Abstract
Context
Adrenocortical carcinoma (ACC) is a rare and highly aggressive endocrine neoplasm, with limited therapeutic options. Activating β-catenin somatic mutations are found in ACC and have been associated with a poor clinical outcome. In fact, activation of the Wnt/β-catenin signaling pathway seems to play a major role in ACC aggressiveness, and might, thus, represent a promising therapeutic target.
Objective
Similar to patient tumor specimen the H295 cell line derived from an ACC harbors a natural activating β-catenin mutation. We herein assess the in vitro and in vivo effect of β-catenin inactivation using a doxycyclin (dox) inducible shRNA plasmid in H295R adrenocortical cancer cells line (clone named shβ).
Results
Following dox treatment a profound reduction in β-catenin expression was detectable in shβ clones in comparison to control clones (Ctr). Accordingly, we observed a decrease in Wnt/βcatenin-dependent luciferase reporter activity as well as a decreased expression of AXIN2 representing an endogenous β-catenin target gene. Concomitantly, β-catenin silencing resulted in a decreased cell proliferation, cell cycle alterations with cell accumulation in the G1 phase and increased apoptosis in vitro. In vivo, on established tumor xenografts in athymic nude mice, 9 days of β-catenin silencing resulted in a significant reduction of CTNNB1 and AXIN2 expression. Moreover, continous β-catenin silencing, starting 3 days after tumor cell inoculation, was associated with a complete absence of tumor growth in the shβ group while tumors were present in all animals of the control group.
Conclusion
In summary, these experiments provide evidences that Wnt/β-catenin pathway inhibition in ACC is a promising therapeutic target.
Introduction
Adrenocortical carcinoma (ACC) is a rare and highly aggressive endocrine neoplasm, with a 5-year overall survival of around 40% [1]–[4]. Therapeutic options for these patients are scarce, and available chemotherapies of limited effectiveness. A better understanding of tumor biology and molecular prognostic factors would help to select relevant therapeutic targets and to develop innovative therapeutic strategies.
Activation of the Wnt/β-catenin signaling pathway in adrenocortical tumorigenesis has recently been investigated in detail [5]–[10], and seems to play a major role in adrenocortical carcinoma prognosis. In animal models, a constitutive activation of the Wnt/β-catenin pathway in the adrenal cortex of transgenic mice leads to the development of adrenocortical tumors with malignant characteristics [11]. In humans, this pathway is frequently activated mainly trough β-catenin gene (CTNNB1, i.e. Catenin (cadherin-associated protein), beta 1) mutations [5], [7], and is associated with specific clinical and pathological characteristics and a poor outcome [8]. Likewise, a specific transcriptomic signature of tumors with CTNNB1 mutation has recently been shown [12], and might be responsible for the particular poor prognosis of affected patients. Overall, these observations suggest Wnt/β-catenin signaling pathway inactivation as a promising therapeutic target in ACC.
The aim of this study was to assess the in vivo and in vitro effects of Wnt/β-catenin signaling pathway specific inactivation using short hairpin RNA (shRNA) in a tumor model for adrenocortical carcinoma (H295R).
Materials and Methods
Cell Culture and generation of H295R Clones
Adrenocortical carcinoma cell line H295R stably transfected with the Tet repressor (H295R/TR) was kindly provided by Dr. Lalli [13]. Cells were grown as previously described [14]. pTer-β-catenin vector, which expresses a doxycyclin inducible shRNA targeted CTNNB1 (β-catenin; targeted sequence: 5′-GTGGGTGGTATAGAGGCTC-3′) mRNA, and the control vector (pTer) were obtained from Dr. van de Wetering [15]. H295R/TR were transfected with the pTer-β-catenin or pTer and clones were selected by zeocin (50 µg/ml, InvivoGen). Three shRNA-βcatenin clones (shβ) were selected in which CTNNB1 expression was down-regulated at least 5 fold in a doxycyclin (dox, 0.2 µg/ml, Sigma) dependent manner in comparison to three control clones (Ctr) transfected with pTer vector. All cell clones were investigated for their ability to express specific steroidogenic genes (StAR and CYP11B1) and their responses to the cAMP/PKA pathway which was found to be comparable to that of the parental cell line, H295R [16] (data not shown). S45P CTNNB1 (β-catenin) gene activating mutation, previously identified in the parental H295R cell line [5], [8], was confirmed by direct sequencing in all Ctr and shβ clones (data not shown). While data are presented for a single Ctr and shβ clone all in vitro experiments were confirmed with equivalent results in 2–3 individual clones (Ctr and shβ).
Analysis of RNA and protein levels
Total RNA or protein extractions and analysis from cell lines were performed as previously described [14] with primers and antibodies described in Table S1.
Cell Transfection, and reporter assays
As a Wnt/β-catenin pathway reporter construct driving expression of luciferase gene, the TopFlash plasmid (Top) was used which contains two copies of the β-catenin/T-cell factor TCF-binding sites whereas the FopFlash plasmid (Fop) contains two mutated copies of the β-catenin/TCF-binding sites [5]. Rous sarcoma virus (RSV)-Renilla (Promega) was used as a control of transfection efficiencies. Cells were cotransfected and Firefly and Renilla luciferase activities were sequentially measured as previously described [14].
Cell proliferation, cell cycle and apoptosis analysis
Proliferation was measured by MTT assay (Promega). The cell cycle and apoptosis were analysed by flow cytometry as previously reported [17].
Xenograft, pathological examination and immunohistochemical staining
Female athymic NMRI nu/nu mice (6–8 weeks) were purchased from Harlan Winkelmann (Borchen, Germany) and housed under pathogen-free conditions. All experiments were carried out following protocols approved by the Regierung von Oberbayern and in accordance with the german guidelines for animal studies. 15×106 cells of the individual clones were inoculated in a volume of 200 µl PBS subcutaneously into the neck of each mouse. For short-term therapeutic experiments dox treatment was initiated when the longest tumor diameters ranged between 0.2–0.9 cm in size (after 21–31 days). Doxycyline was added in a final concentration of 2 mg/ml to the drinking water in amber water bottles. After 9 days of dox treatment tumors were excised, fixed in formalin, embedded in paraffin, and 4 µm sections cut and stained with Hematoxylin-Eosin-Saffron. Immunohistochemistry for β-catenin was performed as previously described [5]. Cells for long-term therapeutic experiments were inoculated and 3 days after tumor induction mice were starting from then continuously treated with dox water. Tumor size was measured every other day using a calliper as described earlier [18]. At day 31 days after tumor induction, when first tumors reached a longest tumor diameter of 1.5 cm, mice were sacrificed and tumors excised.
Statistical analysis
All in vitro data with statistical analyses represent the quantification of at least three experiments. Control conditions were set as 100% and data were analyzed using Fisher's test. The statistical analysis for comparison of tumor weight after long-term therapeutic experiment in vivo was performed by Mann-Whitney test. Significance was set at P<0.05 (represented by * in figures); P<0.01 (**) and P<0.001 (***).
Results
Efficient inactivation of CTNNB1 by shRNA in adrenocortical cancer cells
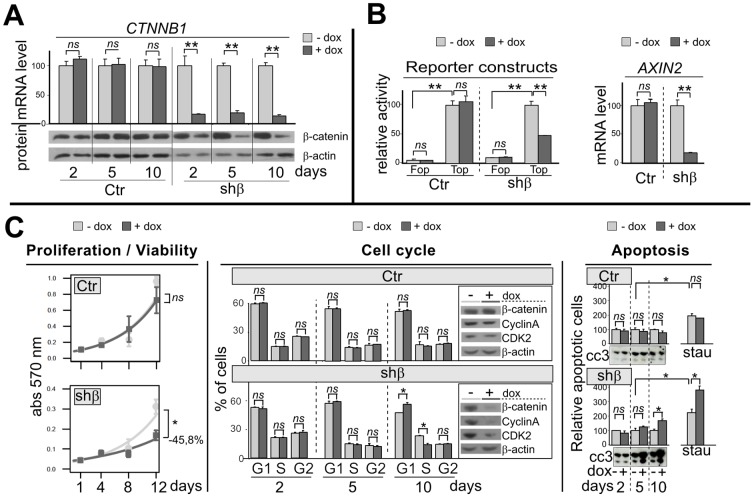
H295R cells, harboring a heterozygous CTNNB1 gene mutation on the GSK3β phosphorylation site (S45P), exhibit a constitutive transcriptional activity of β-catenin-LEF/TCF [5]. Cell clones expressing a doxycylin inducible shRNA targeting β-catenin were generated. Two days following shRNA-β-catenin induction by doxycyclin (dox) treatment, CTNNB1 mRNA (−85%; p<0.01) and protein levels were significantly decreased (figure 1-A). This decrease in CTNNB1 mRNA and protein levels persisted with dox treatment up to 10 days. In contrast, dox treatment had no effect on CTNNB1 expression in control clone (Ctr) (figure 1-A).
Figure 1. CTNNB1 silencing alters the Wnt/β-catenin signaling pathway, proliferation, cell cycle and apoptosis.
A, Histogram and Western blot panels represent CTNNB1 (β-catenin) mRNA and protein accumulation, in Ctr and shβ clones after 2, 5 or 10 days after addition of doxycyclin (dox) in the culture medium (0.2 µg/ml). B-left, cells were transiently co-transfected with an artificial Wnt/-β-catenin pathway reporter construc (Top) or his control mutated (Fop). After 24 h, cells were treated by vehicle or dox (0.2 µg/ml) for 24 h and luciferase activity was measured. -right, Histogram represent AXIN2 mRNA accumulation after 2 days of dox treatment. C-left, Cell survival curve of cells, as assessed by the MTT assay without or with dox (0.2 µg/ml) for 1, 4, 8 or 12 days. -center, The distribution of cells in the various phases of the cell cycle was analysed by flow cytometric analysis of propidium iodide staining after vehicle or dox treatment for 2, 5 and 10 days. Western blots show β-catenin, CyclinA and CDK2 protein levels at 10 day. -right, Histograms represent apoptotic cells measured by flow cytometric annexin V incorporation, after vehicle or dox treatment for 2, 5 and 10 days, without or with staurosporin co-treatment for last 6 h (0.5 µg/ml). Western blots show the cleaved caspase 3 (cc3) in same condition.
CTNNB1 silencing decreases Wnt/β-catenin-LEF/TCF dependent transcription
The Wnt/β-catenin-LEF/TCF dependent transcription was studied by using the Top-Flash/Fop-Flash plasmids (Top and Fop). As expected, transfected H295R cells showed higher transcriptional activity of the β-catenin-LEF/TCF dependent luciferase reporter construct Top in comparison to the mutated Fop construct (figure 1-B, Ctr-Fop: 5% compared to Ctr-Top: 100%, p<0.01; and shβ-Fop: 9% compared to shβ-Top: 100%, p<0.01). H295R cells which expressed shRNA-β-catenin (shβ with dox) showed lower transcriptional activity of the reporter construct Top (shβ-Top: −53%, p<0.01). Dox treatment did not affect activity of the Top construct in the control clone (Ctr-Top) or on the Fop construct with mutated LEF/TCF sites in both clones (Ctr-Fop and shβ-Fop).
Moreover, CTNNB1 silencing for two days significantly decreased mRNA level of an endogenous canonical downstream target gene of Wnt/β-catenin pathway, i.e AXIN2, in the shβ clone (figure 1-B, shβ: −82%, p<0.01) compared to control clones (Ctr, ns).
CTNNB1 silencing alters proliferation, cell cycle and apoptosis
A time course study of CTNNB1 silencing in H295R cell line demonstrated a significant decrease in proliferation (−45,8% at 12 days, p<0.05) compared to shβ clone without CTNNB1 silencing (−dox) or the control clone (figure 1-C), determined by MTT conversion.
Flow cytometric analysis of cell cycle by propidium iodide staining showed no effect on cell cycle until 5 days. However following 10 days of dox treatment, CTNNB1 silencing resulted in the accumulation of cells in the G1 phases and a decrease of cell proportion in the S phase (figure 1-C, shβ-10d-G1: 55.3±0.4% vs 65.8±2.2%; -S: 27.5±0.2% vs 16.5±1.6%, p<0.05). No such difference was observed in the control clone (Ctr). At this time point, we observed a reduction of two important proteins for G1/S transition, Cyclin A and CDK2 only in cells silenced for CTNNB1 (figure 1-C).
Similarly, no effect on apoptosis assayed by the flow cytometric analysis of annexine V incorporation in cells, was observed until 5 days while 10 days of dox induced CTNNB1 silencing increased the proportion of apoptotic cells (figure 1-C, shβ-10d: 167%, p<0.05) when compared to cells without dox treatment. No such difference was observed in the control clone. This increase of apoptosis by CTNNB1 silencing was also confirmed by the gain of a proapoptotic protein level, cleaved caspase3 (cc3), which was detectable already at an earlier time point (5 days, figure 1-C). Likewise, CTNNB1 silencing increased the apoptotic effect of staurosporin (figure 1-C; shβ-5d+stau: 224% vs 377%, p<0.05) while no significant difference was observed in control clones.
CTNNB1 silencing abolish xenograft development of ACC cell line
To evaluate the functional significance of β-catenin knock-down on tumor development we proceeded with investigation in a subcutaneous xenograft tumor model in athymic nude mice.
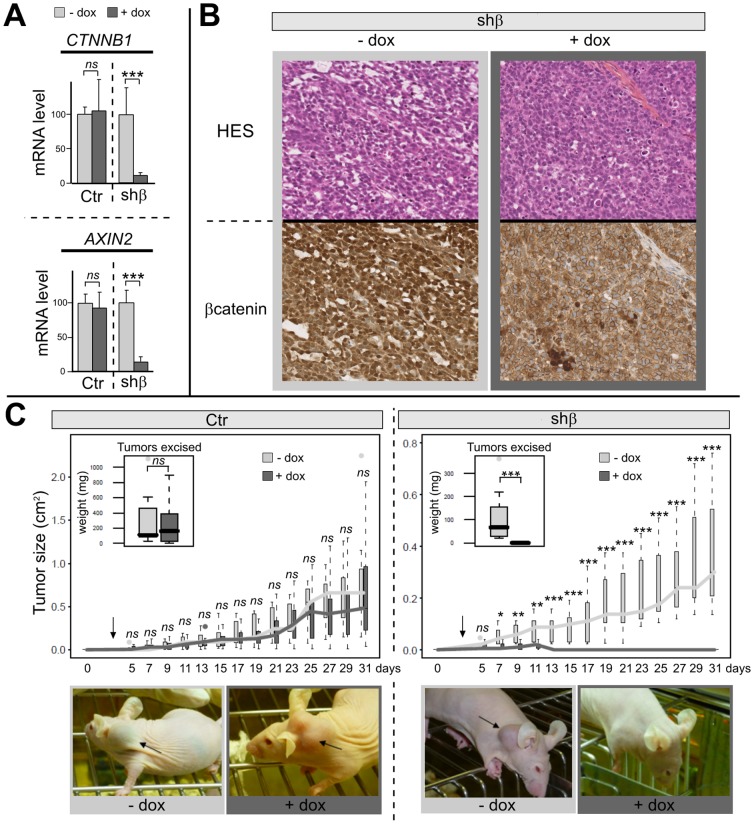
In a first step, short term experiments with CTNNB1 inactivation on established tumors (21 to 31 days after xenografting) for a duration of 9 days were performed to mirror the time course from our in vitro experiments (figure 1-C). Similar to the in vitro setting mRNA expression analyzes revealed a dox dependent significant decrease in tumoral CTNNB1 and AXIN2 expression in shβ clone (figure 2-A; CTNNB1: −89%, p = 0.007; AXIN2: −87%, p<0.001) while control clones remained unaffected by dox treatment. Moreover, and in accordance with the cell culture experiments, immunohistochemical analysis (figure 2-B) revealed a dox treatment dependent reduction of β-catenin protein. But, we have not observed any differences for the Ki67 and the cleaved caspase3 expression by immunohistochemistry analyzes (data not shown), suggesting that 9 days of dox is not sufficient in vivo to induce effects on proliferation and on apoptosis. Because this time course is too short to investigate a potential effect on tumor growth in an in vivo model, long term dox treatment was performed. Ctr and shβ clones were subcutaneously injected and 3 days after tumor induction mice were treated with dox in a continuous manner. There is no significant difference after 31 days in tumor size between shβ and Ctr clones in absence of dox treatment, but it's seem that the shβ clone grow slower than the Ctr clone, probably because of a clonal effect. For this reason the doxycyclin-inducible system is suitable, because the clones are their own control. While dox treatment did not affect tumor growth and weight in the control clone (figure 2-C-left, medians weight: 112 mg vs 162.7 mg, ns), there was a significant impact of CTNNB1 silencing upon dox treatment in the shβ clone (figure 2-C-rigth). Indeed, for shβ clone, during the first 10 days, tumor growth was observable in both groups (without or with dox) but, after 13 days, no tumor was detectable in any of the mice of shβ group treated with dox, including after dissection and pathological analysis (figure 2-C-rigth, median weight 67.4 mg vs 0 mg, p<0.001).
Figure 2. CTNNB1 silencing abolish xenograft development of ACC cell line.
A, Histograms represent CTNNB1 (β-catenin) and AXIN2 mRNA accumulation in xenograft for both Ctr (−dox n = 6; +dox, n = 5) and shβ (−dox, n = 5; +dox, n = 5) clones on established tumors and after 9 days of dox treatment. B, hematoxylin–eosin–saffron and β-catenin, staining (×20) on representative tumors of shβ clone without and with dox treatment (same experiment as B). C, Boxplots represent the tumor sizes for Ctr and shβ xenografts in mice continuously treated with vehicle or dox after 3 days of tumor induction. Boxplots in the left corner represent the weights of tumors excised. Ctr (−dox n = 7, + dox n = 8), shβ (−dox, n = 7; +dox, n = 8).
Discussion
In many cancers, the Wnt/β-catenin signalling pathway plays an important role regulating cell growth, motility, and differentiation [19]. We and others have previously demonstrated the importance of the Wnt/β-catenin signaling pathway activation in adrenal cortex tumorigenesis [5]–[10]. This activation is associated with a specific molecular signature and a worse outcome with lower overall survival [12]. Doghman and colleagues previously showed that a TCF antagonist inhibits proliferation of adrenocortical cells H295R, suggesting a central role of Wnt/β-catenin pathway in adrenocortical tumorigenesis [20]. However, this pharmacological approach might not be specific for Wnt signaling. We herein, using both in vitro and in vivo experiments, demonstrate that direct and specific β-catenin inactivation lead to alterations of proliferation, cell cycle and apoptosis which lead to a dramatic decrease in tumor development in a tumor model for ACC. Further experiments are needed in order to know if β-catenin inactivation suppresses the growth or prevents engraftment of the cells. Nevertheless, these results confirm 1/the biological consequence of Wnt/β-catenin pathway activation in adrenocortical tumorigenesis, and 2/the major therapeutic interest to target this pathway.
Inhibition of Wnt/β-catenin pathway in a subgroup of aggressive ACC seems to be a interesting therapeutic target and should be evaluated in more detail in the future.
Supporting Information
PCR conditions and antibodies used.
(XLS)
Acknowledgments
Dr. E Lalli for the generous gift of the H295R/TR cells and Dr. H Clevers for the generous gift of the pTer-shβcatenin vector.
Funding Statement
This work was supported in part by the Contrat d'Initiation à la Recherche Clinique (grant CIRC 05045 – AP-HP), the Plan Hospitalier de Recherche Clinique (AOM06179) to the COMETE Network, the Recherche Translationnelle DHOS/INCA 2009 (RTD09024), ESF (07-RNP-067), Association pour la Recherche sur le Cancer (SFI20111203542), the Weigand Trust Germany, the Sander Stiftung (2011.003.1) and the Ligue contre le cancer (RS12/75–105). Furthermore, research leading to these results has received funding from the Seventh Framework Programme (FP7/2007–2013) under grant agreement number 259735 (ENS@T-CANCER). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Abiven G, Coste J, Groussin L, Anract P, Tissier F, et al. (2006) Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab 91: 2650–2655. [DOI] [PubMed] [Google Scholar]
- 2. Libe R, Fratticci A, Bertherat J (2007) Adrenocortical cancer: pathophysiology and clinical management. Endocr Relat Cancer 14: 13–28. [DOI] [PubMed] [Google Scholar]
- 3. Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, et al. (2008) Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer 113: 3130–3136. [DOI] [PubMed] [Google Scholar]
- 4. Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, et al. (2009) Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer 115: 243–250. [DOI] [PubMed] [Google Scholar]
- 5. Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, et al. (2005) Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res 65: 7622–7627. [DOI] [PubMed] [Google Scholar]
- 6. Gaujoux S, Tissier F, Groussin L, Libe R, Ragazzon B, et al. (2008) Wnt/beta-catenin and 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A signaling pathways alterations and somatic beta-catenin gene mutations in the progression of adrenocortical tumors. J Clin Endocrinol Metab 93: 4135–4140. [DOI] [PubMed] [Google Scholar]
- 7. Tadjine M, Lampron A, Ouadi L, Bourdeau I (2008) Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clin Endocrinol (Oxf) 68: 264–270. [DOI] [PubMed] [Google Scholar]
- 8. Gaujoux S, Grabar S, Fassnacht M, Ragazzon B, Launay P, et al. (2011) {beta}-catenin activation is associated with specific clinical and pathological characteristics and a poor outcome in adrenocortical carcinoma. Clin Cancer Res [DOI] [PubMed] [Google Scholar]
- 9. Bonnet S, Gaujoux S, Launay P, Baudry C, Chokri I, et al. (2011) Wnt/beta-catenin pathway activation in adrenocortical adenomas is frequently due to somatic CTNNB1-activating mutations, which are associated with larger and nonsecreting tumors: a study in cortisol-secreting and -nonsecreting tumors. J Clin Endocrinol Metab 96: E419–426. [DOI] [PubMed] [Google Scholar]
- 10. Kim A, Giordano TJ, Kuick R, Serecky K, Hammer GD (2009) Wnt/betacatenin signaling in adrenocortical stem/progenitor cells: implications for adrenocortical carcinoma. Ann Endocrinol (Paris) 70: 156. [DOI] [PubMed] [Google Scholar]
- 11. Berthon A, Sahut-Barnola I, Lambert-Langlais S, de Joussineau C, Damon-Soubeyrand C, et al. (2010) Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet 19: 1561–1576. [DOI] [PubMed] [Google Scholar]
- 12. Ragazzon B, Libe R, Gaujoux S, Assie G, Fratticci A, et al. (2010) Transcriptome Analysis Reveals that p53 and {beta}-Catenin Alterations Occur in a Group of Aggressive Adrenocortical Cancers. Cancer Res 70: 8276–8281. [DOI] [PubMed] [Google Scholar]
- 13. Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, et al. (2007) Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol 21: 2968–2987. [DOI] [PubMed] [Google Scholar]
- 14. Ragazzon B, Cazabat L, Rizk-Rabin M, Assie G, Groussin L, et al. (2009) Inactivation of the Carney complex gene 1 (protein kinase A regulatory subunit 1A) inhibits SMAD3 expression and TGF beta-stimulated apoptosis in adrenocortical cells. Cancer Res 69: 7278–7284. [DOI] [PubMed] [Google Scholar]
- 15. van de Wetering M, Oving I, Muncan V, Pon Fong MT, Brantjes H, et al. (2003) Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep 4: 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Groussin L, Massias JF, Bertagna X, Bertherat J (2000) Loss of expression of the ubiquitous transcription factor cAMP response element-binding protein (CREB) and compensatory overexpression of the activator CREMtau in the human adrenocortical cancer cell line H295R. J Clin Endocrinol Metab 85: 345–354. [DOI] [PubMed] [Google Scholar]
- 17. Rizk-Rabin M, Assie G, Rene-Corail F, Perlemoine K, Hamzaoui H, et al. (2008) Differential expression of parathyroid hormone-related protein in adrenocortical tumors: autocrine/paracrine effects on the growth and signaling pathways in H295R cells. Cancer Epidemiol Biomarkers Prev 17: 2275–2285. [DOI] [PubMed] [Google Scholar]
- 18. Hantel C, Lewrick F, Schneider S, Zwermann O, Perren A, et al. (2010) Anti insulin-like growth factor I receptor immunoliposomes: a single formulation combining two anticancer treatments with enhanced therapeutic efficiency. J Clin Endocrinol Metab 95: 943–952. [DOI] [PubMed] [Google Scholar]
- 19. Behrens J, Lustig B (2004) The Wnt connection to tumorigenesis. Int J Dev Biol 48: 477–487. [DOI] [PubMed] [Google Scholar]
- 20. Doghman M, Cazareth J, Lalli E (2008) The T cell factor/beta-catenin antagonist PKF115–584 inhibits proliferation of adrenocortical carcinoma cells. J Clin Endocrinol Metab 93: 3222–3225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR conditions and antibodies used.
(XLS)