Abstract
The spinal motoneuron has long been a good model system for studying neural function because it is a neuron of the central nervous system with the unique properties of (1) having readily identifiable targets (the muscle fibers) and therefore having a very well-known function (to control muscle contraction); (2) being the convergent target of many spinal and descending networks, hence the name of "final common pathway"; and (3) having a large soma which makes it possible to penetrate them with sharp intracellular electrodes. Furthermore, when studied in vivo, it is possible to record simultaneously the electrical activity of the motoneurons and the force developed by their muscle targets. Performing intracellular recordings of motoneurons in vivo therefore put the experimentalist in the unique position of being able to study, at the same time, all the compartments of the "motor unit" (the name given to the motoneuron, its axon, and the muscle fibers it innervates1): the inputs impinging on the motoneuron, the electrophysiological properties of the motoneuron, and the impact of these properties on the physiological function of the motoneurons, i.e. the force produced by its motor unit. However, this approach is very challenging because the preparation cannot be paralyzed and thus the mechanical stability for the intracellular recording is reduced. Thus, this kind of experiments has only been achieved in cats and in rats. However, the study of spinal motor systems could make a formidable leap if it was possible to perform similar experiments in normal and genetically modified mice.
For technical reasons, the study of the spinal networks in mice has mostly been limited to neonatal in vitro preparations, where the motoneurons and the spinal networks are immature, the motoneurons are separated from their targets, and when studied in slices, the motoneurons are separated from most of their inputs. Until recently, only a few groups had managed to perform intracellular recordings of motoneurons in vivo2-4 , including our team who published a new preparation which allowed us to obtain very stable recordings of motoneurons in vivo in adult mice5,6. However, these recordings were obtained in paralyzed animals, i.e. without the possibility to record the force output of these motoneurons. Here we present an extension of this original preparation in which we were able to obtain simultaneous recordings of the electrophysiological properties of the motoneurons and of the force developed by their motor unit. This is an important achievement, as it allows us to identify the different types of motoneurons based on their force profile, and thereby revealing their function. Coupled with genetic models disturbing spinal segmental circuitry7-9, or reproducting human disease10,11, we expect this technique to be an essential tool for the study of spinal motor system.
Keywords: Neuroscience, Issue 70, Physiology, Biophysics, Anatomy, Medicine, Motor System, Spinal Cord, Intracellular Recordings, Motoneurons, EMG, Force, lumbar, neuron, brain, mouse, animal model
Protocol
1. Step One
Pre-anesthetic medication: 10-15 min before the induction of anesthesia, inject atropine (0.20 mg/kg) and methylprenidsolone (0.05 mg) sub-cutaneously to prevent salivation and oedema, respectively.
2. Step Two
Induction of anesthesia: inject pentobarbital sodium (70 mg/kg) or a mixture of ketamine/xylazine (100 mg/kg and 10 mg/kg, respectively) intra-peritoneally. Let the mouse go under until no toe pinch reflex can be obtained. If the anesthesia seems too light, supplement with 1/4 of the dose.
3. Step Three
*Note: this surgery is a terminal procedure.
When a surgical plane of anesthesia has been reached, transfer the mouse on a raised warm blanket in a prone position.
Cover the snout of the mouse with a mask delivering pure O2 at a flow around 100 ml/min.
Using a hair clipper, shave the dorsal region from the nape of the neck to the base of the tail.
Shave also the right hindlimb.
Flip mouse to supine position but take care to leave the oxygen mask in place.
4. Step Four
Secure the mouse in place with sutures looped around the limbs and secured at the four corners of the work surface.
5. Step Five
Insert a temperature probe to monitor the mouse core temperature. Adjust heating blanket/lamp power to maintain core temperature between 36 °C and 38 °C.
6. Tracheotomy and Artificial Ventilation
Using blunt scissors, make a cut over the trachea and pull the skin away on both sides.
Using blunt forceps, tear apart the salivary gland so as to expose two thin muscles (Sternohyoid) covering the trachea.
Using blunt forceps, separate the two muscles along their medial separation to reveal the trachea.
Using a Dumont 7 forceps, slide two length of 4.0 silk suture under the trachea.
Make a transversal cut in the trachea in between two cartilaginous rings but take care not to completely section the trachea.
Insert the tracheal tube down the trachea, then secure it on both sides of the insertion opening by tying the sutures over it. The tracheal tube is connected to a mouse ventilator (SAR-830/AP, CWE Inc.) and a capnograph (μcapstar, CWE Inc.). The ventilator is connected, through a compliance bag, to a source of pure O2. Adjust the parameters of the ventilator (breathing rate 100-150 bpm, end-tidal volume 170-310 μl) so that the mouse is not fighting against the artificial ventilation and the end-tidal pCO2 is stable between 4 and 5%.
7. Placement of the Intravenous Lines
Using blunt dissection techniques, expose the jugular vein on one side of the neck. At this level, the jugular vein splits into two major trunks, the anterior and posterior facial veins.
- Do the following steps twice, one set for each of these trunks:
- Using Dumont 4 or 5 forceps, carefully separate the vein from its surrounding conjunctive tissue.
- Using Dumont 7 forceps, slide two length of 6.0 silk sutures under the vein. separate them as far as possible along the length of the vein.
- Place a small vessel clip on the proximal side of the vein (the side closest to the heart), and tie off the distal side of the vein.
- Using very fine iris scissors, make a small transverse incision in the vein, taking great care not to section the vein completely.
- Insert a pre-filled 1Fr catheter (premicath, Vygon) in the opening, up to the vessel clip.
- Holding the vein and the catheter together in a Dumont 4 forceps, carefully remove the vessel clip, then push the catheter a few more millimeters into the vein.
- Secure the catheter by tying it with both sutures on both sides of the insertion.
One of the catheters is connected to a syringe or a syringe pump to inject supplemental doses of anesthetics whenever necessary (usually every 10-30 min). The IV dose is either 6 mg/kg of pentobarbital sodium or 1250+40 μg/kg/min of ketamine/xylazine12. The other catheter is connected to a syringe pump for a slow intravenous infusion (50 μl/hr) of a 4 % glucose solution containing NaHCO3 (1 %) and plasmion (14 %).
8. Close the Neck Skin with Needle and Suture, and Return the Mouse to Prone Position
9. Dissection of the Hind-limb Muscle and Nerves
Using scissors, make an incision from the top of the thigh to the Achilles tendon. Separate the skin from the underlying muscles, taking care not to damage blood vessels. Cauterize as needed.
Identify the anterior edge of the Biceps Femoris, which appears as a white line running down the thigh. Using scissors, carefully open along the line from the knee all the way to the hip bone. Separate the muscles. The sciatic nerve is now visible under the Biceps Femoris.
Carefully dissect the Biceps Femoris. Cauterize/ligature as needed to prevent bleeding. The Biceps Femoris can be totally removed or simply reclined to expose the sciatic nerve and the Triceps Surae muscles.
Dissect out the Sural nerve.
Using 8/0 silk thread, tie up the distal part of the Common Peroneal nerve and cut distal to the knot. Dissect the nerve all the way up, as close to the hip as possible.
Identify the Tibial nerve in between the Common Peroneal and Sural nerves. Among the different branches of the Tibial nerve, identify the branches innervating the Triceps Surae from the branches that go deeper (henceforth referred to as Tibial nerve).
Using 8/0 silk thread, tie together all the branches of the Tibial nerve while keeping intact the branches innervating the Triceps Surae. Cut the the Tibial nerve distally to the knot and dissect the nerve up as far as possible.
Cover the whole hindlimb with a gauze imbibed with saline to prevent dessication while proceeding with the next step.
10. Laminectomy
Using scissors, make an incision along the backbone. Separate the skin from the underlying muscles.
Make two incisions on each side of the vertebrae to separate the subcutaneous muscles. Then cut each tendon of the muscles that attach on the side of the vertebrae on each side.
Using blunt forceps and a fine curette, remove the remainder of the muscle on the dorsal side of the vertebrae around the spinous processes to clearly identify each individual vertebra.
Identify vertebrae T13 and L1. T13 is the last vertebra to have ribs attached. The vertebral column will be immobilized using the Cunningham Spinal Cord vertebral clamps (Stoelting Co.). Place the spinal clamps on each side of T13 and L1. Be careful not to compress the spinal cord, but put a little bit of tension in the longitudinal axis. Check that the spinal column is well secured by pressing down gently with forceps.
Using fine rongeurs, remove the spinal processes then the laminae over T13 and L1, thereby exposing the spinal cord.
Cover the exposed spinal cord with small bits of cotton or spongel imbibed with saline to prevent desiccation.
Place a custom made plastic bath on top of the back, surrounding the spinal cord. Secure the bath in place using 4/0 silk thread. Ensure that the bath is waterproof by sealing it with Kwik-Cast sealant (WPI).
Once the Kwik-Cast has dried, remove the cotton covering the spinal cord, and fill the bath with mineral oil.
Using Dumont 5 forceps and very fine iris scissors, gently pull on the dura mater surrounding the spinal cord, and open it as far as possible in both direction. Fold the dura on each side of the spinal cord.
Lower the raised platform on which the mouse is lying so as to keep it suspended by the vertebral clamp.
Use another vertebral clamp to clamp a spinous process of the sacral region to support the back region of the animal.
A third clamp is used to immobilize the right hindlimb and ankle, flexed at a 90° angle at the knee.
11. Achilles Tendon Dissection
With the limb bent at 90° at the knee and at the ankle, remove the gauze covering the hindlimb area, and dissect the Achilles tendon free of surrounding tissue. Dissect as much as possible the Triceps Surae from surrounding tissue as well.
Cut the tendon of the Plantaris muscle close to the calcaneus, then cut it again to remove the tendon completely.
Using a threaded needle, attach 6/0 silk thread through the Achilles tendon and make a triple knot around the tendon.
Place the force transducer close to the knot, cut the distal part of the tendon, and attach it to the force transducer using the silk thread.
Insert two lengths of stainless steel wire under the fascia of the Triceps Surae muscles. These wires are connected to an extracellular AC amplifier for EMG recording.
Place the Common Peroneal nerve and the Tibial nerve on two bipolar hook electrodes.
Place the Triceps Surae nerve on the cathode of a hook electrode, with the anode touching a nearby muscle.
Connect all stimulating electrodes to an isolation unit.
Place a ball electrode on the dorsal surface of the spinal cord, connected to an extracellular AC amplifier to record cord dorsum potentials. Place an Ag/AgCl reference electrode in contact with a back muscle.
12. Step Twelve
Stimulate the Triceps Surae nerve using a 50 μsec square pulse of increasing intensity at a low frequency (<1 Hz) until maximum twitch amplitude is observed. Slowly move the force transducer to stretch the muscle while monitoring the amplitude of the twitch response until the twitch amplitude reaches a maximum.
13. Intracellular Recordings of Motoneurons
From this point on, standard electrophysiological techniques are used to prepare an intracellular electrode, penetrate a neuron in the spinal cord and identify it as a motoneuron.
Pull a glass micropipette to a ~1 μm tip using a pipette puller (P-97 Micropipette Puller, Sutter Instruments). Fill the electrode with a KCl 3M solution (resistance of the electrode 10-20 MΩ).
Using a micropositioner, drive the micropipette, connected to an intracellular amplifier (Axoclamp 2B, Axon Instruments) into the spinal cord. Monitor the local field potentials elicited by the stimulation of each nerve to locate the motor pool of the Triceps Surae.
Carefully approach putative motoneurons while monitoring the resistance of the microelectrode. When pushing against a membrane, the resistance increases. Penetration can sometime be facilitated by using the "buzz" function of the intracellular amplifier.
14. Euthanasia Procedure
At the end of the experiment, the animal is euthanized by an overdose of pentobarbital (210 mg/kg IV), followed by decapitation.
Representative Results
Figure 1 shows how to identify a motoneuron from the Triceps Surae group after penetration. At low stimulation intensity, only a monosynaptic EPSP can be observed (Figure 1A). At higher intensity, the EPSP might be large enough to trigger an "orthodromic" spike (Figure 1B). At even higher stimulation intensity, an all-or-none antidromic spike appears, with a shorter latency than the monosynaptic EPSP (Figure 1C). If enough current is injected through the microelectrode to trigger a spike, an EMG activity can be recorded on the muscle after a short delay, followed by a muscle twitch (Figure 1D). After identification of the motoneuron, its electrophysiological properties can be characterized. For example, Figure 2 illustrate how the input resistance can be measured using hyperpolarizing and depolarizing pulses of current (Figure 2A), and plotting the variations of the membrane potential vs. the amount of injected current (Figure 2B).
The contractile properties of the motor unit can also be studied using various protocols of stimulation. For example, the force-frequency relationship can be obtained by injecting short pulses of current at various frequencies in the motoneuron (Figure 3A). The steady state force can then be plotted against the frequency of the current pulses to reveal a sigmoidal force-frequency curve (Figure 3B).
If the anesthesia is well under control, it is possible to record from a single motor unit for more than 15 min. In this lapse of time it should be possible to run a large array of protocols to characterize both the motoneuron and the contractile properties of the muscle fibers it innervates.
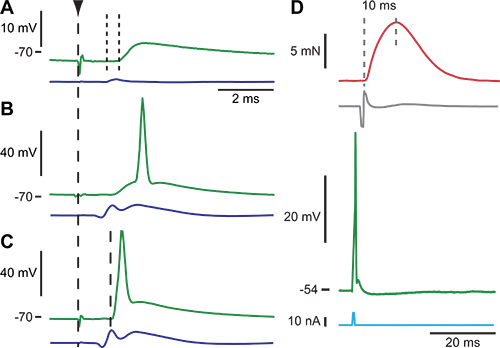 Figure 1. Example of the procedure to identify a motoneuron. Panels A to C three successive response of a motoneuron to an increasing electrical stimulation of the sciatic nerve. The vertical dashed line indicates the time of the stimulation. Each panel is the intracellularly recorded membrane potential (top trace) and the afferent volley recorded on the surface of the lumbar cord (bottom trace). A. At an intensity just above threshold for recruitment of group I afferents (1.1 x T), an EPSP appeared in response to the electrical stimulation. The EPSP was monosynaptic because its central latency, 0.4 msec (small vertical dotted lines), was too short for a pathway involving more than one synapse. B. Increasing the stimulation intensity (2.0 x T) increased the size of the EPSP until it was large enough to trigger an orthodromic spike. C. When the stimulation intensity was increased even more (2.1 x T), the axon was recruited, and an antidromic action potential appeared with a 1.2 msec latency with respect to the stimulation time. Given a conduction length of 38mm, the axonal conduction velocity was 32 m/sec. D. When current is injected (bottom trace) into a Triceps Surae motoneuron (different motoneuron than in A-C), an action potential can be elicited in the soma (second trace from the bottom). This action potential travels down the axon, crosses the neuro-muscular junction, and triggers muscle action potentials in the muscle fibers innervated by the recorded motoneuron. The compound action potential (second trace from the top) can be recorded using the EMG electrodes. The twitch contraction of the muscle fibers is shown in the upper trace. The contraction time of the motor unit can be estimated from the twitch response between the two vertical dashed lines. Figure adapted in part from ref5.
Figure 1. Example of the procedure to identify a motoneuron. Panels A to C three successive response of a motoneuron to an increasing electrical stimulation of the sciatic nerve. The vertical dashed line indicates the time of the stimulation. Each panel is the intracellularly recorded membrane potential (top trace) and the afferent volley recorded on the surface of the lumbar cord (bottom trace). A. At an intensity just above threshold for recruitment of group I afferents (1.1 x T), an EPSP appeared in response to the electrical stimulation. The EPSP was monosynaptic because its central latency, 0.4 msec (small vertical dotted lines), was too short for a pathway involving more than one synapse. B. Increasing the stimulation intensity (2.0 x T) increased the size of the EPSP until it was large enough to trigger an orthodromic spike. C. When the stimulation intensity was increased even more (2.1 x T), the axon was recruited, and an antidromic action potential appeared with a 1.2 msec latency with respect to the stimulation time. Given a conduction length of 38mm, the axonal conduction velocity was 32 m/sec. D. When current is injected (bottom trace) into a Triceps Surae motoneuron (different motoneuron than in A-C), an action potential can be elicited in the soma (second trace from the bottom). This action potential travels down the axon, crosses the neuro-muscular junction, and triggers muscle action potentials in the muscle fibers innervated by the recorded motoneuron. The compound action potential (second trace from the top) can be recorded using the EMG electrodes. The twitch contraction of the muscle fibers is shown in the upper trace. The contraction time of the motor unit can be estimated from the twitch response between the two vertical dashed lines. Figure adapted in part from ref5.
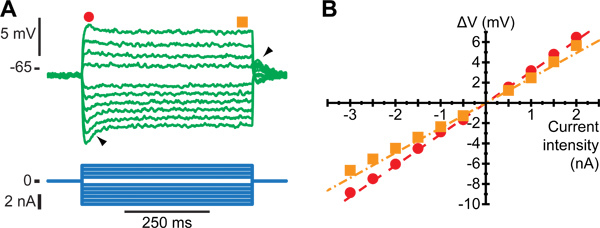 Figure 2. Estimation of the input resistance of a motoneuron. A. Average response to a series of current pulses (bottom traces) lasting 500 msec and ranging from -3 to +2nA. Notice the sag on the voltage response of the motoneuron (filled arrow): the voltage rapidly reached a peak (black dot) before stabilizing to a lower plateau value (black square). After the current pulse has been terminated, a rebound (empty arrow) appears. B. Plot of the deflection of the voltage (ΔV) vs. the intensity of the current pulse. Dots have been measured at the peak of the response, while squares have been measured at the end of the pulse, as depicted by the symbols on top of Figure B1. Straight lines are the best linear fits of the peak response (dashed) and the plateau response (dash-dotted). The slopes of these lines are the peak input resistance and the plateau input resistance of the motoneuron, respectively. Figure adapted from ref5.
Figure 2. Estimation of the input resistance of a motoneuron. A. Average response to a series of current pulses (bottom traces) lasting 500 msec and ranging from -3 to +2nA. Notice the sag on the voltage response of the motoneuron (filled arrow): the voltage rapidly reached a peak (black dot) before stabilizing to a lower plateau value (black square). After the current pulse has been terminated, a rebound (empty arrow) appears. B. Plot of the deflection of the voltage (ΔV) vs. the intensity of the current pulse. Dots have been measured at the peak of the response, while squares have been measured at the end of the pulse, as depicted by the symbols on top of Figure B1. Straight lines are the best linear fits of the peak response (dashed) and the plateau response (dash-dotted). The slopes of these lines are the peak input resistance and the plateau input resistance of the motoneuron, respectively. Figure adapted from ref5.
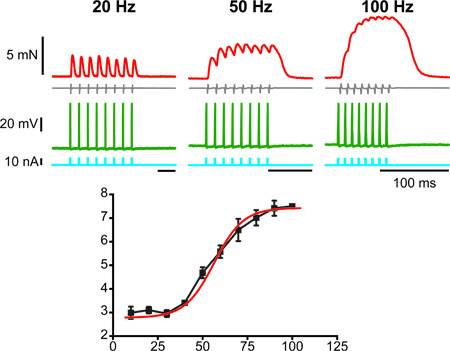 Figure 3. Characterization of the force-frequency relationship of a motor unit. The top three panels show: on the bottom trace, the pulses of current repeated at the frequency indicated on top of each panel and used to elicit action potentials in the motoneuron; on the second trace from the bottom, the membrane potential of the motoneuron, showing action potentials at the frequency of the pulses; on the second trace from the top, the EMG activity; on the top trace the force produced by the train of action potential. The bottom panel shows the summary graph of the amount of force reached during the individual trains plotted against the frequency of the action potentials in each train. The curve is sigmoidal.
Figure 3. Characterization of the force-frequency relationship of a motor unit. The top three panels show: on the bottom trace, the pulses of current repeated at the frequency indicated on top of each panel and used to elicit action potentials in the motoneuron; on the second trace from the bottom, the membrane potential of the motoneuron, showing action potentials at the frequency of the pulses; on the second trace from the top, the EMG activity; on the top trace the force produced by the train of action potential. The bottom panel shows the summary graph of the amount of force reached during the individual trains plotted against the frequency of the action potentials in each train. The curve is sigmoidal.
Discussion
The preparation described here is the first that allows, in the adult mouse, simultaneous intracellular recording of a lumbar motoneuron and the measurement of the force produced by the muscle fibers innervated by its axon.
Because of the small size of the animal, the surgical skills required for this preparation can be challenging to acquire. However, once those skills are mastered, the whole surgery can be performed in three hours, and the animals can survive for up to 7 more hours after the end of the surgical procedure for recordings. The success of this technique is essentially contingent on the management on the anesthesia. It is of critical importance to carefully monitor as many physiological parameters as possible (core temperature, pCO2, heart rate, etc.) and to maintain them is their respective physiological range. Any deviation must be remedied immediately for example by increasing/decreasing the power to the heating blanket, adjusting the parameters of the respirator, or adding more anesthetics. It is our experience that the physiological state of the mouse can take a surprisingly quick turn for the worse if close attention is not paid at all times to these parameters.
Mechanical stability is of paramount importance when trying to achieve intracellular recordings. This is especially true in vivo, where motoneurons can move under the influence of blood pressure, respiration, and muscle contractions. Even though it is impossible to guaranty perfect stability, our procedure allows stable recordings of motoneurons for ten minutes or more. This is achieved by a combination of four clamps immobilizing the vertebral column and the leg bones. Two clamps are located as close as possible from the laminectomy site to immobilize the spinal cord in that area. We found that reducing the length of spinal cord between the two clamps as well as exerting a little bit of tension on the bones provided very good stabilization, as we were able to maintain intracellular recordings for several hours in paralyzed animals 5. However, in the present case, the animal is not paralyzed, and the muscles are free to contract, especially when stimulating the sciatic nerve. This is the reason why we stabilize the whole leg skeleton using two clamps, one at the sacrum level, immobilizing the whole hip and therefore preventing muscle contractions from being transmitted to the vertebral column, and one at the ankle to immobilize the leg at a 90° angle.
Using this technique, it is possible to identify the physiological type of a motoneuron based on the force profile of its motor unit13. Motoneurons can be classified as Slow or Fast based on their twitch contraction time, and in Fatigable or Fatigue Resistant based on their ability to sustain a given force during repetitive stimulation. As such, this preparation provides a definite advantage over in vitro preparations. In in vitro conditions, the spinal cord is extracted from the body of the animal and placed in a dish either whole or sliced. Because of the layer of myelin surrounding the gray matter, proper oxygenation can only be obtained in neonate animals where the myelination is not complete14. Recent technical developments have allowed recordings of spinal motoneurons in adult slices15-17, however, this approach does not alleviate the major drawback of in vitro recordings, which is that there is no way to identify the physiological type of the recorded motoneuron (S, FR, or FF, or even alpha vs. gamma), and force the experimentalist to pool together recordings from motoneurons that are intrinsically different, in terms of function, electrophysiological properties, and protein content (see Manuel & Zytnicki, 201118 for a review of the different types of motoneurons).
Finally, it should be noted that the possibilities offered by this technique are many. Indeed, these in vivo recordings could be performed in genetically modified animals to study the direct impact of this specific alteration on the function of the motor system: produce force. This preparation is also very promising for the study of neurodegenerative human diseases like Amyotrophic Lateral Sclerosis (ALS) or Spinal Muscular Atrophy (SMA). Genetic models have indeed been generated that replicate the hallmark symptoms of these diseases10,11. The new preparation described here opens up the possibility of studying the role of the neuromuscular junctions in these diseases by testing the behavior of motoneurons and muscles fibers (both independently and together) during the progression of the disease. Recently, two independent groups were able to develop a decerebrated in vivo mouse preparation that is exhibiting spontaneous locomotion or fictive locomotion19,20. If the kind of recording stability that we observe using the procedure described here can be achieved after decerebration, this would constitute a formidable tool for the study not only of the motoneurons, but of all the pre-motor spinal circuits involved in generating the locomotor rhythm.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was made possible thanks to financial support from the Fondation pour la Recherche Médicale (FRM), the Milton Safenowitz PostDoctoral Fellowship for ALS Research (ALS Association), NIH NINDS Grants NS05462 and NS034382, and ANR Grant HyperMND.
References
- Liddel EGT, Sherrington CS. Recruitment and some other factors of reflex inhibition. Proc. R. Soc. London. 1925;B:488–518. [Google Scholar]
- Huizar P, Kuno M, Miyata Y. Electrophysiological properties of spinal motoneurones of normal and dystrophic mice. The Journal of physiology. 1975;248:231–246. doi: 10.1113/jphysiol.1975.sp010971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alstermark B, Ogawa J. In vivo recordings of bulbospinal excitation in adult mouse forelimb motoneurons. Journal of neurophysiology. 2004;92:1958–1962. doi: 10.1152/jn.00092.2004. [DOI] [PubMed] [Google Scholar]
- Meehan CF, Sukiasyan N, Zhang M, Nielsen JB, Hultborn H. Intrinsic properties of mouse lumbar motoneurons revealed by intracellular recording in vivo. Journal of neurophysiology. 2010;103:2599–2610. doi: 10.1152/jn.00668.2009. [DOI] [PubMed] [Google Scholar]
- Manuel M, et al. Fast kinetics, high-frequency oscillations, and subprimary firing range in adult mouse spinal motoneurons. J. Neurosci. 2009;29:11246–11256. doi: 10.1523/JNEUROSCI.3260-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias C, et al. Mixed mode oscillations in mouse spinal motoneurons arise from a low excitability state. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:5829–5840. doi: 10.1523/JNEUROSCI.6363-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone SA, Zhong G, Harris-Warrick R, Sharma K. In mice lacking V2a interneurons, gait depends on speed of locomotion. J. Neurosci. 2009;29:7098–7109. doi: 10.1523/JNEUROSCI.1206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpalar AE, et al. Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron. 2011;71:1071–1084. doi: 10.1016/j.neuron.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Rabe N, Gezelius H, Vallstedt A, Memic F, Kullander K. Netrin-1-dependent spinal interneuron subtypes are required for the formation of left-right alternating locomotor circuitry. J. Neurosci. 2009;29:15642–15649. doi: 10.1523/JNEUROSCI.5096-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Cifuentes-Diaz C, et al. Neurofilament accumulation at the motor endplate and lack of axonal sprouting in a spinal muscular atrophy mouse. Hum. Mol. Genet. 2002;11:1439–1447. doi: 10.1093/hmg/11.12.1439. [DOI] [PubMed] [Google Scholar]
- Simpson DP. Prolonged (12 hours) intravenous anesthesia in the rat. Laboratory animal science. 1997;47:519–523. [PubMed] [Google Scholar]
- Burke RE. Motor Unit Types - Functional Specializations in Motor Control. Trends Neurosci. 1980;3:255–258. [Google Scholar]
- Kerkut GA, Bagust J. The isolated mammalian spinal cord. Prog. Neurobiol. 1995;46:1–48. doi: 10.1016/0301-0082(94)00055-m. [DOI] [PubMed] [Google Scholar]
- Carp JS, et al. An in vitro protocol for recording from spinal motoneurons of adult rats. Journal of Neurophysiology. 2008;100:474–481. doi: 10.1152/jn.90422.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Brownstone RM. An In Vitro Spinal Cord Slice Preparation for Recording from Lumbar Motoneurons of the Adult Mouse. Journal of Neurophysiology. 2011. [DOI] [PubMed]
- Husch A, Cramer N, Harris-Warrick RM. Long duration perforated patch recordings from spinal interneurons of adult mice. Journal of Neurophysiology. 2011. [DOI] [PMC free article] [PubMed]
- Manuel M, Zytnicki D. Alpha, beta and gamma motoneurons: functional diversity in the motor system's final pathway. J. Integr. Neurosci. 2011;10:243–276. doi: 10.1142/S0219635211002786. [DOI] [PubMed] [Google Scholar]
- Nakanishi ST, Whelan PJ. A decerebrate adult mouse model for examining the sensorimotor control of locomotion. Journal of Neurophysiology. 2012;107:500–515. doi: 10.1152/jn.00699.2011. [DOI] [PubMed] [Google Scholar]
- Meehan CF, Grondahl L, Nielsen JB, Hultborn H. Fictive locomotion in the adult decerebrate and spinal mouse in vivo. The Journal of Physiology. 2012;590:289–300. doi: 10.1113/jphysiol.2011.214643. [DOI] [PMC free article] [PubMed] [Google Scholar]


