Abstract
Background:
Many older patients with early stage non-small cell lung cancer (NSCLC) do not receive curative therapy. New surgical techniques and radiation therapy modalities, such as video-assisted thoracoscopic surgery (VATS), potentially allow more patients to receive treatment. The adoption of these techniques and their impact on access to cancer care among Medicare beneficiaries with stage I NSCLC are unknown.
Methods:
We used the Surveillance, Epidemiology and End Results-Medicare database to identify patients with stage I NSCLC diagnosed between 1998 and 2007. We assessed temporal trends and created hierarchical generalized linear models of the relationship between patient, clinical, and regional factors and type of treatment.
Results:
The sample comprised 13,458 patients with a mean age of 75.7 years. The proportion of patients not receiving any local treatment increased from 14.6% in 1998 to 18.3% in 2007. The overall use of surgical resection declined from 75.2% to 67.3% (P < .001), although the proportion of patients undergoing VATS increased from 11.3% to 32.0%. Similarly, although the use of new radiation modalities increased from 0% to 5.2%, the overall use of radiation remained stable. The oldest patients were less likely to receive surgical vs no treatment (OR, 0.12; 95% CI, 0.09-0.16) and more likely to receive radiation vs surgery (OR, 13.61; 95% CI, 9.75-19.0).
Conclusion:
From 1998 to 2007, the overall proportion of older patients with stage I NSCLC receiving curative local therapy decreased, despite the dissemination of newer, less-invasive forms of surgery and radiation.
Surgery and radiation can be curative in stage I non-small cell lung cancer (NSCLC); untreated patients with stage I NSCLC have a 5-year survival of only 6% compared with an overall survival of 43% to 73% for all patients with stage I cancer.1‐3 Curative therapy should be offered to all patients for whom it is clinically appropriate. However, up to 20% of patients with early stage NSCLC have been described as medically inoperable.4 Although new technologies have disseminated into clinical practice over the past decade, it is unknown whether these have led to increased access to treatment.
Video-assisted thoracoscopic surgery (VATS) reduces the morbidity of lung cancer surgery and improves quality of life for patients with lung cancer.5,6 It is unknown how adoption of VATS is affecting access to surgical resection in older persons with NSCLC; that is, VATS may have been adopted primarily for the treatment of patients who traditionally would have received open surgery, resulting in no net increase in the use of surgical resection. Alternatively, the adoption of VATS on the population level may have increased the proportion of patients receiving surgery.
Stereotactic body radiation therapy (SBRT) uses multiple radiation beams and sophisticated accounting for motion to deliver biologically effective doses of radiation several times higher than standard external beam radiation therapy (EBRT).7 Intensity-modulated radiation therapy (IMRT) is a method of radiation planning that decreases normal tissue exposure.8 It is also unclear whether these new radiation modalities are associated with an increased proportion of patients receiving radiation therapy or whether the new modalities are merely replacing existing modalities without any net gain in access to treatment.
Materials and Methods
Overview
We analyzed the Surveillance, Epidemiology and End Results (SEER)-Medicare data set for 1998 to 2007 to ascertain trends in the treatment of patients with stage I NSCLC. We used The Dartmouth Atlas of Health Care hospital referral regions (HRRs) to group patients into areas of similar health-care utilization. We constructed separate models to identify patient and regional factors independently associated with each radiation modality and to assess trends over time, and we estimated separate models to assess whether residence in an area of high VATS use was associated with surgical resection. The Yale Human Investigation Committee determined that this study did not constitute human subjects research.
Data Source and Study Population
The SEER-Medicare database links data from the SEER registries, covering 28% of the US population, with Medicare claims.9 We identified patients aged 67 to 94 years diagnosed with stage I NSCLC between 1998 and 2007, using the American Joint Committee on Cancer stage variable in SEER prior to 2004 and the derived American Joint Committee on Cancer variable in SEER in 2004 and later. Patients were excluded if they had an unknown month of diagnosis, a prior cancer diagnosis, a second cancer diagnosis (other than lung cancer) during the study period, or a diagnosis based on autopsy specimen or death certificate or if they were not continuously enrolled in fee-for-service Medicare Parts A and B from 24 months prior to diagnosis through 12 months after diagnosis.
Construction of Variables
The primary independent variable was treatment modality. Patients were assigned to treatment groups based on Medicare claims and the following hierarchy: surgery, SBRT/proton therapy, IMRT, and EBRT. We selected the following patient variables a priori as factors that might influence decisions regarding treatment: age, sex, race, urban vs rural residence, marital status, and income. Clinical variables included comorbidity, tumor size, histology (e-Appendix 1 (328.7KB, pdf) ), receipt of chemotherapy or invasive mediastinal staging, prior receipt of the influenza vaccine, admission for COPD exacerbation in the year prior to diagnosis, and life expectancy. We used Healthcare Common Procedure Coding System and International Classification of Diseases, Ninth Revision, codes to identify treatments and invasive staging (e-Appendix 2 (328.7KB, pdf) ).
US Department of Agriculture rural-urban continuum codes were used to classify patient area of residence. Comorbidity was assessed in two ways. First, Medicare claims for service in the previous 24 months through 3 months prior to diagnosis were used to identify the comorbid conditions recommended by Elixhauser et al,10 which we previously determined to be significantly associated with survival. International Classification of Diseases, Ninth Revision, codes were used if they appeared on an inpatient claim or two or more outpatient or physician claims billed > 30 days apart. Second, we created a variable to indicate whether a patient had been admitted for a COPD exacerbation in the year prior to diagnosis. A dichotomous variable to indicate whether a claim had been submitted for influenza vaccine for service in the previous 24 months through 3 months prior to diagnosis was created; this variable has been used previously as a marker for health-care system access.11,12
We used HRRs to examine geographic variation in the use of VATS.13 In patients receiving a diagnosis in 2002 and later, we calculated the percentage of surgery patients in each HRR (with at least 20 surgical patients) who underwent VATS. We also examined the following HRR variables: hospital bed and physician density, Centers for Medicare & Medicaid Services Hospital Compare technical process quality measures composite quality score (for acute myocardial infarction, congestive heart failure, and pneumonia in 2007), percentage of Medicare enrollees with one or more ambulatory visits, percentage of doctors in primary care specialty, percent mortality among non-health maintenance organization participants, and overall Medicare expenditures per beneficiary.14 We also constructed a dichotomous variable to indicate whether each patient’s state of residence required a certificate of need to construct new radiation facilities.
Statistical Analysis
We summarized patient demographic, clinical, and utilization characteristics according to treatment group (no treatment, surgery, IMRT, EBRT, or SBRT/proton therapy) and calculated the number of patients per physician and per HRR and the percentage of patients in each treatment group for each year (1998-2007). Rather than assume that patient treatment was independent of the physician and geographic region, we examined the degree to which variance in treatment was attributable to each of these factors using hierarchical generalized linear models (HGLMs).15 Because of the technical difficulties in estimating polytomous HGLMs, we estimated a series of logistic HGLMs. We modeled receipt of surgery using no treatment as the reference category and receipt of IMRT, EBRT, or SBRT/proton therapy using surgery as the reference category. To avoid overfitting and to assess the relationship between HRR factors and treatment, we estimated a series of two-level logistic HGLMs, one for each HRR factor and each treatment group, and included those that were significant (P < .05) in the corresponding full models. Finally, to assess any attenuating effect of the use of VATS, we conducted a subgroup analysis, extending these models to include a variable representing the percentage of patients receiving VATS, restricting this final analysis to the subset of HRRs for which VATS information was available. To account for calendar time, all models included elapsed years (from the beginning of the study period) and elapsed years squared. We used the models to calculate adjusted treatment rates for each calendar year and a Wilcoxon test for trend to assess whether these adjusted rates changed over time.16 All analyses were performed with SAS, version 9.2 (SAS Institute Inc) and Stata 12 (StataCorp LP) statistical software.
Results
We identified 13,458 patients with stage I NSCLC treated by 5,716 physicians and residing in 165 HRRs. Of these patients, 2,320 (17.2%) received no local treatment, 9,251 (68.7%) received surgery, and 1,887 (14.0%) received radiation therapy (Table 1). The mean ± SD age was 75.7 ± 5.7 years. The majority of the patients (84%) resided in urban areas. The prevalence of admission for COPD exacerbation in the last year before diagnosis was low in this cohort, with only 1.7% of surgically treated patients, 5.1% of patients not receiving local therapy, and 6.7% of patients receiving radiation therapy.
Table 1.
—Characteristics of the Sample by Treatment Group
| Characteristic | No Local Treatment | Surgery | EBRT | IMRT | SBRT/Proton Therapy |
| No. patients | 2,320 (17.2) | 9,251 (68.7) | 1,653 (12.3) | 108 (0.8) | 126 (0.9) |
| Age | |||||
| 67-69 y | 271 (13.0) | 1,616 (77.4) | 172 (8.2) | 15 (0.7) | 14 (0.7) |
| 70-74 y | 543 (13.2) | 3,138 (76.5) | 368 (9.0) | 30 (0.7) | 24 (0.6) |
| 75-79 y | 635 (16.2) | 2,750 (70.4) | 462 (11.8) | 33 (0.8) | 28 (0.7) |
| 80-84 y | 507 (22.0) | 1,356 (58.7) | 393 (17.0) | …a | …a |
| 85-94 y | 364 (34.7) | 391 (37.2) | 258 (24.6) | …a | …a |
| Sex | |||||
| Male | 1,234 (19.4) | 4,238 (66.8) | 781 (12.3) | 50 (0.8) | 44 (0.7) |
| Female | 1,086 (15.3) | 5,013 (70.5) | 872 (12.3) | 58 (0.8) | 82 (1.2) |
| Race | |||||
| White | 1,954 (16.2) | 8,407 (69.6) | 1,511 (12.5) | 93 (0.8) | 115 (1.0) |
| Black | 255 (32.5) | 416 (53.1) | (11-12)a | …a | …a |
| Other | 111 (18.7) | 428 (72.1) | (7-9)a | …a | …a |
| Income | |||||
| < $33,000 | 666 (22.2) | 1,849 (61.6) | 428 (14.3) | 34 (1.1) | 23 (0.8) |
| $33,000-$40,000 | 377 (18.9) | 1,313 (65.9) | 272 (13.6) | 11 (0.6) | 20 (1.0) |
| $40,000-$50,000 | 480 (16.6) | 1,984 (68.7) | 365 (12.6) | 28 (1.0) | 33 (1.1) |
| $50,000-$63,000 | 407 (15.7) | 1,826 (70.4) | 320 (12.3) | 19 (0.7) | 23 (0.9) |
| > $63,000b | 390 (13.1) | 2,279 (76.5) | 268 (9.0) | 16 (0.5) | 27 (0.9) |
| Marital status | |||||
| Married | 1,018 (14.4) | 5,198 (73.6) | 757 (10.7) | 39 (0.6) | 51 (0.7) |
| Not married | 1,221 (20.5) | 3,764 (63.2) | (13-15)a | …a | …a |
| Unknown | 81 (18.3) | 289 (65.4) | (13-15)a | …a | …a |
| Residence | |||||
| Urban | 1,921 (17.0) | 7,797 (69.1) | 1,365 (12.1) | 92 (0.8) | 111 (1.0) |
| Rural | 111 (17.9) | 428 (69.1) | (10-13)a | …a | …a |
| Other | 288 (18.5) | 1,026 (66.1) | (12-14)a | …a | …a |
| Comorbidities | |||||
| 0 | 770 (17.6) | 3,263 (74.4) | 314 (7.2) | 13 (0.3) | 26 (0.6) |
| 1-2 | 844 (14.5) | 4,168 (71.6) | 696 (11.9) | 58 (1.0) | 59 (1.0) |
| ≥ 3 | 706 (21.7) | 1,820 (56.1) | 643 (19.8) | 37 (1.1) | 41 (1.3) |
| Tumor size | |||||
| T1a | 658 (13.8) | 3,620 (76.1) | 398 (8.4) | 31 (0.7) | 50 (1.1) |
| T1b | 796 (17.7) | 3,031 (67.3) | 591 (13.1) | 37 (0.8) | 52 (1.2) |
| T2a | 866 (20.6) | 2,600 (62.0) | 664 (15.8) | 40 (1.0) | 24 (0.6) |
| Histology | |||||
| Adenocarcinoma | 654 (12.0) | 4,224 (77.8) | 470 (8.7) | 29 (0.5) | 52 (1.0) |
| Bronchioloalveolar carcinoma | 115 (7.8) | 1,312 (89.1) | (1-3)a | …a | …a |
| Squamous cell | 627 (15.4) | 2,821 (69.2) | 568 (13.9) | 33 (0.8) | 29 (0.7) |
| Large cell | 72 (13.5) | 381 (71.6) | (12-15)a | …a | …a |
| Other | 852 (43.8) | 513 (26.4) | 507 (26.1) | 37 (1.9) | 37 (1.9) |
| Chemotherapy | |||||
| No | 2,095 (17.5) | 8,505 (70.9) | 1,190 (9.9) | …a | …a |
| Yes | 225 (15.4) | 746 (51.0) | 463 (31.7) | …a | …a |
| Invasive mediastinal staging | |||||
| No | 2,230 (17.8) | 8,540 (68.1) | 1,555 (12.4) | …a | …a |
| Yes | 90 (9.8) | 711 (77.7) | 98 (10.7) | …a | …a |
| Influenza vaccine | |||||
| No | 1,214 (23.1) | 3,393 (64.7) | 567 (10.8) | 37 (0.7) | 34 (0.6) |
| Yes | 1,106 (13.5) | 5,858 (71.3) | 1,086 (13.2) | 71 (0.9) | 92 (1.1) |
| COPD admission | |||||
| No | 2,201 (16.8) | 9,095 (69.6) | 1,551 (11.9) | …a | …a |
| Yes | 119 (30.2) | 156 (39.6) | 102 (25.9) | …a | …a |
Data are presented as No. (%), unless otherwise indicated. EBRT = external beam radiation therapy; IMRT = intensity-modulated radiation therapy; SBRT = stereotactic body radiation therapy.
Number not reported because of the Centers for Medicare & Medicaid Services prohibition against the publication of cell sizes < 11 beneficiaries. In some cases, a range is provided for the percent value.
Highest income category for no treatment and surgery also includes < 11 patients with unknown income.
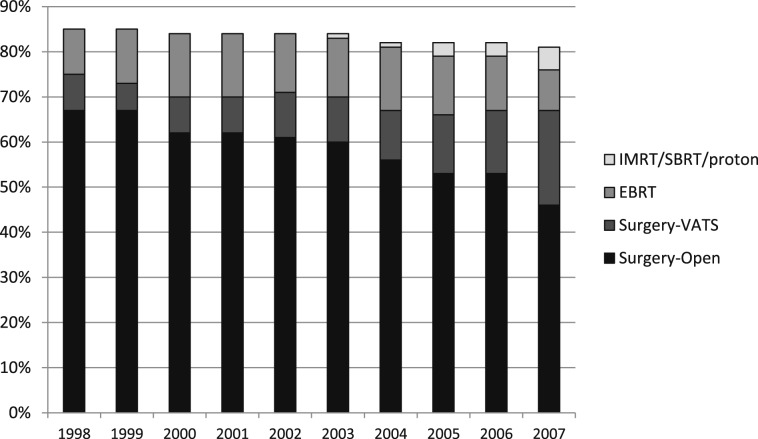
There was a trend over time toward increased numbers of patients not receiving any local treatment (14.6% in 1998 vs 18.3% in 2007) and fewer patients undergoing surgery (75.2% in 1998 vs 67.3% in 2007) (Fig 1). The proportion of patients aged > 85 years increased from 4.5% to 9%, and the percentage with three or more comorbidities increased from 15% to 30% over the study period. However, even after adjusting for age and comorbidities, this trend of decreasing use of surgery compared with no treatment was statistically significant (P < .001).
Figure 1.
Use of different treatment modalities among patients with stage I non-small cell lung cancer, 1998 to 2007. EBRT = external beam radiation therapy; IMRT = intensity-modulated radiation therapy; proton = proton therapy; SBRT = stereotactic body radiation therapy; Surgery-Open = surgery performed through thoracotomy; Surgery-VATS = surgery performed with video-assisted thoracoscopic surgery.
After a slight increase from 1998 to 2000, the total proportion of patients receiving any type of radiation therapy remained stable at about 16% between 2000 and 2007. An increasing proportion of patients received newer radiation modalities (5.2% of all patients and 32.5% of radiation-treated patients) by 2007.
Factors Associated With Surgical Resection
Unmarried patients (OR, 0.65; 95% CI, 0.57-0.75), black patients (OR, 0.43; 95% CI, 0.34-0.54), and patients aged > 85 years (OR, 0.12; 95% CI, 0.09-0.16) were less likely to undergo surgery (Table 2). Receipt of influenza vaccine (a proxy for access to health care) was associated with a greater likelihood of undergoing surgery (OR, 1.77; 95% CI, 1.56-2.01). Patients with an increased number of comorbidities or history of admission for COPD exacerbation were less likely to receive surgery compared with no treatment. Patients with larger tumors were less likely to undergo surgery (OR, 0.57; 95% CI, 0.48-0.66).
Table 2.
—Factors Associated With Receipt of Each Treatment Strategy
| Factor | Surgery Compared With No Local Treatment | EBRT Compared With Surgery | IMRT Compared With Surgery | SBRT/Proton Therapy Compared With Surgery |
| Age | ||||
| 67-69 y | Ref | Ref | Ref | Ref |
| 70-74 y | 0.84 (0.68-1.05) | 1.23 (0.96-1.58) | 1.20 (0.58-2.49) | 1.22 (0.49-3.04) |
| 75-79 y | 0.57 (0.46-0.70) | 2.07 (1.62-2.64) | 1.50 (0.71-3.08) | 1.35 (0.55-3.35) |
| 80-84 y | 0.32 (0.26-0.40) | 5.06 (3.87-6.61) | 2.22 (0.98-5.05) | 4.51 (1.68-12.12) |
| 85-94 y | 0.12 (0.09-0.16) | 13.80 (9.94-19.15) | 3.27 (1.16-9.17) | 21.65 (6.2-74.92) |
| Sex | ||||
| Male | Ref | Ref | Ref | Ref |
| Female | 1.14 (1.0-1.30) | 1.19 (1.02-1.39) | 0.81 (0.50-1.31) | 1.66 (0.93-2.95) |
| Race | ||||
| White | Ref | Ref | Ref | Ref |
| Black | 0.43 (0.34-0.54) | 1.17 (0.84-1.62) | 1.60 (0.70-3.65) | 1.79 (0.61-5.25) |
| Other | 0.57 (0.42-0.78) | 0.73 (0.46-1.13) | 0.99 (0.30-3.23) | 0.30 (0.04-2.26) |
| Marital status | ||||
| Married | Ref | Ref | Ref | Ref |
| Not married | 0.65 (0.57-0.75) | 1.31 (1.12-1.54) | 2.22 (1.34-3.70) | 1.15 (0.65-2.03) |
| Unknown | 0.66 (0.47-0.93) | 1.68 (1.14-2.48) | 2.04 (0.53-7.89) | 1.16 (0.23-5.79) |
| Residence | ||||
| Urban | Ref | Ref | Ref | Ref |
| Rural | 1.19 (0.85-1.65) | 0.85 (0.57-1.26) | 0.50 (0.12-2.06) | 0.18 (0.03-1.18) |
| Other | 1.02 (0.82-1.25) | 0.95 (0.74-1.22) | 0.98 (0.45-2.11) | 0.76 (0.30-1.94) |
| Comorbidities | ||||
| 0 | Ref | Ref | Ref | Ref |
| 1-2 | 0.85 (0.73-0.98) | 1.96 (1.63-2.36) | 3.21 (1.61-6.40) | 1.61 (0.82-3.18) |
| ≥ 3 | 0.47 (0.39-0.55) | 4.81 (3.91-5.92) | 3.33 (1.58-7.02) | 1.60 (0.75-3.40) |
| Tumor size | ||||
| T1a | Ref | Ref | Ref | Ref |
| T1b | 0.64 (0.55-0.75) | 1.68 (1.40-2.00) | 1.66 (0.94-2.93) | 1.19 (0.66-2.13) |
| T2a | 0.57 (0.48-0.66) | 1.86 (1.55-2.23) | 1.96 (1.09-3.52) | 0.58 (0.28-1.18) |
| Histology | ||||
| Adenocarcinoma | Ref | Ref | Ref | Ref |
| Bronchioloalveolar carcinoma | 1.65 (1.28-2.12) | 0.22 (0.14-0.32) | 0.64 (0.25-1.65) | 0.29 (0.09-0.90) |
| Squamous cell | 0.81 (0.70-0.94) | 1.67 (1.41-1.98) | 1.32 (0.75-2.34) | 0.79 (0.42-1.49) |
| Large cell | 0.78 (0.57-1.07) | 1.41 (1.01-1.98) | 0.79 (0.21-2.93) | 0.12 (0.01-1.58) |
| Other | 0.08 (0.07-0.10) | 11.82 (9.38-14.91) | 12.59 (6.26-25.30) | 12.34 (4.80-31.73) |
| Chemotherapy | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.52 (0.42-0.63) | 8.06 (6.56-9.90) | 3.17 (1.74-5.78) | 0.23 (0.05-0.97) |
| Influenza vaccine | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 1.77 (1.56-2.01) | 0.95 (0.82-1.11) | 0.99 (0.61-1.61) | 1.19 (0.67-2.10) |
| COPD admission | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 0.32 (0.23-0.44) | 3.29 (2.29-4.72) | 4.46 (1.71-11.64) | 7.36 (2.0-27.20) |
| HRR characteristicsa | ||||
| Medicare expenditures | 1.00 (1.00-1.00) | 1.00 (1.00-1.00) | ||
| Hospital Compare quality scoreb | 1.02 (0.97-1.07) | 0.88 (0.82-0.94) | 0.77 (0.66-0.89) | |
| Percentage of surgeries performed using VATSc | ||||
| < 8.6 | Ref | Ref | Ref | Ref |
| 8.7-12.3 | 1.06 (0.82-1.35) | 0.93 (0.61-1.42) | 0.97 (0.36-2.67) | 2.70 (0.80-9.14) |
| 13.6-18.1 | 1.11 (0.84-1.46) | 1.07 (0.66-1.71) | 0.96 (0.29-3.23) | 1.48 (0.38-5.69) |
| 18.4-26.6 | 0.95 (0.73-1.24) | 0.93 (0.58-1.50) | 1.15 (0.38-3.51) | 0.60 (0.14-2.60) |
| 26.7-70.5 | 1.28 (0.95-1.73) | 0.83 (0.49-1.41) | 1.36 (0.40-4.56) | 0.44 (0.09-2.28) |
Data are presented as OR (95% CI). HRR = hospital referral region; Ref = reference; VATS = video-assisted thoracoscopic surgery. See Table 1 legend for expansion of other abbreviations.
Models were adjusted for the following HRR variables: percentage of doctors in primary care specialty, total Medicare reimbursement, percentage non-health maintenance organization mortality (adjusted) (2006), Centers for Medicare & Medicaid Services Hospital Compare composite and state requirement for certificate of need for radiation. For clarity, only two are shown in the table.
Centers for Medicare & Medicaid Services Hospital Compare technical process quality measures composite quality score (acute myocardial infarction, congestive heart failure, and pneumonia) (2007).
VATS ORs are from separate models using the same variables but only including HRRs where at least 20 VATS were performed.
Regional Medicare expenditures and Hospital Compare quality scores were not associated with surgery vs no local therapy. There was significant variability in the use of VATS across HRRs, ranging from 0% to > 70% (median, 11.7%; interquartile range, 8.0-20.4). However, the proportion of surgeries performed using VATS was not significantly related to the proportion of patients who underwent surgery in a given HRR. For instance, the ORs for surgery (compared with no treatment) ranged from 0.95 (95% CI, 0.73-1.24) to 1.28 (95% CI, 0.95-1.73) in the fourth and fifth quintiles of VATS utilization compared with the lowest quintile. The majority of both open surgery and VATS was performed at teaching hospitals. Twenty percent of surgeries at teaching hospitals were VATS, and 11.9% of surgeries at nonteaching hospitals were VATS.
Factors Associated With Receipt of Radiation Therapy
Several patient factors were independently associated with the receipt of EBRT or IMRT compared with surgery (Table 2). Larger tumor size (OR, 1.86; 95% CI, 1.55-2.23) and admission for COPD exacerbation (OR, 3.29; 95% CI, 2.29-4.72) were associated with increased use of radiation therapy compared with surgery.
Patients who lived in regions with higher Hospital Compare quality scores were less likely to be treated with radiation therapy compared with surgery (OR, 0.88; 95% CI, 0.82-0.94). Patients residing in regions with high Medicare expenditures were more likely to undergo treatment with newer radiation modalities.
Discussion
An increasing proportion of patients with stage I NSCLC did not receive curative therapy from 1998 to 2007. Newer surgery and radiation modalities did not lead to an increase in the proportion of patients being treated. The failure of new technologies to increase access to therapy for NSCLC has not been seen in other fields. For example, the introduction of surgical robots and laparoscopic cholecystectomy has been associated with increased rates of prostatectomy and cholecystectomy, respectively.17,18
Potential causes of the decline in treatment of stage I NSCLC include increased diagnosis of lung cancer in patients believed unlikely to benefit from therapy, reluctance to treat older patients, and changing patient preferences. The increased age and comorbidity burden of the population have likely played a role in this decline as well, although the trends remained significant after adjusting for these factors. Patients with lung cancer have been reported to experience inappropriate therapeutic nihilism and stigma related to tobacco use, but we do not know how these factors are changing over time.19 Increased use of CT scanning may have led to more diagnoses of cancer in patients unwilling to undergo treatment.
The present findings suggest that the increased use of VATS during the study period represented a shift in surgical approach among patients who would have undergone surgery anyway rather than a broadening of access to surgical care. The finding that patients with larger tumors were less likely to undergo surgery may reflect the use of surgery to diagnose smaller lesions that may not be easily amenable to nonsurgical approaches to tissue diagnosis.
The association of higher Hospital Compare quality scores for care of nonmalignant disease with use of surgery instead of radiation therapy suggests that use of surgical resection for stage I NSCLC may be associated with higher-quality health care. The association of higher overall Medicare expenditures and newer radiation modalities suggests that use of newer, more-expensive technology for cancer care may be associated with the use of newer, more-expensive technology in general medical care.
The higher rate of chemotherapy use among patients treated with radiation may reflect concern about the effectiveness of radiation as a curative modality and merits further study. However, this could also be related to more accurate staging among surgical patients. The SEER database uses the best available information for determining stage. In surgically treated patients, this is pathologic staging after resection, whereas in radiation-treated patients, this information might be limited to imaging alone.
There was increased use of nonspecific histology codes in radiation-treated patients compared with surgically treated patients. This increase may reflect the use of less-invasive diagnostic techniques, such as needle biopsy, in patients treated with radiation and is consistent with prior reports that the diagnosis of carcinoma not otherwise specified was used in 22% of NSCLC cases overall and 37% of cytologically diagnosed cases.20
Unfortunately, race continues to be associated with lower use of surgical resection.21 Whether black patients are less likely to be offered surgery is controversial; however, it has also been shown that black patients are more likely to refuse surgery when offered.22 The present finding that radiation use was similar for both black and white patients may reflect greater acceptance of radiation therapy as opposed to surgery among black patients. Perhaps computerized decision aids or different educational processes would result in different treatment decisions and outcomes.
The present study has several limitations. For most of the study period, there was no specific Healthcare Common Procedure Coding System procedure code for linac-based SBRT, so we may have missed some early adopters of this modality. This does not alter our observation that despite increased use of newer radiation modalities, the overall proportion of patients undergoing radiation remained stable. For analytic purposes, we classified IMRT (a planning technique that can be used with either EBRT or SBRT) as a separate radiation modality because it indicated use of newer technology. When looking at the adoption of VATS, we included all VATS procedures, not just lobectomies. Because lobectomy is considered the appropriate surgery for stage I NSCLC, the availability of surgeons performing VATS wedge resection might not be an incentive for patients to undergo surgery. However, this does not explain or alter the finding that use of surgery actually declined. Finally, we were not able to determine why clinical decisions were made or whether they were appropriate.
The data showing a decline in the use of surgical resection, despite the introduction of VATS, for potentially curable NSCLC tumors raise concerns that important barriers to optimal care exist. Similarly, the increased use of newer radiation modalities did not result in an increase in the portion of patients receiving radiation. Although beyond the scope of our study, one possible explanation for this finding is that increasing numbers of early stage lung cancers were diagnosed in patients who were too ill from other diseases to benefit from treatment. It is imperative to further our understanding of challenges to accessing care for stage I NSCLC as well as to further refine risk stratification schema to identify patients who are likely to benefit from therapy. This understanding has both clinical and policy implications given the data showing a survival benefit for CT scan screening among high-risk smokers aged 55 to 74 years.23 As lung cancer screening begins to disseminate into clinical practice, further information will be needed to optimize care for the increasing numbers of patients with early stage lung cancer.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Gross had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Vest: contributed to the study design, data interpretation, and drafting and final review of the manuscript.
Dr Herrin: contributed to the study design, data interpretation, and review of the final manuscript.
Ms Soulos: contributed to the study design, data interpretation, and review of the final manuscript.
Dr Decker: contributed to the study design, data interpretation, and review of the final manuscript.
Dr Tanoue: contributed to the study design, data interpretation, and review of the final manuscript.
Dr Michaud: contributed to the study design, data interpretation, and review of the final manuscript.
Dr Kim: contributed to the study design, data interpretation, and review of the final manuscript.
Dr Detterbeck: contributed to the study design, data interpretation, and review of the final manuscript.
Dr Morgensztern: contributed to the study design, data interpretation, and review of the final manuscript.
Dr Gross: contributed to the study design, data interpretation, and review of the final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Gross has received support from Medtronic, Inc, to assist with strategies for dissemination of trial data and from FAIR Health, Inc. for serving on their Scientific Advisory board. Drs Vest, Herrin, Decker, Tanoue, Michaud, Kim, Detterbeck, and Morgensztern and Ms Soulos have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services, Inc; and the SEER program tumor registries in the creation of the SEER-Medicare database. This study used the SEER-Medicare-linked database. The interpretation and reporting of these data are the sole responsibility of the authors.
Additional information: The e-Appendixes can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- EBRT
external beam radiation therapy
- HGLM
hierarchical generalized linear model
- HRR
hospital referral region
- IMRT
intensity-modulated radiation therapy
- NSCLC
non-small cell lung cancer
- SBRT
stereotactic body radiation therapy
- SEER
Surveillance, Epidemiology and End Results
- VATS
video-assisted thoracoscopic surgery
Footnotes
Funding/Support: This work was supported by a grant from the National Cancer Institute [R01CA149045].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K; American College of Chest Physicians Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3):234S-242S [DOI] [PubMed] [Google Scholar]
- 2.Raz DJ, Zell JA, Ou SH, Gandara DR, Anton-Culver H, Jablons DM. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest. 2007;132(1):193-199 [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Crowley J, Chansky K, et al. ; International Association for the Study of Lung Cancer International Staging Committee ; Participating Institutions The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706-714 [DOI] [PubMed] [Google Scholar]
- 4.Mehta HJ, Ross C, Silvestri GA, Decker RH. Evaluation and treatment of high-risk patients with early-stage lung cancer. Clin Chest Med. 2011;32(4):783-797 [DOI] [PubMed] [Google Scholar]
- 5.Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thorac Surg. 2008;85(2):S719-S728 [DOI] [PubMed] [Google Scholar]
- 6.Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86(6):2008-2016 [DOI] [PubMed] [Google Scholar]
- 7.Das M, Abdelmaksoud MH, Loo BW, Jr, Kothary N. Alternatives to surgery for early stage non-small cell lung cancer-ready for prime time?. Curr Treat Options Oncol. 2010;11(1-2):24-35 [DOI] [PubMed] [Google Scholar]
- 8.Gewanter RM, Rosenzweig KE, Chang JY, et al. ACR appropriateness criteria: nonsurgical treatment for non-small-cell lung cancer: good performance status/definitive intent. Curr Probl Cancer. 2010;34(3):228-249 [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute Surveillance Epidemiology and End Results. National Cancer Institute website. http://seer.cancer.gov/about. Accessed November 26, 2011
- 10.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27 [DOI] [PubMed] [Google Scholar]
- 11.Vest MT, Tanoue L, Soulos PR, et al. Thoroughness of mediastinal staging in stage IIIA non-small cell lung cancer. J Thorac Oncol. 2012;7(1):188-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith BD, Haffty BG, Smith GL, Hurria A, Buchholz TA, Gross CP. Use of postmastectomy radiotherapy in older women. Int J Radiat Oncol Biol Phys. 2008;71(1):98-106 [DOI] [PubMed] [Google Scholar]
- 13.Dartmouth Institute for Health Policy and Clinical Practice Data by region. The Dartmouth Atlas of Health Care website. http://www.dartmouthatlas.org/data/region/. Accessed December 6, 2011
- 14.Dartmouth Institute for Health Policy and Clinical Practice Downloads. The Dartmouth Atlas of Health Care website. http://www.dartmouthatlas.org/tools/downloads.aspx. Accessed April 28, 2011
- 15.Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. London, UK: Sage; 1999 [Google Scholar]
- 16.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4(1):87-90 [DOI] [PubMed] [Google Scholar]
- 17.Makarov DV, Yu JB, Desai RA, Penson DF, Gross CP. The association between diffusion of the surgical robot and radical prostatectomy rates. Med Care. 2011;49(4):333-339 [DOI] [PubMed] [Google Scholar]
- 18.Legorreta AP, Silber JH, Costantino GN, Kobylinski RW, Zatz SL. Increased cholecystectomy rate after the introduction of laparoscopic cholecystectomy. JAMA. 1993;270(12):1429-1432 [PubMed] [Google Scholar]
- 19.Chambers SK, Dunn J, Occhipinti S, et al. A systematic review of the impact of stigma and nihilism on lung cancer outcomes. BMC Cancer. 2012;12(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ou SH, Zell JA. Carcinoma NOS is a common histologic diagnosis and is increasing in proportion among non-small cell lung cancer histologies. J Thorac Oncol. 2009;4(10):1202-1211 [DOI] [PubMed] [Google Scholar]
- 21.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341(16):1198-1205 [DOI] [PubMed] [Google Scholar]
- 22.McCann J, Artinian V, Duhaime L, Lewis JW, Jr, Kvale PA, DiGiovine B. Evaluation of the causes for racial disparity in surgical treatment of early stage lung cancer. Chest. 2005;128(5):3440-3446 [DOI] [PubMed] [Google Scholar]
- 23.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement