Abstract
Alanine aminotransferase (AlaAT) has been studied in a variety of organisms due to the involvement of this enzyme in mammalian processes such as non-alcoholic hepatocellular damage, and in plant processes such as C4 photosynthesis, post-hypoxic stress response and nitrogen use efficiency. To date, very few studies have made direct comparisons of AlaAT enzymes and fewer still have made direct comparisons of this enzyme across a broad spectrum of organisms. In this study we present a direct kinetic comparison of glutamate:pyruvate aminotransferase (GPAT) activity for seven AlaATs and two glutamate:glyoxylate aminotransferases (GGAT), measuring the KM values for the enzymes analyzed. We also demonstrate that recombinant expression of AlaAT enzymes in Eschericia coli results in differences in bacterial growth inhibition, supporting previous reports of AlaAT possessing bactericidal properties, attributed to lipopolysaccharide endotoxin recognition and binding. A probable lipopolysaccharide binding region within the AlaAT enzymes, homologous to a region of a lipopolysaccharide binding protein (LBP) in humans, was also identified in this study. The AlaAT enzyme differences identified here indicate that AlaAT homologues have differentiated significantly and the roles these homologues play in vivo may also have diverged significantly. Specifically, the differing kinetics of AlaAT enzymes and how this may alter the nitrogen use efficiency in plants is discussed.
Introduction
Alanine aminotransferase (AlaAT) [E.C. 2.6.1.2], also referred to as glutamate:pyruvate aminotransferase (GPAT), is a pyridoxal-5′-phosphate-dependent (PLP) enzyme that catalyzes the reversible transfer of an amino group from alanine to 2-oxoglutarate to form glutamate and pyruvate [1], [2]. AlaAT is widespread, with homologues in all three biological domains (Eukarya, Archaea and Eubacteria) and functions as a part of several major metabolic pathways. Existing in both the cytosol and the mitochondria, AlaAT plays a critical role in linking carbon and nitrogen metabolism (assimilation and catabolism) within both eukaryotes and prokaryotes [3]. AlaAT is involved in a number of cellular processes including glycolysis, gluconeogenesis, amino acid metabolism [2], hepatocellular damage in mammals [4], photorespiration in plants [5] and nitrogen use efficiency (NUE) in plants, including cereal crops [6], [7], [8]. This latter process is of particular interest, as it has been previously shown that both canola (Brassica napus) and rice (Oryza sativa) plants over-expressing barley (Hordeum vulgare) AlaAT (HvAlaAT) in a tissue-specific manner have increased NUE under nitrogen (N) limiting conditions, both in controlled environments and field trials for rice [6], [7], [8]. Due to the increased awareness of the detrimental effects of increased N fertilizers in the environment as well as the concerns surrounding increasing world population and subsequent food shortages, the ability to obtain increased yields under limiting N fertilizer conditions is particularly important. Any further understanding of the key enzymes involved in these processes may be of significance in additional improvements in NUE [9], [10], [11].
To date, AlaAT enzymes and their kinetics have been characterized in a variety of species. However most work on this enzyme has focused on the medical implications of the AlaAT isoforms found in humans (HsAlaAT) [12]. Increases of both HsAlaAT1 (cytoplasmic) and HsAlaAT2 (mitochondrial) in mammalian serum samples have shown to be reliable indicators of liver damage, muscle damage and celiac disease [13]. Moreover, significant increases in activity of mouse (Mus musculus) mitochondrial AlaAT (MmAlaAT2), but not mouse cytoplasmic AlaAT (MmAlaAT1) in fatty livers of obese mice, indicate possible differences in the roles/effects these two isozymes have in the cell. The evolution of differences in the kinetics of various isozymes of AlaAT would be driven in part by the distinct cellular roles these isozymes play [4].
Good et al., [7] and Shrawat et al., [8] observed a nitrogen use efficient (NUE) phenotype in plants with over-expression of the HvAlaAT enzyme in canola and rice, respectively, using a tissue specific promoter. However, the specific basis for this phenotype remains unclear and there is a continued effort to understand the intracellular mechanisms which cause this phenotype. One question of particular interest is whether different AlaAT enzyme isoforms have different kinetics and if so, could these different isoforms favor an NUE phenotype? More specifically, are there optimal kinetic properties of AlaAT which can produce an increase in NUE when expressed within plants? Given that the previous NUE phenotypes were observed in canola and rice utilizing a promoter which increased expression in the roots [14], the benefits of targeting the expression of a gene of interest to a particular tissue have become clear. For example, genes involved in producing modified oils are usually expressed with a seed specific promoter [15]. What has been less studied is the importance of choosing an enzyme that works with optimal efficiency in the appropriate environment and tissue. The importance of studying enzyme variants is illustrated by the example of Golden Rice. Development of this rice involved the insertion of a daffodil phytoene synthase (psy) gene for the efficient production of ß-carotene, a product used to synthesize Vitamin A [16]. A number of psy genes were analyzed in order to determine which produced the highest levels of ß-carotene, and which variant was rate-limiting; further analysis revealed that an even more efficient psy gene may exist in maize (“Golden Rice 2”) [16]. Therefore, identifying enzyme variants that overcome a metabolic bottle-neck could prove to be an effective strategy for trait improvement.
To investigate further the basis for an increased NUE phenotype, we chose to evaluate different enzyme variants of AlaAT with a view to using these variants to gain insights into the underlying metabolic changes that affect NUE in plants. Because AlaAT has an equilibrium constant near one, the reaction of this enzyme in vivo will be driven by substrate concentrations [17]. Therefore, it follows that an AlaAT homologue with increased specificity or different kinetic properties could allow for increased NUE properties in a plant system. This approach was recently taken by Duff et al., who examined the kinetic properties and crystal structure of different AlaAT enzyme variants [17]. Here we present a kinetic comparison of AlaAT enzymes from a broader variety of organisms, placing emphasis on the difference in KM values between homologues enzymes instead of the specific activity of the enzyme which has been analyzed elsewhere [17]. Furthermore, AlaAT enzymes used in this analysis were not tagged as has been done previously, which can affect enzyme activity. Finally, only L-amino acid enzymes were used in this analysis given that many plant pathways are L-enantiomer stereospecific, including shikimate, aspartate, pyruvate and glutamate [18], and that a very small percent (∼0.5–3) of the total amino acids within many plants are not of the L-type [19]. To our knowledge, this is the most comprehensive kinetic analysis of AlaAT homologues. We show that AlaAT homologues and two glutamate: aminotransferase (GGAT) enzymes (which have secondary glutamate:pyruvate aminotransferase activity) have relative KM values for co-substrates that indicate that in vivo the rate and direction of the reaction catalyzed by each enzyme, under similar substrate concentrations, may differ dramatically. These results reaffirm the results obtained by Duff et al., [17] for some of the variants tested with the addition of KM values for eight AlaAT’s not studied previously. The effects of various enzymes with diverging kinetic behaviours were also assessed for functional consequences in E. coli under different environmental conditions.
Results and Discussion
Homologous AlaAT Primary Sequence Comparison
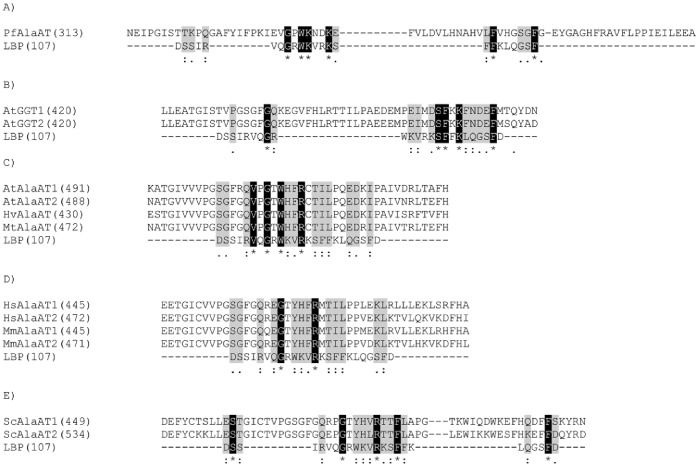
Glutamate:pyruvate aminotransferases (AlaAT/GPAT) and glutamate:glyoxylate aminotransferases (GGAT) are subgroup I aminotransferases, containing eleven invariant residues essential for binding the coenzyme PLP and for stabilizing the enzyme:substrate transition state [1], [5]. Both AlaAT and GGAT enzymes share similar primary and secondary structures, as well as hydropathy with other subgroup I aminotransferases such as aspartate aminotransferase and tyrosine aminotransferase [1]. All 13 enzymes examined in this study showed this conservation and maintained the 11 invariant residues previously defined for subgroup I aminotransferases (see Ward et al., 2000 [20]) (Figure S1). Primary sequence analysis (Figure 1) indicates that of the sequences studied, P. furiosus (PfAlaAT) is the most divergent, which is not surprising considering this was the only non-eukaryotic sequence examined. Interestingly, the protein sequences of AtGGAT1 and AtGGAT2 are more similar to plant AlaAT enzymes than are mammalian and archaean AlaAT enzymes to plant homologues, even though GGAT enzymes are capable of both glutamate:glyoxylate and glutamate:pyruvate aminotransferase reactions [5]. It appears that the kinetic differences identified here are due to differences in non-conserved residues which may cause changes in substrate binding affinity and/or catalytic rate, perhaps as a result of changes in enzyme folding.
Figure 1. Phylogenetic dendrogram of eleven AlaAT enzymes and two GGAT enzymes.

A phylogenetic dendrogram was constructed using neighbour joining (NJ) based on amino acid sequence similarity using Vector NTI Advance v. 11.0, AlignX software. The sequences used to construct the dendrogram were obtained from the NCBI database except that of Medicago which was obtained courtesy of Anis Limami at the Université d' Angers.
AlaAT Enzymes have Varying Substrate KM Values
KM values from eight AlaAT and two GGAT enzymes are compared in Table 1. Although KM values for several of the enzymes analyzed here have been reported previously, our study facilitates a comparison of data obtained with a single assay system. To date, most studies on AlaAT have been organism and tissue-specific, focusing on a single enzyme or isozymes, making comparisons between AlaAT enzymes from different species difficult. Comparisons between enzymes have also been limited due to purification and expression differences, as in the case of AtGGAT1. The AlaAT activity of this enzyme has been examined by purification of this protein from both shoot tissues [21] and recombinant E. coli [5]. These differences in enzyme source and purification procedure can manifest as alterations in enzymatic kinetic behaviour, compounded by differences in the assay conditions used during kinetic analyses [20], [22], [23]. Other errors may result from the presence of homologous proteins which were not separated from the AlaAT of interest during purification, due to similar biochemical properties and increases in activity from environmental changes (e.g. greening of leaves) [24]. Due to these confounding factors, kinetic data obtained with enzymes from various sources cannot be reliably compared across different studies (Table 2).
Table 1. Summary of kinetic assay results.
| Enzyme | Alanine | 2-oxoglutarate | Glutamate | Pyruvate | ||||
| KM (mM) | SE KM | KM (mM) | SE KM | KM (mM) | SE KM | KM (mM) | SE KM | |
| AtAlaAT1 | 2.4 | ±0.5 | 0.1 | ±0.0 | 2.5 | ±0.4 | 0.1 | ±0.0 |
| AtAlaAT2 | 10.4 | ±1.2 | 1.0 | ±0.1 | 4.9 | ±1.1 | 5.1 | ±0.9 |
| AtGGAT1 | 1.9 | ±0.5 | 0.2 | ±0.1 | 0.2 | ±0.1 | 0.6 | ±0.1 |
| AtGGAT2 | 1.2 | ±0.1 | 8.8 | ±1.8 | 0.2 | ±0.1 | 18.5 | ±2.7 |
| HvAlaAT | 5.6 | ±0.6 | 0.2 | ±0.0 | 4.9 | ±0.5 | 0.1 | ±0.0 |
| MtAlaAT1 | 1.0 | ±0.2 | 0.2 | ±0.0 | 0.1 | ±0.0 | 18.6 | ±2.7 |
| MtAlaAT2 | 1.5 | ±0.2 | 0.3 | ±0.0 | 0.3 | ±0.1 | 18.0 | ±1.9 |
| MmAlaAT1 | 26.5 | ±2.3 | 0.1 | ±0.1 | 13.0 | ±2.4 | 12.5 | ±2.0 |
| PfAlaAT | 4.0 | ±0.5 | 0.02 | ±0.0 | 0.9 | ±0.1 | 16.5 | ±2.2 |
| ScAlaAT1 | 0.3 | ±0.1 | 0.5 | ±0.1 | 0.7 | ±0.1 | 11.0 | ±1.7 |
KM values are shown for each substrate, for each of the ten enzymes examined. Kinetic values represent the average of three independent trials. The correlation coefficient (r2) was >0.80 for all trials, except AtGGAT1 glutamate, AtGGAT2 glutamate and MmAlaAT1 pyruvate. Raw data are plotted in Figure S2.
Table 2. Comparison of KM values for AlaAT and GGAT enzymes.
| Organism | KM (mM) | Previous KM (mM) | Source of AlaAT | Reference | ||||||
| Alanine | 2-Oxoglutarate | Pyruvate | Glutamate | Alanine | 2-Oxoglutarate | Pyruvate | Glutamate | |||
| Arabidopsis AlaAT1 | 2.4 | 0.1 | 0.1 | 2.5 | 1.5 | 0.2 | NA | NA | A. thaliana leaves (protein purified) | Wiśniewski et al., (2006) |
| Barley AlaAT | 5.6 | 0.2 | 0.1 | 4.9 | 17 | 5 | 0.1 | 1 | Barley roots (protein purified) | Good and Muench (1992) |
| Arabidopsis GGAT1 | 1.9 | 0.2 | 0.6 | 0.2 | 4.8 | 0.3 | 0.3 | 2.0 | E. coli (recombinant expression) | Liepman and Olsen (2003) |
| NA | NA | NA | 1.2 | A. thaliana leaves (protein purified) | Wiśniewski et al., (2006) | |||||
| Arabidopsis GGAT2 | 1.2 | 8.8 | 18.5 | 0.2 | 3.6 | 0.5 | 0.4 | 3.3 | E. coli (recombinant expression) | Liepman and Olsen (2003) |
| Pyrococcus furiosus AlaAT | 4.0 | 0.02 | 16.5 | 0.9 | 3.2 | 1.1 | 4.7 | 5.6 | E. coli (recombinant expression) | Ward et al., (2000) |
KM values obtained during this study were compared with previous KM values from various published sources. Discrepancies between kinetic values can be attributed partially to differences in procedure as well as differences in original data collection. Nevertheless, significant differences in KM values for all enzymes are evident. Variation amongst results highlights the benefit of using one system and one procedure when comparing kinetic data from several different enzymes. (NA = no data available from previous studies.).
KM values reported here indicate significant diversity between the different enzymes for the same substrates. Between AtAlaAT1 (cytoplasmic) and AtAlaAT2 (mitochondrial) the minimal KM discrepancy between substrates was reported for glutamate, with an approximate two-fold increase in KM (2.5 mM to 4.9 mM respectively) (Table 1). The KM values obtained from both M. truncatula proteins were similar for all substrates, the greatest difference being seen for the substrate glutamate, with a three-fold change in KM (0.1 mM for MtAlaAT1, 0.3 mM for MtAlaAT2). The largest difference between substrates for a single enzyme was seen for PfAlaAT. For this enzyme, there was an 825-fold difference between KM values for 2-oxoglutarate and pyruvate (0.02 mM and 16.5 mM respectively). The second largest difference in KM values for a given enzyme between substrates was seen for MmAlaAT, with a 265-fold difference (seen between the KM values for alanine and 2-oxoglutarate, 26.5 mM and 0.1 mM, respectively). No groupings or patterns could be established among the KM values obtained, and relative differences were not consistent for a single enzyme and multiple substrates, or for the KM values of multiple enzymes for a single substrate. KM values for 2-oxoglutarate appeared to be reasonably constant (difference in KM values of 8.78 mM) with AtGGAT2 having a KM of 8.8 mM. The next largest value belonged to AtAlaAT2, with a KM for 2-oxoglutarate of 1.0 mM. The range of KM values for alanine, pyruvate and glutamate were much greater (differences in KMs of 26.2 mM, 12.9 mM and 18.5 mM respectively).
The KM values reported here for HvAlaAT and ScAlaAT1 share some similarity to those recently reported by Duff et al., [17], with the largest difference between values being the KM for ScAlaAT1 and the substrate pyruvate, here reported as a KM of 11.0 and previously reported as a KM of 0.4, a 27.5 fold difference. All other KM’s for the remaining substrates alanine, 2-oxoglutarate and glutamate showed lower fold differences when KM values for ScAlaAT were compared, 12, 2.5 and 2.9 respectively. The KM values reported for HvAlaAT did not show as great a deviation between studies for the various substrates with, 6.7, 1.1, 9.1 and 2.1 fold differences for alanine, 2-oxoglutarate, glutamate and pyruvate respectively. These discrepancies in KM values could be the result of numerous protocol differences as outlined above, and re-emphasize the importance of obtaining enzymatic data from a single source for the purpose of direct comparisons.
Vmax values for all enzymes assayed are presented in Table S1. Since enzyme fractions were not purified, the concentrations of the enzymes used, and thus catalytic rate constants are unknown, therefore the usefulness of Vmax values in making meaningful comparisons between different enzymes is diminished. Purification of individual proteins in order to establish enzyme concentrations, thereby allowing determination of kcat and comparison of Vmax, was not done due to the absence of an antibody that would specifically bind each of the different variants for purification purposes. Furthermore, enzymes were not tagged with either His or Myc-C sequences since such alterations may affect enzyme kinetics. Given that these were recombinant proteins expressed in a bacterial system, protein folding may have been altered affecting kinetic results. Since whole protein fractions were utilized during this study, the possibility that enzyme inhibitors were present or that non-AlaAT transaminase activities may have contributed to substrate turnover as well as NAD/NADH concentrations and influenced calculated kinetic constants must be considered.
It is therefore of interest to determine the effects on NUE phenotypes of AlaAT enzymes that display kinetics similar to those of HvAlaAT, compared with enzymes that have very different characteristics. Based on the results from the kinetic assays, AtAlaAT1 appears to be most similar to HvAlaAT. Both AtAlaAT enzymes have higher KM values for glutamate and alanine, and lower KM values for 2-oxoglutarate and pyruvate, compared with HvAlaAT. MmAlaAT1 has rather different KM values, raising the possibility of a distinct in vivo role(s). Compared with other AlaATs examined, this enzyme had the highest KM values for both alanine (26.5 mM) and glutamate (13.0 mM).
It is difficult to extrapolate in vitro kinetic data and predict the consequences of altered substrate KM values, without knowing the cellular concentrations of substrates under various environmental conditions. For example, while MmAlaAT appears to display very different kinetic behavior compared with HvAlaAT, in vivo analysis will be needed in order to verify whether or not such differences have any effect on plant phenotype when the gene is ectopically expressed. Alanine and glutamic acid concentrations in rice seeds show average millimolar amounts of alanine as ∼0.57 mM and glutamic acid as ∼1.53 mM, with vitamin B6, a precursor to the AlaAT cofactor PLP, at ∼0.72 mM [25]. Other studies conducted by Narsai et al., [26] show that in rice seedlings these concentrations are altered during the growth and development of the plant and are approximately 0.09 mM for glutamate, 2.27 mM for alanine, 0.79 mM for pyruvate and 0.65 mM for 2-oxoglutarate. The changes in AlaAT substrate concentrations during different phases of plant growth and in various cellular tissues and organs has also been documented elsewhere [27]. Aminotransferase enzymes with overlapping functions have also been observed in a number of organisms, including E. coli [28], P. furiosus [20] and plants [17], [29]. The effect of any of these enzymes on nitrogen uptake or metabolism can only be speculated upon and would require whole plants studies and in vivo analysis which are currently underway. Analysis of the kinetics of aspartate aminotransferase (AspAT) from higher plants has also been carried out recently with similar intentions of crop improvement [30].
AlaAT Homologues Differ in their Ability to Reduce Growth Rate of Gram Negative Bacteria
As an initial screen to determine if the presence of a specific AlaAT variant in E. coli had a significant effect on the bacteria’s ability to utilize specific substrates, E. coli expressing the various AlaAT enzymes from plasmid constructs were grown in modified M63 medium supplemented with various concentrations of 2-oxoglutarate, with ammonium as the nitrogen source. It was speculated that 2-oxoglutarate might have an effect on the growth rates of E. coli due to its central role in linking both carbon and nitrogen metabolism in bacteria [31], [32], [33], [34]. Furthermore, excess 2-oxoglutarate in the growth medium may have a significant impact on AlaAT enzymes with lower KM values for both alanine and 2-oxoglutarate, if substrate kcat values of these enzymes are not also lower and assuming that substrates are present at sub-saturating concentrations. It was hoped that changes in the availability of AlaAT substrate(s) (2-oxoglutarate) during growth of E. coli over-expressing various AlaAT homologues would allow for differentiation of homologous enzymes in terms of substrate usage, manifest phenotypically as changes in rates of growth. For the reasons outlined below, we were unable to characterize the transgenic E. coli in terms of changes to available substrate concentrations however the results indicate that AlaAT may maintain novel functions within Eukarya, Archaea and Eubacteria. Whether these functions play a role in plant NUE has yet to be explored. Ultimately, no difference in growth rate of E. coli containing HvAlaAT was observed when exposed to concentrations of 2-oxoglutarate (Figure 2). However we did observe a slow growth phenotype in all E. coli cultures expressing the various AlaAT constructs (Figure 2 and Figure 3).
Figure 2. Effect of 2-oxoglutarate on growth rate of E. coli expressing HvAlaAT.
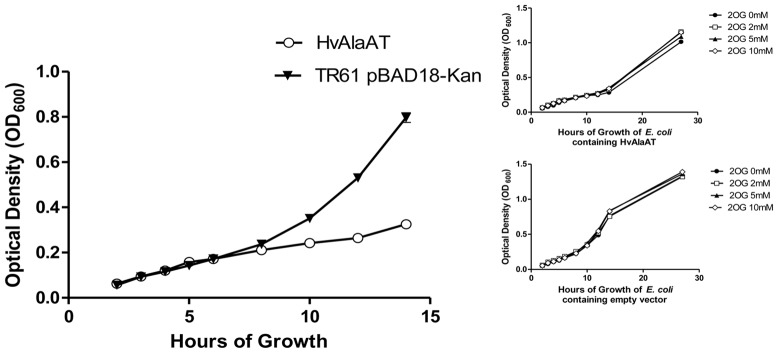
Growth of TR61 cells containing either pBAD:HvAlaAT or TR61 cells containing an empty pBAD18-Kan vector in modified M63 medium. E. coli containing the two constructs grown in media containing 0 mM, 2 mM, 5 mM or 10 mM 2-oxoglutarate (2OG), pH 8.0 (inset).
Figure 3. The average growth of E. coli containing various AlaAT and GGAT enzymes.
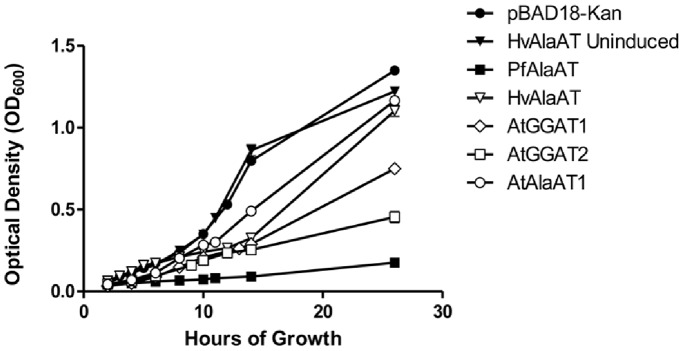
TR61 E. coli were cultured in a M63 revised minimal medium and expression of AlaAT or GGAT was induced by the addition of arabinose. The average optical density at 600 nm was measured and recorded over a 26 hour time period and is representative of trials done in triplicate. Error bars show standard error, where they exceed symbol size. TR61 cells containing no pBAD vector, cells containing a pBAD vector with no AlaAT or GGAT insert and uninduced TR61 cells containing a pBAD::HvAlaAT enzyme were utilized as controls. E. coli expressing AtAlaAT2, MtAlaAT, ScAlaAT and MmAlaAT showed changes in optical density over the 26 hour time period similar to that seen by Arabidopsis thaliana AlaAT1 expressing cells.
Expression of recombinant AlaAT from B. japonicus (AmphiALT), in gram-negative E. coli has been shown to cause cell lysis through lipopolysaccharide (LPS) binding [35]. Jing and Zhang (2011) [35] observed that AmphiALT was able to bind and lyse gram-negative but not gram-positive cells; binding was reported to be specific to the LPS region. From these results it was suggested that AlaAT may also be involved in the acute phase response, particularly in liver tissues. Our study supports this view, indicating that recombinant expression of AlaAT in E. coli inhibits growth, and decreases cell growth rates when grown in minimal medium (Figure 3 and Figure 4). Due to the absence of antibodies for all AlaAT enzymes and thus an inability to purify individual AlaATs, direct binding of E. coli LPS to the various AlaAT enzymes was not examined. However, slow growth phenotypes similar to those described previously in the presence of AlaAT were observed, leading to the conclusion that all of the AlaAT enzymes studied exhibit some bactericidal activity similar to AmphiALT from B. japonicus, and providing evidence for the conservation of AlaAT bactericidal properties. In order to clarify, the growth curves of only five of the ten AlaATs expressed in E. coli are shown in Figure 3. E. coli cells expressing AtAlaAT2, MtAlaAT, ScAlaAT and MmAlaAT1 showed growth curves similar to those obtained with AtAlaAT1.
Figure 4. The rate of change of the growth over time (OD600/Hrs) of E. coli strains containing various alanine aminotransferase enzymes.
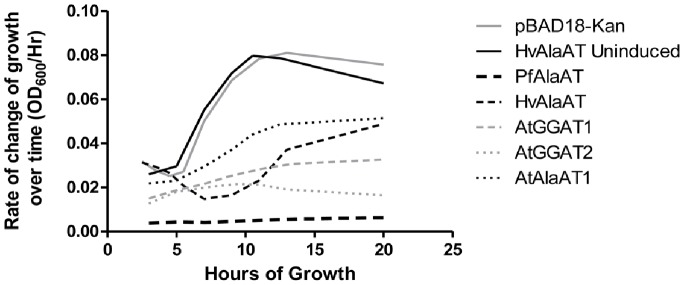
The smooth first derivative of each time trial in Figure 4 was determined, indicating the change in the growth rate of the bacteria containing each of the different AlaATs and the controls over time. E. coli expressing AtAlaAT2, MtAlaAT, ScAlaAT1 and MmAlaAT1 showed changes in the rate of growth over the 26 hour time period similar to that seen by Arabidopsis thaliana AlaAT1 expressing cells.
Among the ten enzymes assayed, inhibition of growth rate varied significantly. PfAlaAT showed the greatest effect on growth rate over time, seen most clearly in Figure 4, when the first derivative of each sample is determined. Both AtGGAT2 and AtGGAT1 also show dramatic decreases in growth rate over time (Figure 4). Comparison of kinetic constants for a particular enzyme with growth assay results has not provided any insights into the reasons for these differences in growth rates. Also interesting was the observed change in the growth rate of the bacteria containing the HvAlaAT over time (Figure 4). While a constant or slight increase in growth rate was observed with the majority of proteins assayed, the over-expression of HvAlaAT resulted in a substantial decrease in growth rate starting at approximately 2 hours and continuing until approximately 7 hrs, at which point growth rate once again began to increase.
To investigate further the bactericidal activity of AlaAT, the primary structure of each enzyme was analyzed for a conserved endotoxin binding region. Through analysis of the known LPS region from human lipopolysaccharide binding protein (LBP) [36], a similar conserved region was found to various extents in all AlaAT enzymes (Figure 5). PfAlaAT shows the greatest conservation of the LPS binding region, with the highest number of residues conserved, and a percent identity of 26% (Figure 5A). PfAlaAT was also the most effective isoform inhibiting growth of E. coli (Figure 4). AtGGAT1 and AtGGAT2 also show a high degree of conservation of identical residues, with percent identities of 22% (Figure 5B). All sequences show a high degree of conservative and semi-conservative sequence similarity, for example with AtAlaAT1, AtAlaAT2, MtAlaAT and HvAlaAT (Figure 5C), HsAlaAT1, HsAlaAT2, MmAlaAT1 and MmAlaAT2 (Figure 5D), and ScAlaAT1 and ScAlaAT2 (Figure 5E). However the ability of these enzymes to bind the LPS region of E. coli and inhibit growth was not as pronounced as that observed for PfAlaAT, AtGGAT1 and AtGGAT2, perhaps demonstrating the importance of a high level of conservation of sequences/residues at these sites. The possibility that other conserved endotoxin binding regions may exist to varying degrees in the AlaAT homologues analyzed should also be considered as this could also contribute to the variation of growth inhibition observed. Given the evidence that AlaAT enzymes may be playing a role in acute phase response to bacterial infections in vivo [35], determining how these differences affect, or are affected by concentration-dependent binding of other molecules within the cell will be important to develop a more complete understanding of enzyme function.
Figure 5. Comparison of AlaAT putative endotoxin binding regions.
Primary sequence comparison of AlaAT homologues to the known endotoxin binding region of human LBP (A–E) using the ClustalW alignment program. Identical residues are highlighted in black with white text while conservative and semi-conservative residues are highlighted in grey with black text. (“*” indicate identical residues: “:” indicates conservative substitutions; “.” indicates semi-conservative substitution.
Conclusion
A kinetic examination of the enzyme alanine aminotransferase is of interest for a number of reasons. AlaAT has been shown to be involved in stress responses in numerous plants, including cereal crops [2], [22], [37], while in mammals, this enzyme is used as an indicator of non-alcoholic hepatocellular liver damage [13] and may be involved in immune system acute phase response [35]. Recently, AlaAT has been shown to increase NUE in cereal crops when over-expressed with a tissue specific promoter [6], [8]. The kinetic results presented here indicate that catalytic properties between AlaAT homologues differ considerably. Our data reveal that when AlaAT is over-expressed in plant systems, various phenotypic results with respect to changes in NUE may be observed. Previous analysis in Brassica napus and rice has already determined the importance of using a tissue-specific promoter in obtaining an NUE phenotype [6], [7], [8], [38], [39]. Preliminary studies in our group indicate that Arabidopsis thaliana over-expressing several of the different AlaAT enzymes with differing kinetics can produce novel phenotypes. The knowledge of enzyme variants gained here, as well as prior knowledge of promoters provides a platform for future NUE studies and the improvement of crop nutrient utilization.
Materials and Methods
pBAD18-Kan:AlaAT Constructs
The alanine aminotransferase enzymes assayed were chosen based on differences in their amino acid sequence (Figure S1) and the availability of a cloned gene. Barley alanine aminotransferase (Hordeum vulgare, HvAlaAT) (GenBank accession no. Z26322) was obtained from a cDNA originally used in our over-expression studies [8] and was described by Muench and Good (1994) [40]. Both Medicago truncatula cDNA sequences were obtained from Anis Limami at the Université d' Angers [41]. Although only one naturally-occurring mitochondrial Medicago truncatula alanine aminotransferase (MtAlaAT) has been described (Medicago truncatula genome sequencing resources, Medtr8g023140), none of the sequences received were identical to the expected sequence, and so two of the most similar sequences were chosen to be expressed and analyzed. MtAlaAT1 contained the point mutation I144V while MtAlaAT2 contained the point mutation F177S. Pyrococcus furiosus AlaAT (PfAlaAT) (GenBank accession no. NP_579226) was amplified from ATCC gDNA (DSM 3638). Arabidopsis thaliana AlaAT1 (AtAlaAT1) (TAIR reference no. AtG17290) and AlaAT2 (AtAlaAT2) (TAIR reference no. At1G72330) sequences were obtained from Yo Miyashita [2]. Yeast (Saccharomyces cerevisiae) sequences (ScAlaAT1, GenBank accession no. NP_013190 and ScAlaAT2, Genbank accession no. NP_010396) were amplified from ATCC gDNA (S288C [MUCL 38902]). Arabidopsis thaliana AtGGAT1 (TAIR reference no. At1G23310) and AtGGAT2 (TAIR reference no. At1G70580) (glutamate:glyoxylate aminotransferase) sequences were obtained from Laura Olsen at the University of Michigan [5]. Mouse (Mus musculus) MmAlaAT1 (GenBank accession no. NP_877957) and MmAlaAT2 (GenBank accession no. NP_776291) sequences were acquired from Rong ze Yang at the University of Maryland [13], as were those for human (Homo sapien) HsAlaAT1 (GenBank accession no. NP_005300) and HsAlaAT2 (GenBank accession no. NP_597700). All genes were sequenced and the primers used for sequencing are listed in Table S2. When available, sequences were compared with BLAST results from the NCBI nucleotide database.
All of the sequences were cloned into the E. coli expression vector pBAD18-Kan using primers containing approximately 22 bps of AlaAT specific gene sequence and a restriction digest cut site at the 5′ end (Table S2). Forward primers contained cut sites for either Kpn1 or Sac1, while Xba1 cut sites were used for the reverse primers. These constructs were transformed into E. coli TR61 strain K-12 cells and used for AlaAT expression, activity and kinetic assays. TR61 cells are derived from the E. coli K-12 strain MC4100, containing a lac reporter gene on a lambda phage insertion and a Tn10 insertion conferring resistance to the sugar arabinose, and were a gift from Tracy Ravio at the University of Alberta. TR61 cells are a previously unpublished de novo cell line and permission for their use was granted by Tracy Ravio and the Biosafety Committee, University of Alberta.
Analysis of AlaAT Primary Structure
Thirteen enzymes with AlaAT activity were obtained for expression studies and kinetic analysis and the amino acid sequences were compared using ClustalW software (full primary sequence comparison is provided in Figure S1). The Medicago truncatula sequence utilized for this analysis was obtained from Anis Limami at the Université d' Angers [41]. Vector NTI Advance v. 11.0 software was used to construct a dendrogram (Figure 1). For both ClustalW and Vector NTI analysis, a gap open penalty of 10 and a gap extension penalty of 0.05 were used. The percent identity of amino acid sequences was defined by dividing the number of identical residues by the number of amino acids in the shortest sequence; gaps were not taken into account.
Induction of AlaAT Constructs in E. coli
Five hundred µL of E. coli TR61 overnight cultures containing the various AlaAT constructs were added to 45 mL LB and grown at 37°C, to an OD600 of 0.5–0.75, at which point 20 mL of culture was added separately to two flasks, one of which received arabinose to a final concentration of 0.2%. Both induced and uninduced cultures were incubated, shaking at 37°C for an additional 4 hrs. Induced and uninduced cultures were harvested by centrifugation after 3–4 hrs, washed a single time with STE buffer (10 mM Tris-HCl pH = 7.5, 1 mM EDTA, 150 mM NaCl), and bacterial pellets were flash frozen and stored at −80°C. Cultures were stored at −80°C for no more than 2 weeks prior to protein extraction.
Protein Extraction
Whole protein fractions were extracted using a freeze-thaw method. Cells were re-suspended in 2 mL protein extraction buffer (100 mM Tris-HCl pH = 7.5, 5 mM EDTA) containing 1 mM DTT and 1 mM PMSF. Ten µL of 10% Triton-X100 and 10 µL of 1 mg/mL lysozyme (Sigma, L-6876) were added to resuspended cells and incubated at room temperature for 15–30 min. Protein was extracted through six cycles of freeze-thaw using liquid nitrogen. Samples were then centrifuged and the supernatant from each cell fraction was removed and applied to a PD-10 desalting column (GE Healthcare, Sephadex G-25 M, PD-10 Columns). The eluate from these columns was used for both AlaAT activity measurements and kinetics assays.
Activity Assays
Extracts were tested for glutamate:pyruvate aminotransferase (GPAT) activity prior to kinetic assays to ensure the induction of AlaAT expression. Varying dilutions of the AlaAT/GGAT protein extractions were assayed alongside the uninduced protein fractions to ensure the presence and activity of the AlaAT/GGAT enzymes. Leaky expression of AlaAT in the uninduced protein fractions was regularly observed, but at very low levels. Activity assays were also conducted in order to determine the optimal degree of dilution of the enzyme necessary for kinetic assays; these typically ranged between 30X–40X. Activity assays were prepared and carried out at 20°C in the direction alanine and 2-oxoglutarate to pyruvate and glutamate. Activity of AlaAT enzyme was determined using a continuous coupled reaction catalyzed by lactate dehydrogenase (LDH, Sigma, L-2518), with the change in absorbance associated with generation of NAD+ from NADH monitored at 340 nm. Assays, done in 96 well microplates (UV-Star, VWR, 82050-788), were initiated by the addition of 10 µL of protein extracts, including the undiluted fraction (maximal activity of sample before dilution) and the undiluted uninduced (negative control) protein samples, to 290 µL of an AlaAT assay buffer (0.1 M Tris-HCl pH 8.0 at 20°C, 10 mM 2-oxoglutarate, 670 mM alanine, 0.27 mg ml−1 NADH, 0.36 U LDH, 10 µM pyridoxal-5′-phosphate (PLP)) such that the final reaction volume was 300 µL per well. The change in absorbance at 340 nm was monitored continuously for 10 min in a SpectraMax Plus absorbance plate reader (Molecular Devices, Sunnyvale, CA).
Kinetic Assays
Kinetic assays were run for both sets of AlaAT substrates, with the concentration of one substrate varied while the other was held constant at a saturating concentration, for 10 of the 13 constructs. Kinetic data were not obtained for MmAlaAT2-pBAD18-Kan, ScAlaAT2-pBAD18-Kan, HsAlaAT1-pBAD18-Kan, or HsAlaAT2-pBAD18-Kan as activity of these constructs was not detected in initial assays. We believe that this inability to detect activity was the result of inclusion body formation with these proteins in E. coli.
Enzyme activity at each substrate concentration was assayed at 20°C in triplicate, over a concentration range(≈ 0.3×KM–8×KM) chosen based on previously published values and preliminary kinetic assessments. To each well, 10 µL of diluted AlaAT protein sample were added, along with 20 or 50 µL of substrate, and kinetic assay buffer to a final volume of 300 µL. When AlaAT activity was measured in the direction alanine to pyruvate, the kinetic assay buffer consisted of either alanine (100 mM) or 2-oxoglutarate (10 mM), 0.1 M Tris-HCl, pH 8.0, 0.27 mg ml−1 NADH, 10 µM PLP and 0.36 U LDH. When activity in the direction of pyruvate to alanine was measured, the assay buffer contained either pyruvate (10 mM) or glutamate (50 mM), 0.1 M Tris-HCl, pH 8.0, 100 mM NH4Cl, 0.27 mg ml−1 NADH, 10 µM PLP and 1.14 U glutamate dehydrogenase (GDH, Sigma, G-2501). The change in absorbance at 340 nm was monitored continuously for 6–10 min in a SpectraMax Plus platereader. The initial pseudo-linear portion of each absorbance-time plot was analyzed by linear regression (SoftMax Pro v. 3.0) to obtain initial rates. Thereafter, plots of initial rate versus [substrate] were fitted to the Michaelis-Menten equation by nonlinear regression (GraphPad Prism v. 5.03) to determine KM and Vmax values (Figure S2).
Escherichia Coli Growth Assays
E. coli TR61 cells containing various pBAD18-Kan:AlaAT or pBAD18-Kan:GGAT constructs were assayed for growth over 26 hrs. One mL of an overnight starter culture grown in LB medium was added to 100 mL of modified M63 minimal medium containing 0.2% glycerol, 0.2% arabinose, 50 µg mL−1 kanamycin and chloramphenicol at 25 µg mL−1. Bacterial cultures were then grown in flasks at 37°C for 26 hrs. After 4 hrs of growth all cultures were re-inoculated with 0.1% arabinose. The OD600 was recorded at 1–4 hr intervals through 14 hrs of growth and then again at 26 hrs for induced cultures, untransformed controls, empty vector controls, and uninduced controls containing a pBAD18-Kan:HvAlaAT.
TR61 cells containing an empty pBAD18-Kan vector as well as TR61 cells containing pBAD18-Kan:HvAlaAT were also assayed for growth differences in M63 liquid minimal medium, supplemented as described above, containing 2-oxoglutarate at different concentrations (Figure 2). Cell growth under these conditions was also assayed for a total of 26 hrs, with measurements taken every 1–3 hrs for the first 14 hrs and then again at 26 hrs.
Supporting Information
Amino acid sequence alignment for eleven AlaAT enzyme sequences and two GGAT enzymes sequences. Amino acid sequences used were obtained from NCBI, except M. truncatula which was provided by Anis Limami, at the Université d' Angers, and analysis was done using ClustalW software. Residues conserved in subtype I aminotransferases are highlighted in white text on a black background. Fully conserved residues are indicated by “*”, conservative substitutions are indicated by “:”, and “.” denotes a semi-conservative substitution.
(TIF)
KM and Vmax of various AlaAT enzymes with alanine, 2-oxoglutarate, pyruvate and glutamate. Data were fitted to the Michaelis-Menten equation with the nonlinear regression facility of GraphPad Prism v. 5.03, in order to calculate KM and Vmax values. Data points are the mean ± standard error (SE) of triplicate determinations.
(TIF)
Primer sequences used in the cloning of AlaAT enzymes. All AlaATs were cloned into the pBAD18-Kan plasmid using the restriction sites indicated. Restriction enzyme sites are shown in lower case lettering.
(TIF)
Average Vmax values for unpurified AlaAT and GGAT enzymes. Vmax values are shown for each substrate, for each of the ten enzymes examined. Kinetic values represent the average of three independent trials. The correlation coefficient (r2) was >0.80 for all trials, except AtGGAT1 glutamate, AtGGAT2 glutamate and MmAlaAT1 pyruvate. Raw data are plotted in Figure S2.
(TIF)
Acknowledgments
We thank Anis Limami, at the Université d' Angers for the Medicago AlaAT cDNAs; Laura Olsen at the University of Michigan for the AtGGAT1 and AtGGAT2 cDNAs; Rong ze Yang at the University of Maryland for the HsAlaAT1, HsAlaAT2, MmAlaAT1 and MmAlaAT2 cDNAs; Tracy Ravio at the University of Alberta for E. coli strain TR61 and Disa Brownfield-Walker at the University of Alberta.
Funding Statement
This research was funded by an NSERC Discovery Grant - NSERC RGPIN 89739 to the Principal Investigator, AGG (http://www.nserc-crsng.gc.ca/), and a Research grant from the Alberta Crop Industry Development Fund; ACIDF2009C001R to AGG (URL http://www.acidf.ca/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mehta PK, Hale TI, Christen P (1993) Aminotransferases: demonstration of homology and division into evolutionary subgroups. FEBS J 214: 549–61. [DOI] [PubMed] [Google Scholar]
- 2. Miyashita Y, Dolferus R, Ismond KP, Good AG (2007) Alanine aminotransferase catalyses the breakdown of alanine after hypoxia in Arabidopsis thaliana. Plant J 49: 1108–21. [DOI] [PubMed] [Google Scholar]
- 3. Kameya M, Arai H, Ishii M, Igarashi Y (2010) Purification of three aminotransferases from Hydrogenobacter thermophilus TK-6-novel types of alanine or glycine aminotransferase: enzymes and catalysis. FEBS J 277: 1876–85. [DOI] [PubMed] [Google Scholar]
- 4. Jadaho SB, Yang RZ, Lin Q, Hu H, Anania FA, et al. (2004) Murine alanine aminotransferase: cDNA cloning, functional expression, and differential gene regulation in mouse fatty liver. Hepatology 39: 1297–302. [DOI] [PubMed] [Google Scholar]
- 5. Liepman AH, Olsen LJ (2003) Alanine aminotransferase homologs catalyze the glutamate:glyoxylate aminotransferase reaction in peroxisomes of Arabidopsis. Plant Physiol 131: 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Good AG, DePauw M, Kridl JC, Theodoris G, Shrawat AK (2007a) Nitrogen-efficient monocot plants. US Patent #20070162995.
- 7. Good AG, Johnson SJ, DePauw M, Carroll RT, Savidov N, et al. (2007b) Engineering nitrogen use efficiency with alanine aminotransferase. Can J Bot 85: 252–62. [Google Scholar]
- 8. Shrawat AK, Carroll RT, DePauw M, Taylor GJ, Good AG (2008) Genetic engineering of improved nitrogen use efficiency in rice by the tissue-specific expression of alanine aminotransferase. Plant Biotech J 6: 722–32. [DOI] [PubMed] [Google Scholar]
- 9. Masclaux-Daubresse C, Daniel-Vedel F, Dechorgnat J, Chardon F, Gaufichon FL (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot 105: 1141–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kant S, Bi Y-M, Rothstein SJ (2011) Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency. Access 62: 1499–1509. [DOI] [PubMed] [Google Scholar]
- 11. McAllister CH, Beatty PH, Good AG (2012) Engineering nitrogen use efficient crop plants; the current status. Plant Biotech J 10: 1011–25. [DOI] [PubMed] [Google Scholar]
- 12. Sohocki MM, Sullivan LS, Harrison WR, Sodergren EJ, Elder FF, et al. (1997) Human glutamate pyruvate transaminase (GPT): localization to 8q24.3, cDNA and genomic sequences, and polymorphic sites. Genomics 40: 247–52. [DOI] [PubMed] [Google Scholar]
- 13. Yang R, Park S, Reagan WJ, Goldstein R, Zhong S, et al. (2009) Alanine aminotransferase isoenzyme: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology 49: 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beatty PH, Shrawat AK, Carroll RT, Zhu T, Good AG (2009) Transcriptome analysis of nitrogen-efficient rice over-expressing alanine aminotransferase. Plant Biotech J 7: 562–76. [DOI] [PubMed] [Google Scholar]
- 15. Vigeolas H, Waldeck P, Zank T, Geigenberger P (2007) Increasing seed oil content in oil-seed rape (Brassica napus L.) by over-expression of a yeast glycerol-3-phosphate dehydrogenase under the control of a seed-specific promoter. Plant Biotech J 5: 431–41. [DOI] [PubMed] [Google Scholar]
- 16. Paine JA (2005) Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nature Biotech 23: 482–87. [DOI] [PubMed] [Google Scholar]
- 17.Duff SMG, Rydel TJ, McClerren AL, Zhang W, Li JY, et al.. (2012) The enzymology of alanine aminotransferase (AlaAT) isoforms from Hordeum vulgare and other organisms, and the HvAlaAT crystal structure. Arch Biochem Biophys, doi: 10.1016/j.abb.2012.06.006. [DOI] [PubMed]
- 18. Brückner H, Westhauser T (2003) Chromatographic determination of L- and D-amino acids in plants. Amino Acids 24: 43–55. [DOI] [PubMed] [Google Scholar]
- 19. Brückner H, Westhauser T (1994) Chromatographic determination of D-amino acids as native constituents of vegetables and fruitsa) . Chromatographia 39: 419–26. [Google Scholar]
- 20. Ward DE, Kengen SW, van Der Oost J, de Vos WM (2000) Purification and characterization of the alanine aminotransferase from the hyperthermophilic archaeon Pyrococcus furiosus and its role in alanine production. J Bacteriol 182: 2559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wiśniewski P, Szklarczyk J, Maciąga M, Paszkowski A (2006) L -alanine?: 2-oxoglutarate aminotransferase isoenzymes from Arabidopsis thaliana leaves. Acta Physiol Plant 28: 577–88. [Google Scholar]
- 22. Good AG, Muench DG (1992) Purification and characterization of an anaerobically induced alanine aminotransferase from barley roots. Plant Physiol 99: 1520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marangoni B (2003) Enzyme Kinetics: A Modern Approach. New Jersey: John Wiley and Sons Inc.
- 24.Singh B (1999) Plant Amino Acids: Biochemistry and Biotechnology. New York Marcel Dekker, Inc.
- 25. Jiao Z, Si X, Li G, Zhang Z, Xu X (2010) Unintended compositional changes in transgenic rice seeds (Oryza sativa L.) studied by spectral and chromatographic analysis coupled with chemometrics methods. J Agric Food Chem 58: 1746–54. [DOI] [PubMed] [Google Scholar]
- 26. Narsai R, Howell KA, Carroll A, Ivanova A, Millar AH, et al. (2009) Defining core metabolic and transcriptomic responses to oxygen availability in rice embryos and young seedlings. Plant Phys 151: 306–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Branco-Price C, Kaiser KA, Jang CJH, Larive CK, Bailey-Serres J (2008) Selective mRNA translation coordinates energetic and metabolic adjustments to cellular oxygen deprivation and reoxygenation in Arabidopsis thaliana. The Plant J 56: 743–55. [DOI] [PubMed] [Google Scholar]
- 28. Kim SH, Schneider BL, Reitzer L (2010) Genetics and regulation of the major enzymes of alanine synthesis in Escherichia coli . J Bacteriol 192: 5304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hatch MD, Mau SL (1977) Association of NADP- and NAD-linked malic enzyme activities in Zea mays: relation to C4 pathway photosynthesis. Arch Biochem Biophys 179: 361–69. [DOI] [PubMed] [Google Scholar]
- 30. Duff SMG, Qi Q, Reich T, Wu X, Brown T, et al. (2011) A kinetic comparison of asparagine synthetase isozymes from higher plants. Plant Physiol Biochem 49: 251–6. [DOI] [PubMed] [Google Scholar]
- 31. Ninfa AJ, Jiang P (2005) PII signal transduction proteins: sensors of alpha-ketoglutarate that regulate nitrogen metabolism. Curr Opin Microbiol 8: 168–73. [DOI] [PubMed] [Google Scholar]
- 32. Bunik VI, Fernie AR (2009) Metabolic control exerted by the 2-oxoglutarate dehydrogenase reaction: a cross-kingdom comparison of the crossroad between energy production and nitrogen assimilation. Biochem J 422: 405–21. [DOI] [PubMed] [Google Scholar]
- 33. Jiang P, Ninfa AJ (2009) Alpha-ketoglutarate controls the ability of the Escherichia coli PII signal transduction protein to regulate the activities of NRII (NrB) but does not control the binding of PII to NRII. Biochem 48: 11514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uhrig RG, Ng KKS, Moorhead GBG (2009) PII in higher plants: a modern role for an ancient protein. Trends Plant Sci 14: 505–11. [DOI] [PubMed] [Google Scholar]
- 35. Jing X, Zhang S (2011) An ancient molecule with novel function: Alanine aminotransferase as a lipopolysaccharide binding protein with bacteriocidal activity. Dev Comp Immunol 35: 94–104. [DOI] [PubMed] [Google Scholar]
- 36. Wong KF, Luk JM, Cheng RH, Klickstein LB, Fan ST (2007) Characterization of two novel LPS-binding sites in leukocyte integrin betaA domain. FASEB J 21: 3231–9. [DOI] [PubMed] [Google Scholar]
- 37. Kikuchi H, Hirose S, Toki S, Akama K, Takaiwa F (1999) Molecular characterization of a gene for alanine aminotransferase from rice (Oryza sativa). Plant Mol Biol 39: 149–59. [DOI] [PubMed] [Google Scholar]
- 38.Lock Y-Y (2011) Engineering nitrogen use efficiency in Oryza sativa by the developmental over-expression of barley alanine aminotransferase using a novel rice promoter. M.Sc. thesis, University of Alberta.
- 39.Wolansky MA (2005) Genetic manipulation of aspartate aminotransferase levels in Brassica napus: effects on nitrogen use efficiency. M.Sc. thesis, University of Alberta.
- 40. Muench DG, Good AG (1994) Hypoxically inducible barley alanine aminotransferase: cDNA cloning and expression analysis. Plant Mol Biol 24: 417–27. [DOI] [PubMed] [Google Scholar]
- 41. Ricoult C, Echeverria LO, Cliquet JB, Limami AM (2006) Characterization of alanine aminotransferase (AlaAT) multigene family and hypoxic response in young seedlings of the model legume Medicago truncatula . J Exp Bot 57: 3079–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Amino acid sequence alignment for eleven AlaAT enzyme sequences and two GGAT enzymes sequences. Amino acid sequences used were obtained from NCBI, except M. truncatula which was provided by Anis Limami, at the Université d' Angers, and analysis was done using ClustalW software. Residues conserved in subtype I aminotransferases are highlighted in white text on a black background. Fully conserved residues are indicated by “*”, conservative substitutions are indicated by “:”, and “.” denotes a semi-conservative substitution.
(TIF)
KM and Vmax of various AlaAT enzymes with alanine, 2-oxoglutarate, pyruvate and glutamate. Data were fitted to the Michaelis-Menten equation with the nonlinear regression facility of GraphPad Prism v. 5.03, in order to calculate KM and Vmax values. Data points are the mean ± standard error (SE) of triplicate determinations.
(TIF)
Primer sequences used in the cloning of AlaAT enzymes. All AlaATs were cloned into the pBAD18-Kan plasmid using the restriction sites indicated. Restriction enzyme sites are shown in lower case lettering.
(TIF)
Average Vmax values for unpurified AlaAT and GGAT enzymes. Vmax values are shown for each substrate, for each of the ten enzymes examined. Kinetic values represent the average of three independent trials. The correlation coefficient (r2) was >0.80 for all trials, except AtGGAT1 glutamate, AtGGAT2 glutamate and MmAlaAT1 pyruvate. Raw data are plotted in Figure S2.
(TIF)