Abstract
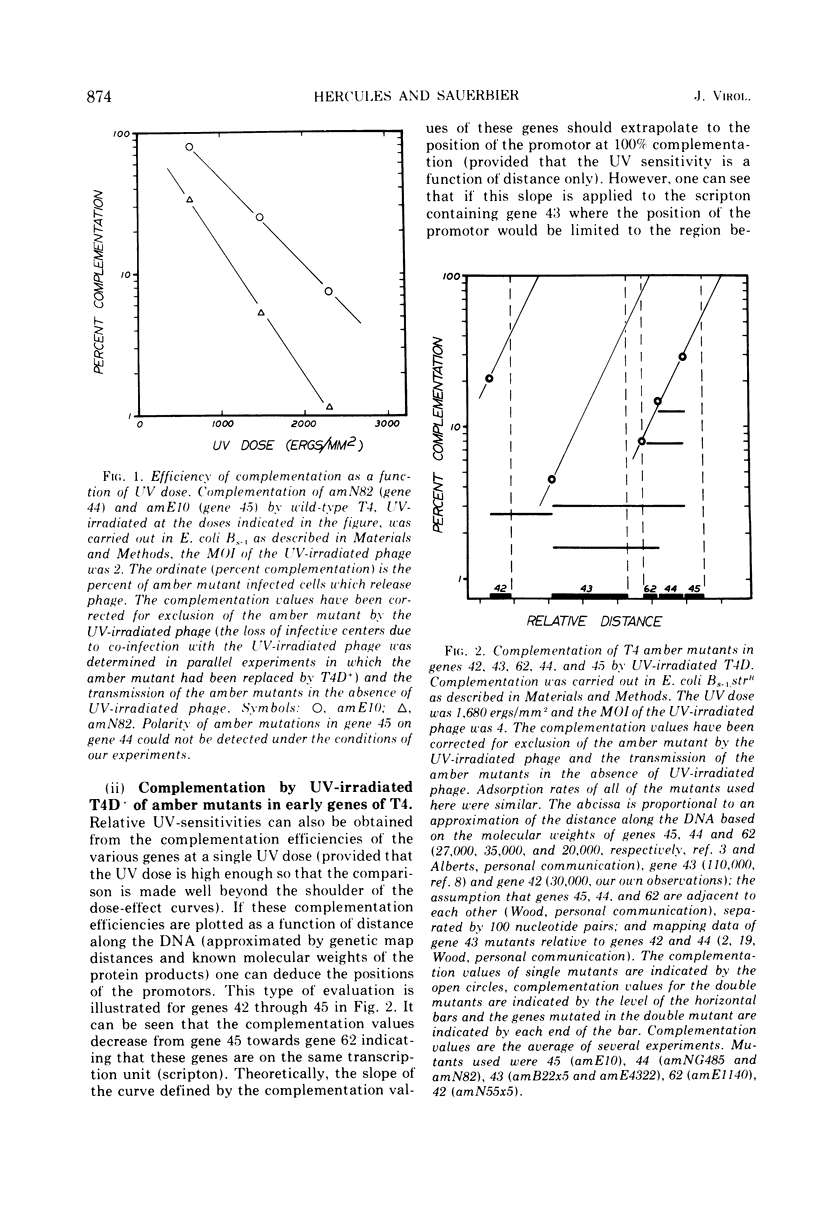
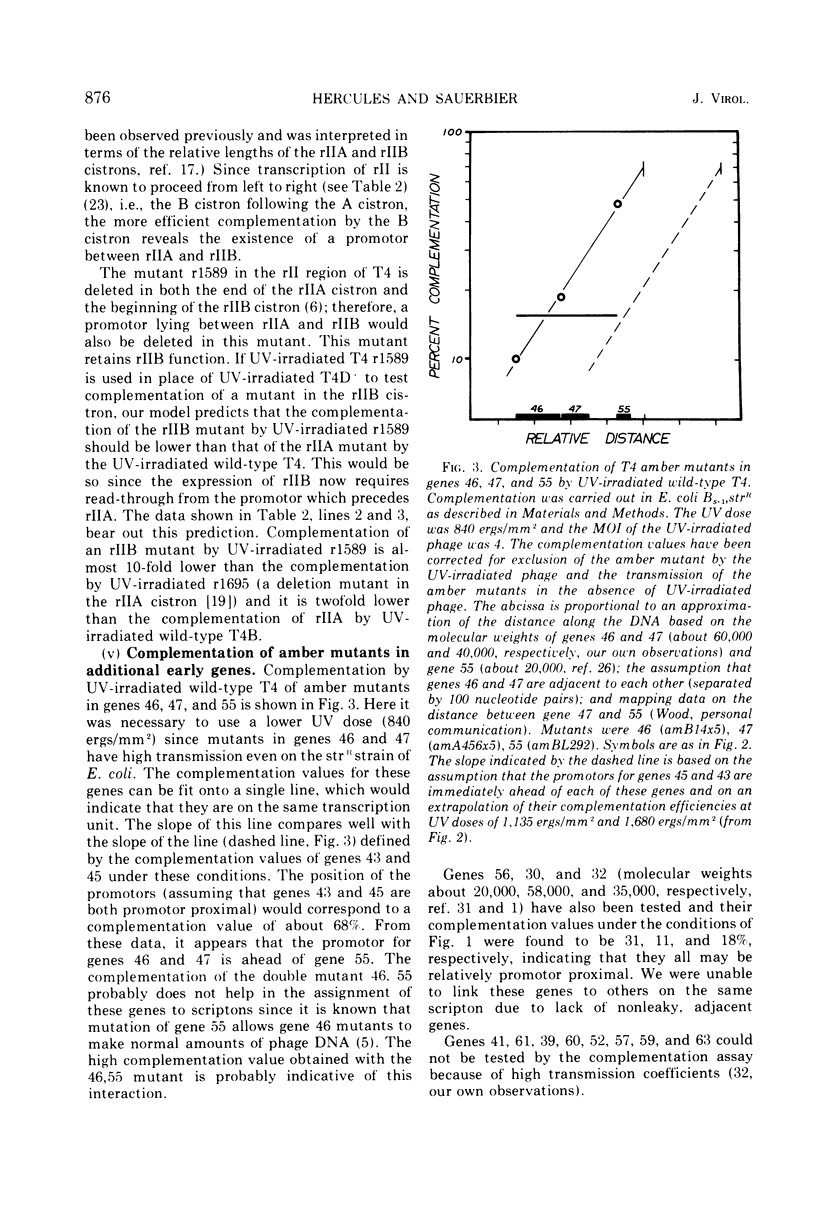
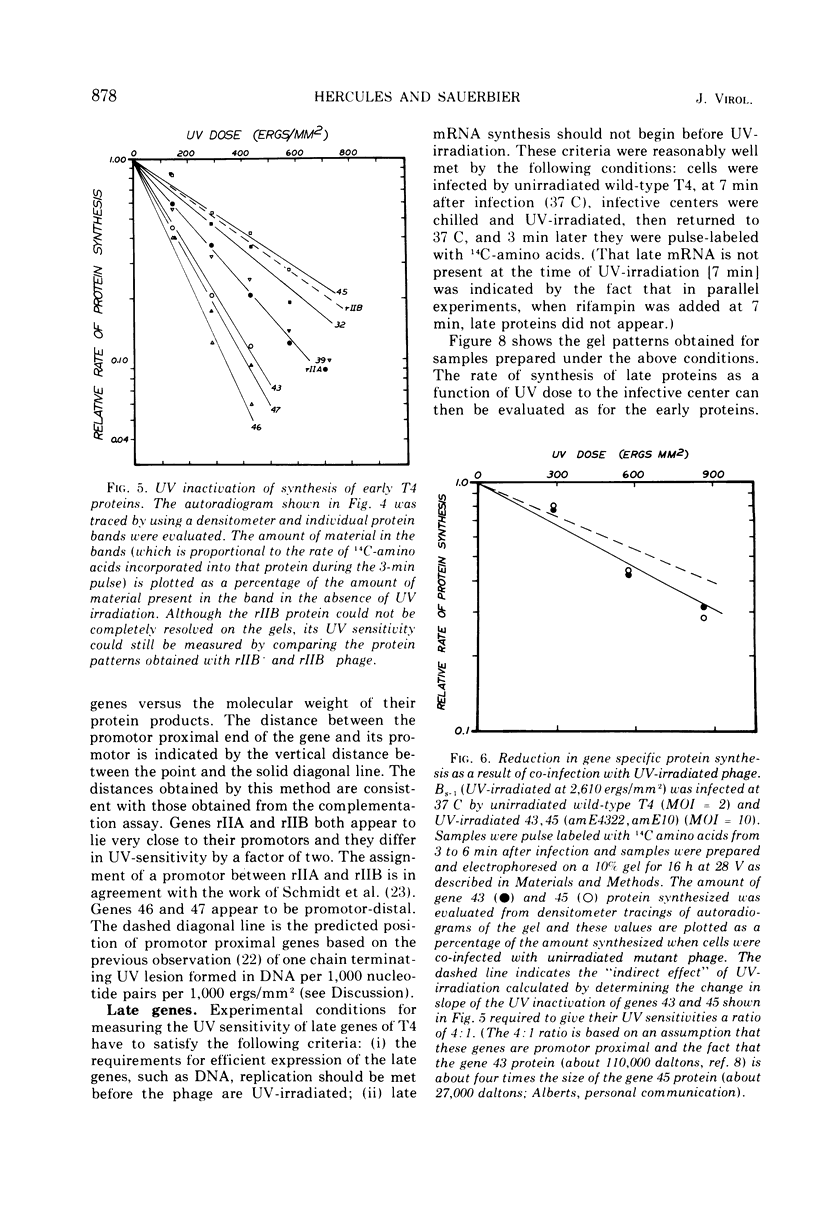
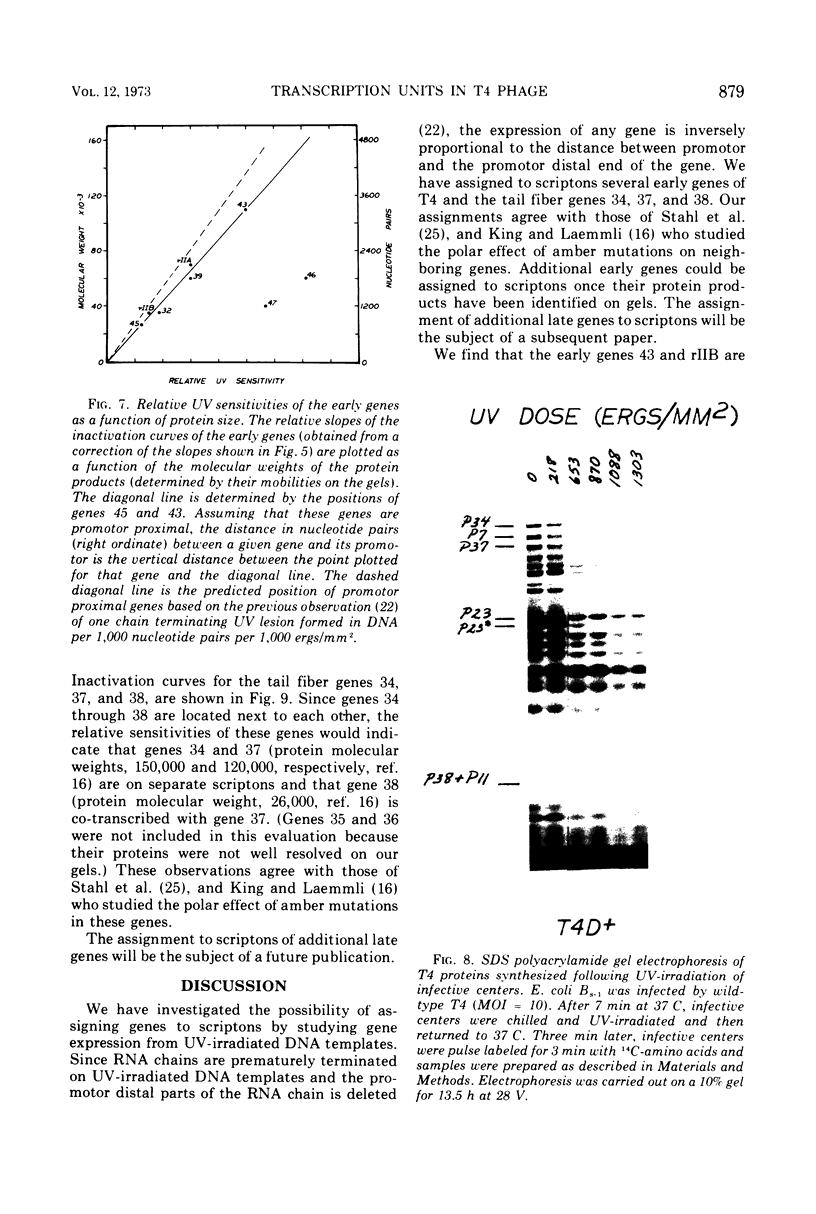
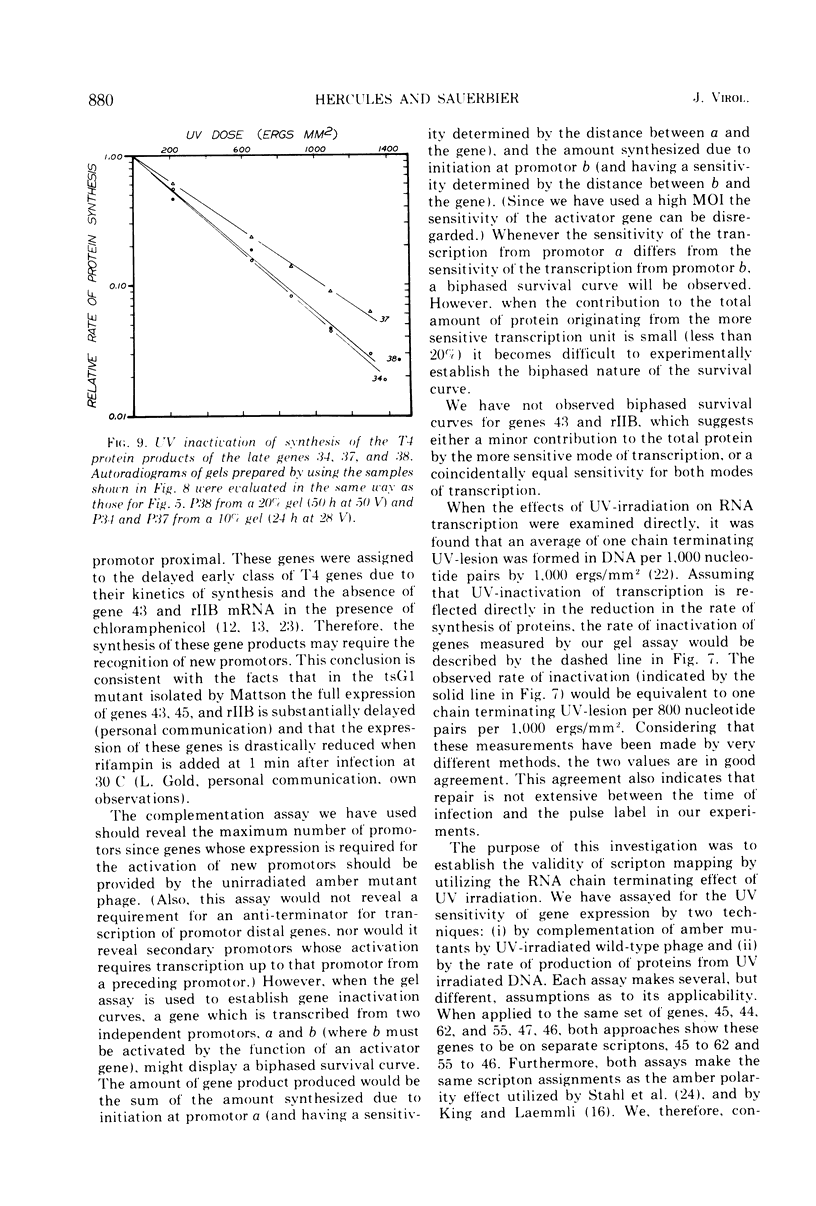
We have investigated the possibility of assigning genes of T4 bacteriophage to their units of transcription (scriptons) by studying gene expression from UV-irradiated DNA templates. Since RNA chains are prematurely terminated on UV-irradiated DNA templates and since the promotor distal part of the RNA chain is deleted, the expression of any gene is inversely proportional to the distance between the promotor and the promotor distal end of the gene. We find that the early genes, 43, 45 and rIIB, are promotor proximal. Since at least genes 43 and rIIB are classified as delayed early genes, these results suggest that their synthesis may require the recognition of new promotors. Additional early genes (44, 62, 42, 46, 47, 55, and rIIA) and some late genes (34, 37, and 38) have also been assigned positions relative to their promotors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Allen E. F., Albrecht I., Drake J. W. Properties of bacteriophage T4 mutants defective in DNA polymerase. Genetics. 1970 Jun;65(2):187–200. doi: 10.1093/genetics/65.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry J., Alberts B. In vitro complementation as an assay for new proteins required for bacteriophage T4 DNA replication: purification of the complex specified by T4 genes 44 and 62. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2717–2721. doi: 10.1073/pnas.69.9.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: requirements for late messenger synthesis. J Mol Biol. 1968 Apr 28;33(2):339–362. doi: 10.1016/0022-2836(68)90193-9. [DOI] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- CHAMPE S. P., BENZER S. An active cistron fragment. J Mol Biol. 1962 Apr;4:288–292. doi: 10.1016/s0022-2836(62)80006-0. [DOI] [PubMed] [Google Scholar]
- Goulian M., Lucas Z. J., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXV. Purification and properties of deoxyribonucleic acid polymerase induced by infection with phage T4. J Biol Chem. 1968 Feb 10;243(3):627–638. [PubMed] [Google Scholar]
- Guha A., Szybalski W., Salser W., Geiduschek E. P., Pulitzer J. F., Bolle A. Controls and polarity of transcription during bacteriophage T4 development. J Mol Biol. 1971 Jul 28;59(2):329–349. doi: 10.1016/0022-2836(71)90054-4. [DOI] [PubMed] [Google Scholar]
- HILL R. F. A radiation-sensitive mutant of Escherichia coli. Biochim Biophys Acta. 1958 Dec;30(3):636–637. doi: 10.1016/0006-3002(58)90112-4. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Levinthal C. Protein synthesis by Escherichia coli infected with bacteriophage T4D. Virology. 1968 Apr;34(4):709–727. doi: 10.1016/0042-6822(68)90092-5. [DOI] [PubMed] [Google Scholar]
- Jayaraman R. Transcription of bacteriophage T4 DNA by Escherichia coli RNA polymerase in vitro: identification of some immediate-early and delayed-early genes. J Mol Biol. 1972 Sep 28;70(2):253–263. doi: 10.1016/0022-2836(72)90537-2. [DOI] [PubMed] [Google Scholar]
- KRIEG D. R. A study of gene action in ultraviolet-irradiated bacteriophage T4. Virology. 1959 May;8(1):80–98. doi: 10.1016/0042-6822(59)90021-2. [DOI] [PubMed] [Google Scholar]
- Karam J. D., O'Donnell P. V. Suppression of amber mutations of bacteriophage T4 gene 43 (DNA polymerase) by translational ambiguity. J Virol. 1973 Jun;11(6):933–945. doi: 10.1128/jvi.11.6.933-945.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T., Bautz E. K. Regulation of gene-specific RNA synthesis in bacteriophage T4. J Mol Biol. 1969 May 14;41(3):401–417. doi: 10.1016/0022-2836(69)90284-8. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Milanesi G., Brody E. N., Grau O., Geiduschek E. P. Transcriptions of the bacteriophage T4 template in vitro: separation of "delayed early" from "immediate early" transcription. Proc Natl Acad Sci U S A. 1970 May;66(1):181–188. doi: 10.1073/pnas.66.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOMURA M., BENZER S. The nature of the "deletion" mutants in the rII region of phage T4. J Mol Biol. 1961 Oct;3:684–692. doi: 10.1016/s0022-2836(61)80031-4. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. V., Karam J. D. On the direction of reading of bacteriophage T4 gene 43 (deoxyribonucleic acid polymerase). J Virol. 1972 Jun;9(6):990–998. doi: 10.1128/jvi.9.6.990-998.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W., Bolle A., Epstein R. Transcription during bacteriophage T4 development: a demonstration that distinct subclasses of the "early" RNA appear at different times and that some are "turned off" at late times. J Mol Biol. 1970 Apr 28;49(2):271–295. doi: 10.1016/0022-2836(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Sauerbier W., Millette R. L., Hackett P. B., Jr The effects of ultraviolet irradiation on the transcription of T4 DNA. Biochim Biophys Acta. 1970;209(2):368–386. doi: 10.1016/0005-2787(70)90735-5. [DOI] [PubMed] [Google Scholar]
- Schmidt D. A., Mazaitis A. J., Kasai T., Bautz E. K. Involvement of a phage T4 sigma factor and an anti-terminator protein in the transcription of early T4 genes in vivo. Nature. 1970 Mar 14;225(5237):1012–1016. doi: 10.1038/2251012a0. [DOI] [PubMed] [Google Scholar]
- Stahl F. W., Crasemann J. M., Yegian C., Stahl M. M., Nakata A. Co-transcribed cistrons in bacteriophage T4. Genetics. 1970 Feb;64(2):157–170. doi: 10.1093/genetics/64.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl F. W., Murray N. E., Nakata A., Crasemann J. M. Intergenic cis-trans position effects in bacteriophage T4. Genetics. 1966 Jul;54(1):223–232. doi: 10.1093/genetics/54.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Bacteriophage sigma factor for RNA polymerase. Nature. 1969 Sep 13;223(5211):1107–1110. doi: 10.1038/2231107a0. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Positive control of transcription by a bacteriophage sigma factor. Nature. 1970 Mar 14;225(5237):1009–1012. doi: 10.1038/2251009a0. [DOI] [PubMed] [Google Scholar]
- WIBERG J. S., BUCHANAN J. M. STUDIES ON LABILE DEOXYCYTIDYLATE HYDROXYMETHYLASES FROM ESCHERICHIA COLI B INFECTED WITH TEMPERATURE-SENSITIVE MUTANTS OF BACTERIOPHAGE T4. Proc Natl Acad Sci U S A. 1964 Mar;51:421–428. doi: 10.1073/pnas.51.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegian C. D., Mueller M., Selzer G., Russo V., Stahl F. W. Properties of the DNA-delay mutants of bacteriophage T4. Virology. 1971 Dec;46(3):900–919. doi: 10.1016/0042-6822(71)90090-0. [DOI] [PubMed] [Google Scholar]