Abstract
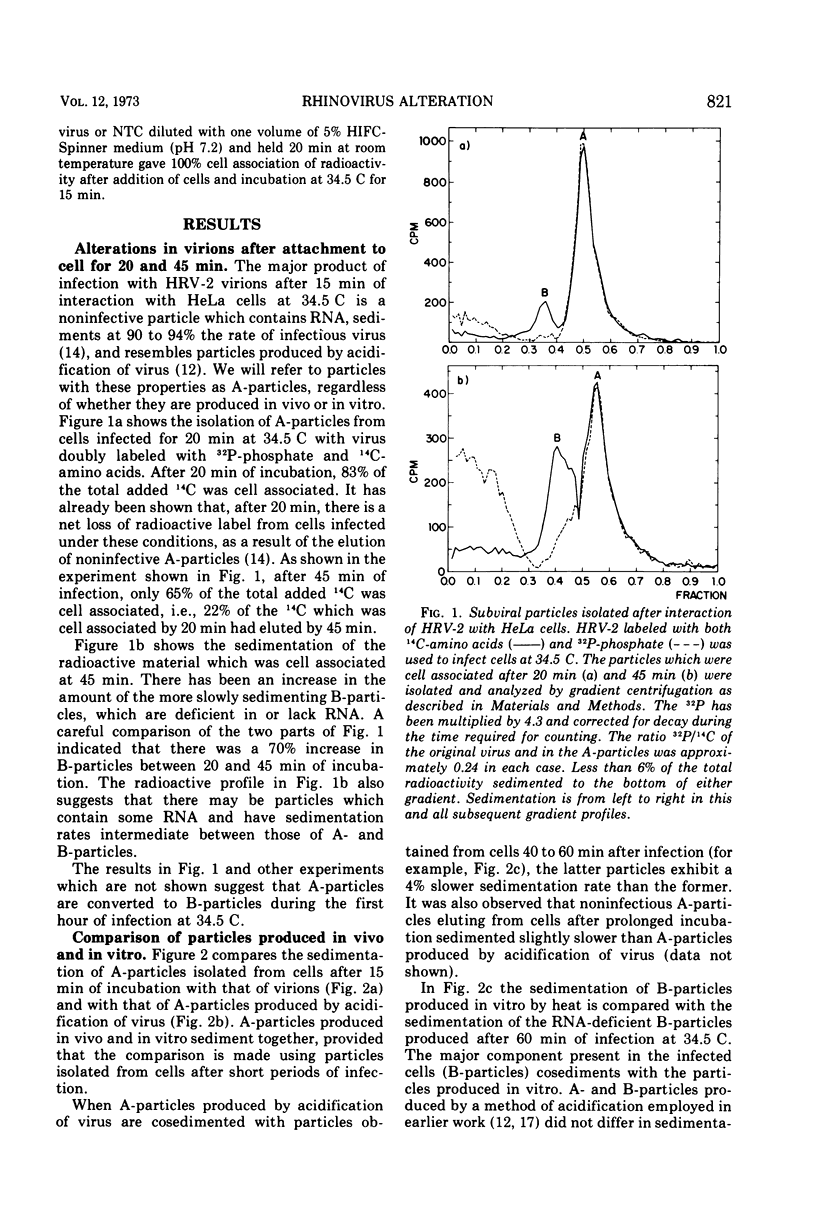
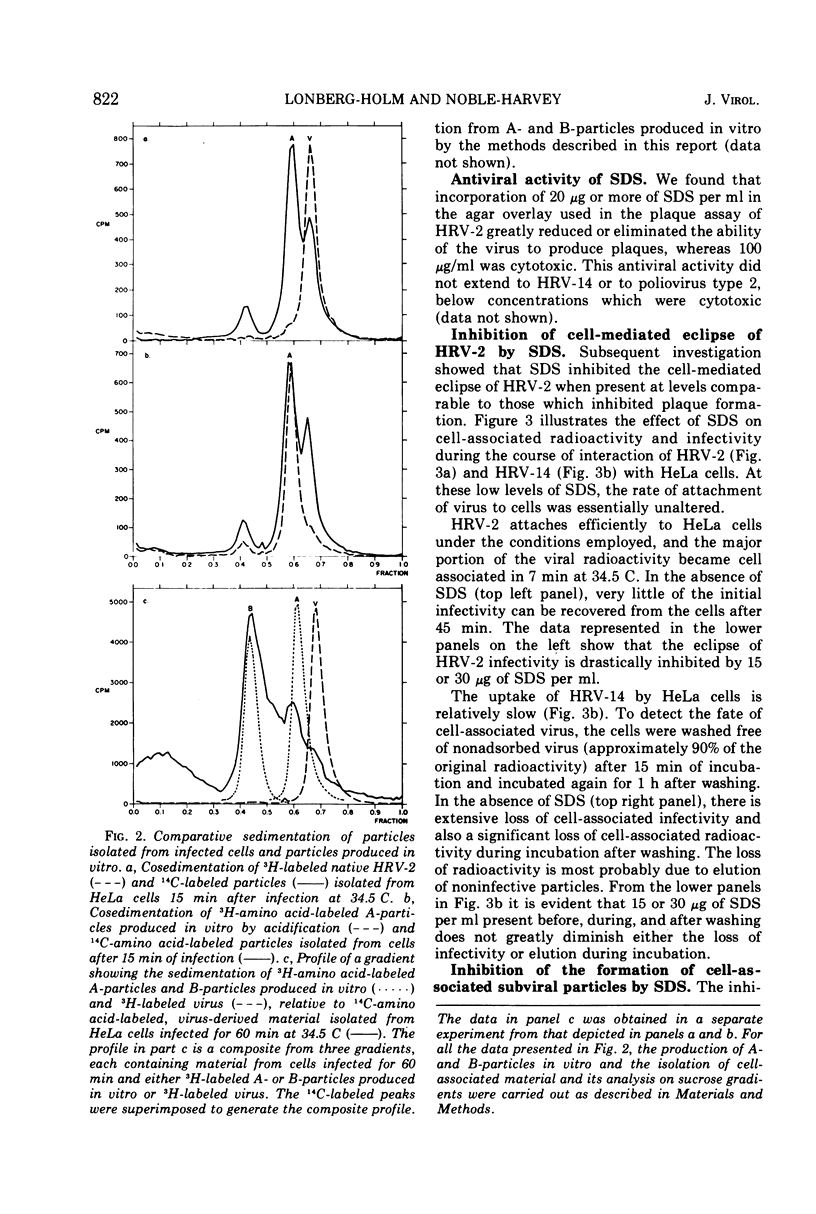
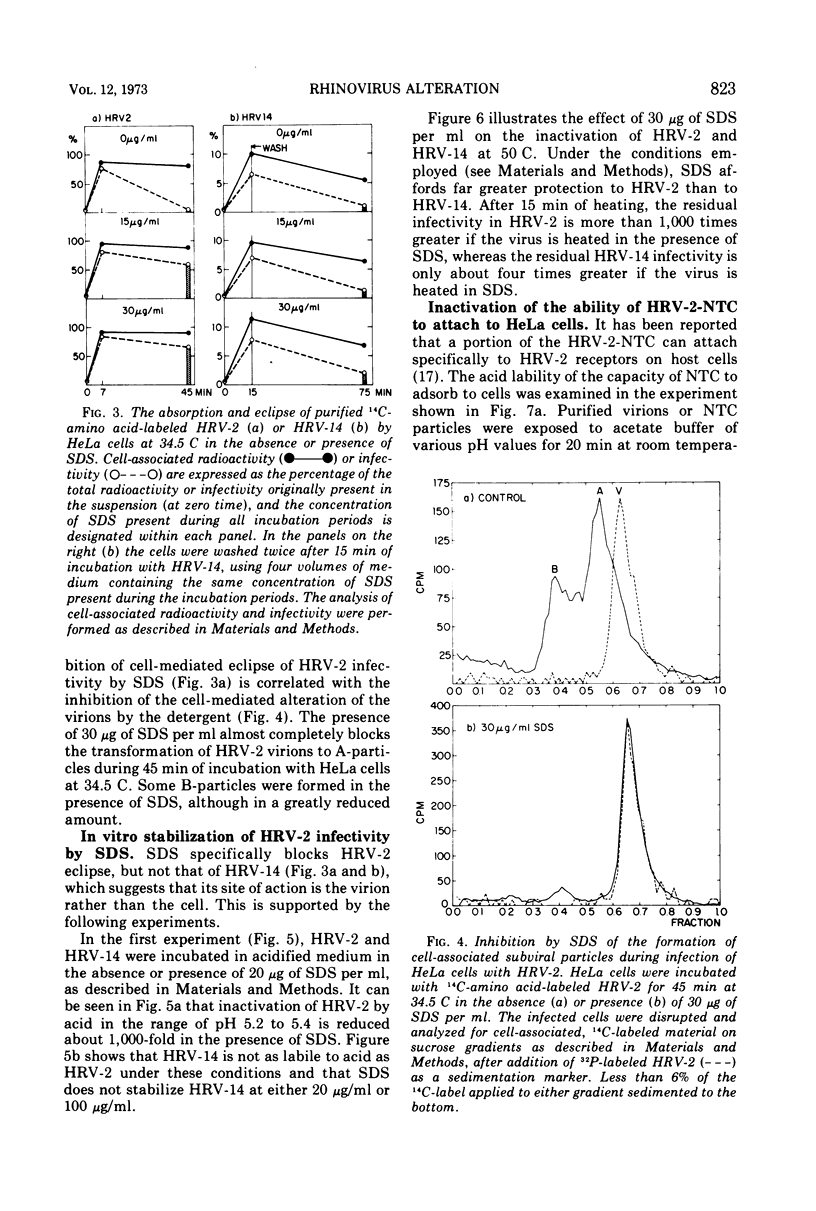
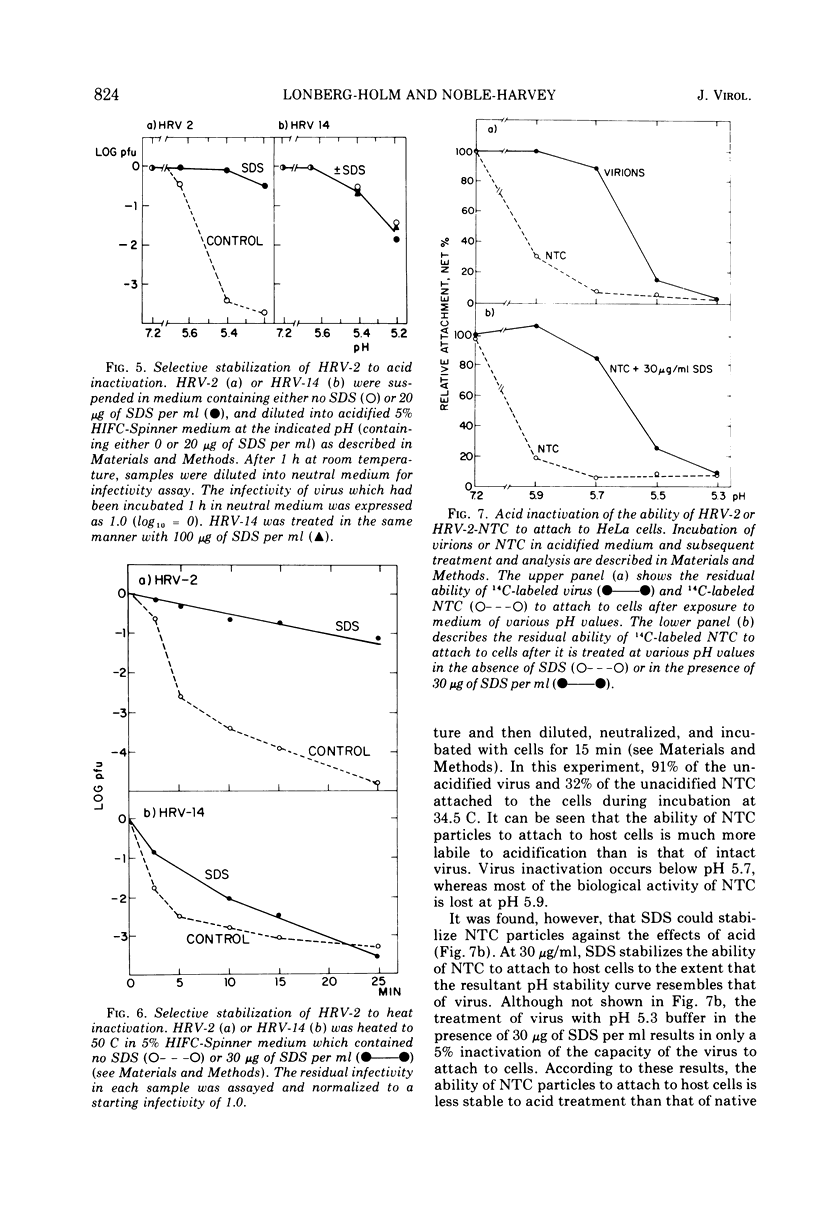
After human rhinovirus type 2 (HRV-2) attaches to HeLa cells, two types of subviral particles are formed which closely resemble particles produced in vitro by acid or heat. One type of particle contains RNA whereas the second sediments as an empty capsid and is RNA-deficient. Sodium dodecyl sulfate (SDS) at 10−4 M inhibits the cell-mediated formation of these particles from HRV-2 virions and the ability of HRV-2 to form plaques, but it does not inhibit the formation of plaques by human rhinovirus 14 (HRV-14). SDS also stabilizes HRV-2 against inactivation by acid or heat to a much greater extent than it does HRV-14. In a similar manner, SDS protects against the acid inactivation of the subpopulation of HRV-2 natural top component particles which attach to virus-specific cellular receptors. This suggests that the loss of native properties of natural top component particles and of virion are related processes. The basis for this alteration and also its role in infection are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breindl M., Koch G. Competence of suspended HeLa cells for infection by inactivated poliovirus particles and by isolated viral RNA. Virology. 1972 Apr;48(1):136–144. doi: 10.1016/0042-6822(72)90121-3. [DOI] [PubMed] [Google Scholar]
- Breindl M. VP 4, the D-reactive part of poliovirus. Virology. 1971 Dec;46(3):962–964. doi: 10.1016/0042-6822(71)90097-3. [DOI] [PubMed] [Google Scholar]
- Butler T. C. pH: Another View. Science. 1973 Mar 2;179(4076):854–855. doi: 10.1126/science.179.4076.854-a. [DOI] [PubMed] [Google Scholar]
- Crowell R. L., Landau B. J., Philipson L. The early interaction of coxsackievirus B3 with HeLa cells. Proc Soc Exp Biol Med. 1971 Jul;137(3):1082–1088. doi: 10.3181/00379727-137-35732. [DOI] [PubMed] [Google Scholar]
- Crowell R. L., Philipson L. Specific alterations of coxsackievirus B3 eluted from HeLa cells. J Virol. 1971 Oct;8(4):509–515. doi: 10.1128/jvi.8.4.509-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENWICK M. L., COOPER P. D. Early interactions between poliovirus and ERK cells: some observations on the nature and significance of the rejected particles. Virology. 1962 Oct;18:212–223. doi: 10.1016/0042-6822(62)90007-7. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Lonberg-Holm K., Noble J., Stasny J. T. Naturally occurring and artificially produced components of three rhinoviruses. Virology. 1972 Apr;48(1):71–86. doi: 10.1016/0042-6822(72)90115-8. [DOI] [PubMed] [Google Scholar]
- LE BOUVIER G. L., SCHWERDT C. E., SCHAFFER F. L. Specific precipitates in agar with purified poliovirus. Virology. 1957 Dec;4(3):590–593. doi: 10.1016/0042-6822(57)90088-0. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Korant B. D. Early interaction of rhinoviruses with host cells. J Virol. 1972 Jan;9(1):29–40. doi: 10.1128/jvi.9.1.29-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Yin F. H. Antigenic determinants of infective and inactivated human rhinovirus type 2. J Virol. 1973 Jul;12(1):114–123. doi: 10.1128/jvi.12.1.114-123.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J. F., Rowlands D. J., Brown F. A physico-chemical sub-grouping of the mammalian picornaviruses. J Gen Virol. 1973 Feb;18(2):171–180. doi: 10.1099/0022-1317-18-2-171. [DOI] [PubMed] [Google Scholar]
- Noble J., Lonberg-Holm K. Interactions of components of human rhinovirus type 2 with Hela cells. Virology. 1973 Feb;51(2):270–278. doi: 10.1016/0042-6822(73)90427-3. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Herbert S., Polet H., Steinhardt J. The binding of divers detergent anions to bovine serum albumin. Biochemistry. 1967 Mar;6(3):937–947. doi: 10.1021/bi00855a038. [DOI] [PubMed] [Google Scholar]
- SCHMIDT N. J., DENNIS J., FROMMHAGEN L. H., LENNETTE E. H. SEROLOGIC REACTIVITY OF CERTAIN ANTIGENS OBTAINED BY FRACTIONATION OF COXSACKIE VIRUSES IN CESIUM CHLORIDE DENSITY GRADIENTS. J Immunol. 1963 Apr;90:654–662. [PubMed] [Google Scholar]



