Bolat F, Kayaselcuk F, Nursal TZ, Yagmurdur MC, Bal N, Demirhan B. Microvessel density, VEGF expression, and tumor-associated macrophages in breast tumors: correlations with prognostic parameters. J Exp Clin Cancer Res. 2006;25:365–372. [PubMed] [Google Scholar]
- 20.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- 21.Bingle L, Lewis CE, Corke KP, Reed MW, Brown NJ. Macrophages promote angiogenesis in human breast tumour spheroids in vivo. Br J Cancer. 2006;94:101–107. doi: 10.1038/sj.bjc.6602901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu X, Kang Y. Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J Biol Chem. 2009;284:29087–29096. doi: 10.1074/jbc.M109.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norris PC, Reichart D, Dumlao DS, Glass CK, Dennis EA. Specificity of eicosanoid production depends on the TLR-4-stimulated macrophage phenotype. J Leukoc Biol. 2011;90:563–574. doi: 10.1189/jlb.0311153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deryugina EI, Quigley JP. Chapter 2. Chick embryo chorioallantoic membrane models to quantify angiogenesis induced by inflammatory and tumor cells or purified effector molecules. Methods Enzymol. 2008;444:21–41. doi: 10.1016/S0076-6879(08)02802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Stenken JA. Affinity-based microdialysis sampling using heparin for in vitro collection of human cytokines. Anal Chim Acta. 2009;651:105–111. doi: 10.1016/j.aca.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 30.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettersson AT, Laurencikiene J, Mejhert N, Naslund E, Bouloumie A, Dahlman I, et al. A possible inflammatory role of twist1 in human white adipocytes. Diabetes. 2009;59:564–571. doi: 10.2337/db09-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes S, Poh TY, Bumstead N, Kaiser P. Re-evaluation of the chicken MIP family of chemokines and their receptors suggests that CCL5 is the prototypic MIP family chemokine, and that different species have developed different repertoires of both the CC chemokines and their receptors. Dev Comp Immunol. 2007;31:72–86. doi: 10.1016/j.dci.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser P, Poh TY, Rothwell L, Avery S, Balu S, Pathania US, et al. A genomic analysis of chicken cytokines and chemokines. J Interferon Cytokine Res. 2005;25:467–484. doi: 10.1089/jir.2005.25.467. [DOI] [PubMed] [Google Scholar]
- 35.Collino F, Revelli A, Massobrio M, Katsaros D, Schmitt-Ney M, Camussi G, et al. Epithelial-mesenchymal transition of ovarian tumor cells induces an angiogenic monocyte cell population. Exp Cell Res. 2009;315:2982–2994. doi: 10.1016/j.yexcr.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Luini W, Sozzani S, Van Damme J, Mantovani A. Species-specificity of monocyte chemotactic protein-1 and-3. Cytokine. 1994;6:28–31. doi: 10.1016/1043-4666(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 37.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 38.Anghelina M, Krishnan P, Moldovan L, Moldovan NI. Monocytes and macrophages form branched cell columns in matrigel: implications for a role in neovascularization. Stem Cells Dev. 2004;13:665–676. doi: 10.1089/scd.2004.13.665. [DOI] [PubMed] [Google Scholar]
- 39.Henry N, Eeckhout Y, van Lamsweerde AL, Vaes G. Co-operation between metastatic tumor cells and macrophages in the degradation of basement membrane (type IV) collagen. FEBS Lett. 1983;161:243–246. doi: 10.1016/0014-5793(83)81017-5. [DOI] [PubMed] [Google Scholar]
- 40.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.