Abstract
Objective To investigate whether work related stress, measured and defined as job strain, is associated with the overall risk of cancer and the risk of colorectal, lung, breast, or prostate cancers.
Design Meta-analysis of pooled prospective individual participant data from 12 European cohort studies including 116 056 men and women aged 17-70 who were free from cancer at study baseline and were followed-up for a median of 12 years. Work stress was measured and defined as job strain, which was self reported at baseline. Incident cancers (all n=5765, colorectal cancer n=522, lung cancer n=374, breast cancer n=1010, prostate cancer n=865) were ascertained from cancer, hospital admission, and death registers. Data were analysed in each study with Cox regression and the study specific estimates pooled in meta-analyses. Models were adjusted for age, sex, socioeconomic position, body mass index (BMI), smoking, and alcohol intake
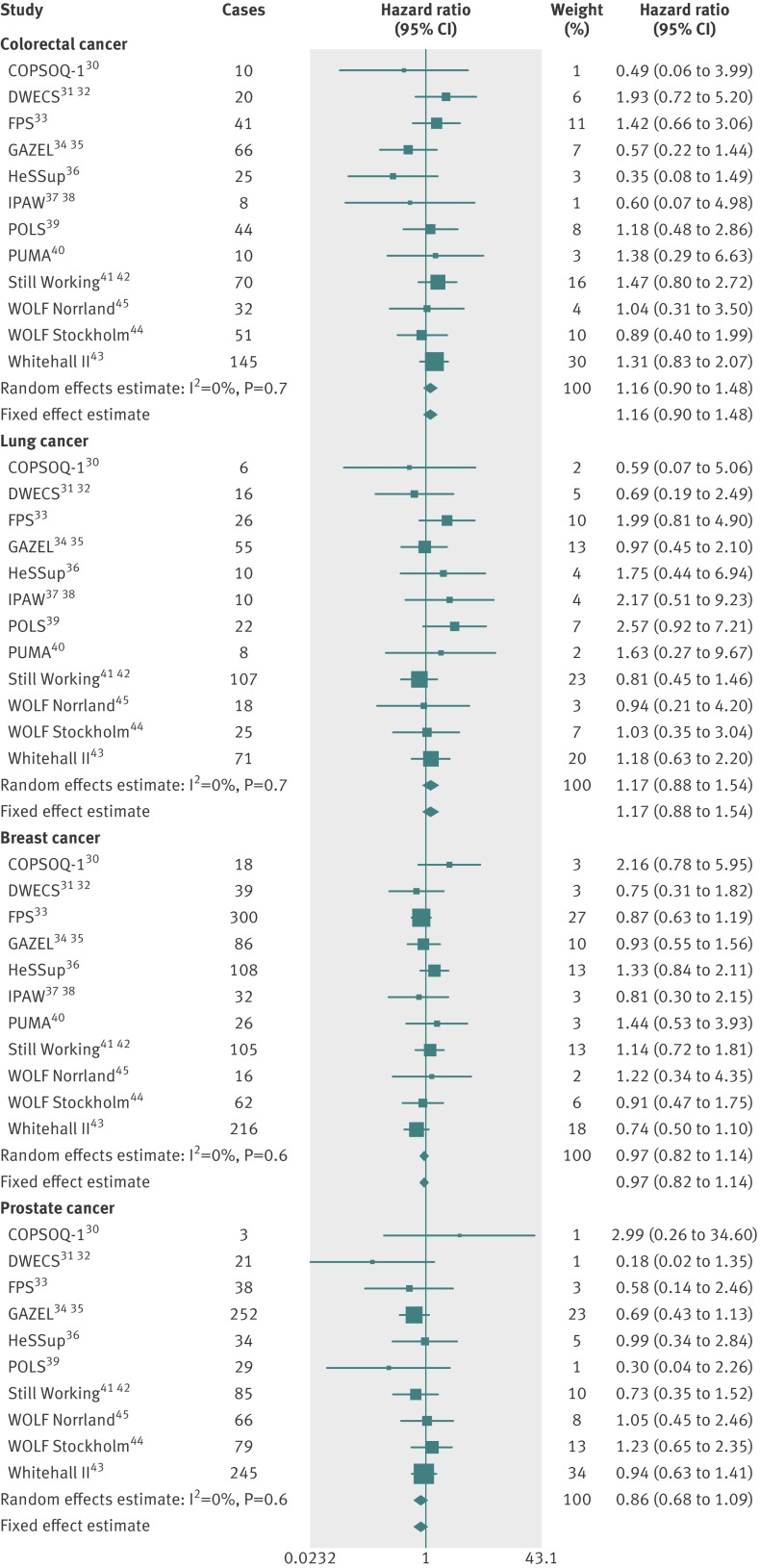
Results A harmonised measure of work stress, high job strain, was not associated with overall risk of cancer (hazard ratio 0.97, 95% confidence interval 0.90 to 1.04) in the multivariable adjusted analyses. Similarly, no association was observed between job strain and the risk of colorectal (1.16, 0.90 to 1.48), lung (1.17, 0.88 to 1.54), breast (0.97, 0.82 to 1.14), or prostate (0.86, 0.68 to 1.09) cancers. There was no clear evidence for an association between the categories of job strain and the risk of cancer.
Conclusions These findings suggest that work related stress, measured and defined as job strain, at baseline is unlikely to be an important risk factor for colorectal, lung, breast, or prostate cancers.
Introduction
Around 90% of cancers have been linked to environmental exposures.1 Many exposures related to environment and lifestyle, such as ultraviolet radiation, viral infections (such as Epstein-Barr virus or human papillomavirus), and tobacco smoke are now well recognised as carcinogens, but the evidence for many others, such as bitumen, surgical implants, or psychosocial factors, is tentative.2 3 One psychosocial factor that could plausibly have a role in the development of cancer is stress.4 Psychosocial stress is associated with the physiological stress response, which is characterised by increased secretion of hypothalamic and pituitary stress hormones.5 These stress biomarkers can trigger and maintain chronic inflammation,5 which has been shown to have various roles in cancer promotion and progression.1 Stressed individuals are also more likely than stress free individuals to smoke tobacco, consume excessive amounts of alcohol, and be obese,6 7 8 all behaviours that are risk factors for cancer9 10 11 12 13 and are associated with chronic inflammation.14
Many patients with cancer and healthcare professionals hold the view that stress has a role in the development of the disease, but these views might be from recall bias (for example, patients possibly over-reporting past exposures to stress), residual confounding in epidemiological studies, and sometimes even misunderstanding of cancer biology.15 16 The epidemiological evidence for an association between stress (irrespective of its cause) and risk of cancer has been inconclusive,4 14 17 18 19 20 21 22 and few studies have examined the associations between various measures of work related psychosocial stress and risk.4 14 17 23 24 Job strain (high demands and low control at work), which is the most widely used definition of psychosocial work stress,25 has been used in two prospective studies of work stress and cancer risk. High levels of strain were associated with a slight increase in the risk of breast cancer in one study26 but unrelated to breast cancer risk in another.27 Because of the different exposure measures used in the studies thus far and inconclusive findings in the studies that used common measures, however, the association between work related stress and risk of cancer remains unclear.
To examine whether work related psychosocial stress is associated with risk of cancer, we conducted individual participant data meta-analyses with harmonised data from 12 independent prospective European studies and over 116 000 participants.
Methods
Studies
We used data from 12 independent studies conducted between 1985 and 2008 in Finland, France, the Netherlands, Sweden, Denmark, and the United Kingdom. All studies were a part of the IPD-Work (individual-participant-data meta-analysis in working populations) consortium.28 29 The consortium used a predefined two stage data acquisition protocol: in the first stage, baseline data on work stress and sociodemographic and lifestyle factors were acquired and harmonised; in the second stage, these data were linked to register data on disease outcomes, including cancer.
Details of the designs and participants in the studies included in the present analyses have been published previously and are described, with references to previous publications, in appendix 1. The studies included in the present analyses were COPSOQ-I (Copenhagen Social Questionnaire I), Danish Work Environment Cohort Study (DWECS), Finnish Public Sector study (FPS), GAZEL, Health and Social Support (HeSSup), IPAW (Intervention Project on Absence and Well-being), Permanent Onderzoek Leefsituatie (POLS), PUMA (Danish acronym for Burnout, Motivation and Job Satisfaction study), Still Working, Whitehall II, and Work Lipids and Fibrinogen (WOLF) Norrland and Stockholm).30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45
Participants
A total of 180 967 men and women responded to the baseline questionnaire in the 12 studies. Individuals with missing data on job strain and those with missing data on covariates were excluded. Some studies included individuals who were not in employment at baseline or who were lost to registry follow-up, and these were also excluded (see appendix 3, fig S1). In all studies individuals with a diagnosis of cancer before the study baseline (n=2700) and those who with a diagnosis of cancer during the first year of follow-up (n=366) were excluded from the analyses to avoid reverse causality from symptoms of an undiagnosed cancer causing stress at work (see appendix 3, fig S1). We included in our analyses the 116 056 individuals with complete data on job strain, age, sex, socioeconomic position, body mass index (BMI), smoking, alcohol intake, and incident cancer outcomes.
Exposures to work stress
Psychosocial stress at work was measured and defined in all studies as job strain—a combination of high demands and low control at work. Job strain was ascertained with sets of questions from the validated Job Content Questionnaire (JCQ) and Demand-Control Questionnaire (DCQ),25 46 which were included in the baseline self report questionnaire in all studies. A detailed description of the job strain measure and its validation is provided elsewhere.28 Briefly participants were asked to rate psychosocial demands and control aspects of their job on a Likert-type scale. Mean response scores were calculated for the questions on job demands and job control for each participant. High demands were defined as having a job demands score higher than the study specific median score and low job control was defined as having a job control score lower than the study specific median score. Binary job strain was defined as job strain (high demands and low control) versus no strain (all other categories combined). Job strain categories, or quadrants based on the job strain model, were defined as follows: high strain job (high demands and low control), active job (high demands and high control), passive job (low demands and low control), and low strain job (low demands and high control).
Cancer outcomes
All studies, apart from GAZEL,34 35 ascertained information on incident cancer events during the follow-up from national cancer or death registries and hospital admissions registries. In the GAZEL study incident cancer events were ascertained from the employer’s medical register, which has been validated for accuracy and completeness,47 and by confirming any self reported cancer diagnoses with the participant’s physician. Individuals were classed as cancer cases according to the type and time of diagnosis of their first cancer, either a cancer registration or admission to hospital for cancer during their lifetime, or cancer on their death certificate. The date of the cancer was defined as the date of diagnosis, hospital admission for cancer, or the date of death, whichever came first. Incident cancer events were coded according to ICD-10 (international classification of diseases, 10th revision). Cancer outcomes were any cancer (codes C00-C97) and colorectal (C18-C20), lung (C34), female breast (C50), and prostate (C61) cancers.
Potential confounders
Rationale for our choice of potential confounders and for the categorisation of these are provided in appendix 2. Information on sex and age was obtained from population registries or interview (COPSOQ-I, DWECS, FPS, GAZEL, IPAW, PUMA, Still Working, WOLF Norrland, and WOLF Stockholm) or from questionnaires completed by participants (in HeSSup, POLS, and Whitehall II). Socioeconomic position was based on occupational title obtained from employers’ or other registers (in COPSOQ-I, DWECS, FPS, GAZEL, IPAW, PUMA, and Still Working) or questionnaires completed by participants (in HeSSup, POLS, Whitehall II, WOLF Norrland, and WOLF Stockholm). In HeSSup, socioeconomic position was based on the participant’s self reported highest educational qualification. The harmonised socioeconomic position was categorised into low, intermediate, and high.7 Participants who were self employed or who had missing data on job title were included in the analyses in the “other” category for socioeconomic position.
Tobacco smoking and alcohol intake were ascertained from questionnaires completed by participants in all studies. Smokers were categorised into never, former, and current smokers.6 Alcohol intake was ascertained from questions on the total number of alcoholic drinks the participants consumed in a week. One drink was defined as about equivalent to one unit or one glass of alcoholic drink or 10 g of ethanol. Participants were categorised according to their alcohol intake as non-drinkers, moderate drinkers (1-14 drinks/week for women; 1-21 drinks/week for men), intermediate drinkers (15-20 and 22-27, respectively), and heavy drinkers (≥21 and ≥28, respectively).8 Because of the way questions on alcohol intake were asked, we could not determine the number of alcoholic drinks per week in two studies. In COPSOQ-I alcohol intake was not measured and in Still Working, we were able to categorise alcohol intake only approximately as none, moderate, and heavy. Body mass index (BMI) was calculated with data on height and weight, which were self reported in seven studies (DWECS, FPS, GAZEL, HeSSup, IPAW, POLS, and PUMA) and measured in three studies (Whitehall II, WOLF Norrland, and WOLF Stockholm). BMI was not measured in two studies (COPSOQ-I and Still Working), and the multivariable adjusted analyses in these studies were not adjusted for it. BMI was categorised according to the WHO recommendations into <18.5 (underweight), 18.5-24.9 (normal weight), 25-29.9 (overweight), and ≥30 (obese).7 Participants with BMI <15 or >50 were excluded from the analysis (n=60).
Statistical analyses
Job strain was modelled both as a binary exposure (strain versus no strain) and as a categorical exposure (high strain, active job, and passive job versus low strain). Any incident cancer and incident colorectal, lung, female breast, and prostate cancers were modelled as binary outcomes. As some countries had previously had incomplete or inconsistent registration of non-melanoma skin cancers (C44), we also conducted sensitivity analyses excluding individuals with non-melanoma skin cancer as their only cancer. Studies in which no one with the exposure experienced a cancer outcome were excluded from the meta-analysis of the relevant outcome but included in all other meta-analyses.
We used a two stage approach to meta-analysis (modelling the associations in each study in turn and subsequently pooling the model results in meta-analyses) because we had access to individual level data from all studies apart from POLS, COPSOQ-I, DWECS, IPAW, and PUMA, in which the study teams conducted all analyses according to our instructions and provided us with aggregate data and results. All associations between job strain exposures and cancer outcomes were investigated with Cox proportional hazards regression, with the participant’s age as the timescale. Each participant was followed up from the date of their baseline assessment to the earliest of cancer event, death, or the end of the registry follow-up. We ran minimum adjusted models and multivariable adjusted models for each job strain exposure and cancer outcome pair. The minimum adjusted models were adjusted for age (as the timescale in the model) and sex. The multivariable adjusted models were further adjusted for socioeconomic position, BMI, smoking, and alcohol intake. Only women were included in the breast cancer models and only men in the prostate cancer models, and these models were not adjusted for sex. We tested for the proportional hazards assumption using Schoenfeld test and found the assumption to reasonably hold for all exposure-outcome pairs. We pooled the study specific effect estimates and their standard errors in fixed effect and random effects meta-analyses.48 Heterogeneity in the effect estimates was quantified with the I2 statistic, which indicates the proportion of the total variation in the estimates that is caused by variation between studies. All statistical analyses were conducted with Stata SE 11.2 (StataCorp, College Station, TX) apart from the study specific analyses in COPSOQ-I, DWECS, IPAW, and PUMA, which were conducted with SAS 9.2 (SAS Institute, Cary, NC) and in POLS, which were conducted with SPSS 17 (SPSS, Chicago, IL).
Results
Table 1 shows the numbers and proportions of incident cancer cases in all the studies included in our analyses, and table 2 gives the characteristics of the participants according to whether they developed cancer and by type of cancer. Participants’ characteristics by study are shown in appendix 3, table S1. We included in our analyses 116 056 men and women who were working and aged 17-70 at study baseline. Of these, 5765 (5%) developed some form of cancer during the average follow-up of 12 years (mean of the median follow-up times).
Table 1.
Participants, incident cancer cases, and duration of follow-up in study of exposure work strain and risk of cancer
| Study* (country) | Median† follow-up (years) | No of participants‡ | No (%) cancer free | No (%) of incident cancer cases | ||||
|---|---|---|---|---|---|---|---|---|
| Any cancer | Colorectal cancer | Lung cancer | Breast cancer | Prostate cancer | ||||
| COPSOQ-I (Denmark)30 | 12 (2-12) | 1699 | 1609 (94.7) | 90 (5.3) | 10 (0.6) | 6 (0.4) | 18 (1.1) | 3 (0.2) |
| DWECS (Denmark)31 32 | 10 (2-10) | 5313 | 5129 (96.5) | 184 (3.5) | 20 (0.4) | 16 (0.3) | 39 (0.7) | 21 (0.4) |
| FPS (Finland)33 | 5 (3-5) | 42 401 | 41 562 (98.0) | 839 (2.0) | 41 (0.1) | 26 (0.1) | 300 (0.7) | 38 (0.1) |
| GAZEL (France)34 35 | 11 (3-12) | 10 700 | 9888 (92.4) | 812 (7.6) | 66 (0.6) | 55 (0.5) | 86 (0.8) | 252 (2.4) |
| HeSSup (Finland)36 | 8 (4-8) | 14 689 | 14 304 (97.4) | 385 (2.6) | 25 (0.2) | 10 (0.1) | 108 (0.7) | 34 (0.2) |
| IPAW (Denmark)37 38 | 13 (2-14) | 1880 | 1769 (94.1) | 111 (5.9) | 8 (0.4) | 10 (0.5) | 32 (1.7) | 7 (0.4) |
| POLS (Netherlands)39 | 11 (3-13) | 8844 | 8574 (96.9) | 270 (3.1) | 44 (0.5) | 22 (0.2) | 2 (0.0) | 29 (0.3) |
| PUMA (Denmark)40 | 11 (3-11) | 1691 | 1609 (95.2) | 82 (4.8) | 10 (0.6) | 8 (0.5) | 26 (1.5) | 6 (0.4) |
| Still Working (Finland)41 42 | 23 (3-23) | 8998 | 8169 (90.8) | 829 (9.2) | 70 (0.8) | 107 (1.2) | 105 (1.2) | 85 (0.9) |
| Whitehall (UK)43 | 23 (4-24) | 10 074 | 8618 (85.5) | 1 456 (14.4) | 145 (1.4) | 71 (0.7) | 216 (2.2) | 245 (2.4) |
| WOLF Norrland (Sweden)45 | 12 (4-13) | 4468 | 4220 (94.4) | 248 (5.6) | 32 (0.7) | 18 (0.4) | 16 (0.4) | 66 (1.5) |
| WOLF Stockholm (Sweden)44 | 15 (2-16) | 5299 | 4840 (91.3) | 459 (8.7) | 51 (1.0) | 25 (0.5) | 62 (1.2) | 79 (1.5) |
| All | 12 (2-24) | 116 056 | 110 291 (95.0) | 5765 (5.0) | 522 (0.5) | 374 (0.3) | 1010 (0.9) | 865 (0.7) |
*See appendix 1 for study acronyms and details.
†1st-99th centile.
‡Participants with complete data on job strain, age, sex, socioeconomic position, BMI (not available in Still Working or COPSOQ-I), smoking, alcohol intake (not available in COPSOQ-I and an approximation in Still Working), and incident cancer events.
Table 2.
Characteristics of participants by cancer type in study of exposure work strain and risk of cancer. Figures are numbers (percentage) of participants unless stated otherwise
| Characteristics | Cancer free | Incident cancer | ||||
|---|---|---|---|---|---|---|
| Any cancer | Colorectal cancer | Lung cancer | Breast cancer | Prostate cancer | ||
| Participants* | 110 291 | 5 765 | 522 | 374 | 1010 | 865 |
| Job strain | 17 763 (16.1) | 843 (14.6) | 78 (14.9) | 64 (17.1) | 181 (17.9) | 79 (9.1) |
| Female | 59 695 (54.1) | 2 588 (44.9) | 188 (36.0) | 109 (29.1) | 1 010 (100.0) | 0 (0) |
| Mean (SD; range) age at baseline (years) | 38.5 (10.3; 17-70) | 48.9 (8.2; 19-67) | 49.9 (7.8; 26-64) | 51.6 (6.8; 29-65) | 49.1 (6.0; 21-68) | 53.2 (5.5; 28-67) |
| Low socioeconomic position at baseline | 34 644 (31.4) | 1778 (30.8) | 151 823 | 182 (48.7) | 297 (29.49 | 194 (22.49 |
| Mean (SD) BMI at baseline | 24.8 (3.9) | 25.0 (3.8) | 24.9 (28.9) | 24.3 (3.8) | 24.1 83.5) | 26.7 (3.9) |
| Normal weight in POLS† | 5294 (61.7) | 140 (51.7) | 21 (47.7) | 11(50.0) | — | 17 (58.6) |
| Smokers at baseline | 26 486 (24.0) | 1562 (27.1) | 122 (23.4) | 251 (67.1) | 224 (22.2) | 159 (18.4) |
| Drinkers at baselineठ| 11 089 (10.1) | 655 (11.4) | 71 (13.6) | 56 (15.0) | 73 (7.2) | 120 (13.9) |
*Participants with complete data on job strain, age, sex, socioeconomic position, BMI (not available in Still Working and COPSOQ-I), smoking, alcohol intake, and incident cancer events.
†BMI recorded as categorical in POLS.39
‡Drinkers here defined as participants who consume more than recommended amounts of alcohol: ≥15 units/week for women; ≥22 units/week for men.
§Approximated for participants in Still Working as we were unable to ascertain number of drinks per time period because of way alcohol intake was determined in baseline questionnaire.
Associations of job strain with cancer risk
Job strain (versus no strain) was not associated with the overall risk of cancer in age and sex adjusted analyses (hazard ratio for any cancer 0.95, 95% confidence interval 0.88 to 1.02) or multivariable adjusted analyses (0.97, 0.90 to 1.04). These associations did not markedly differ in the analyses including and excluding non-melanoma skin cancer (ICD-10 code C44) from the “any cancer” category (appendix 4, figs S2 and S3).
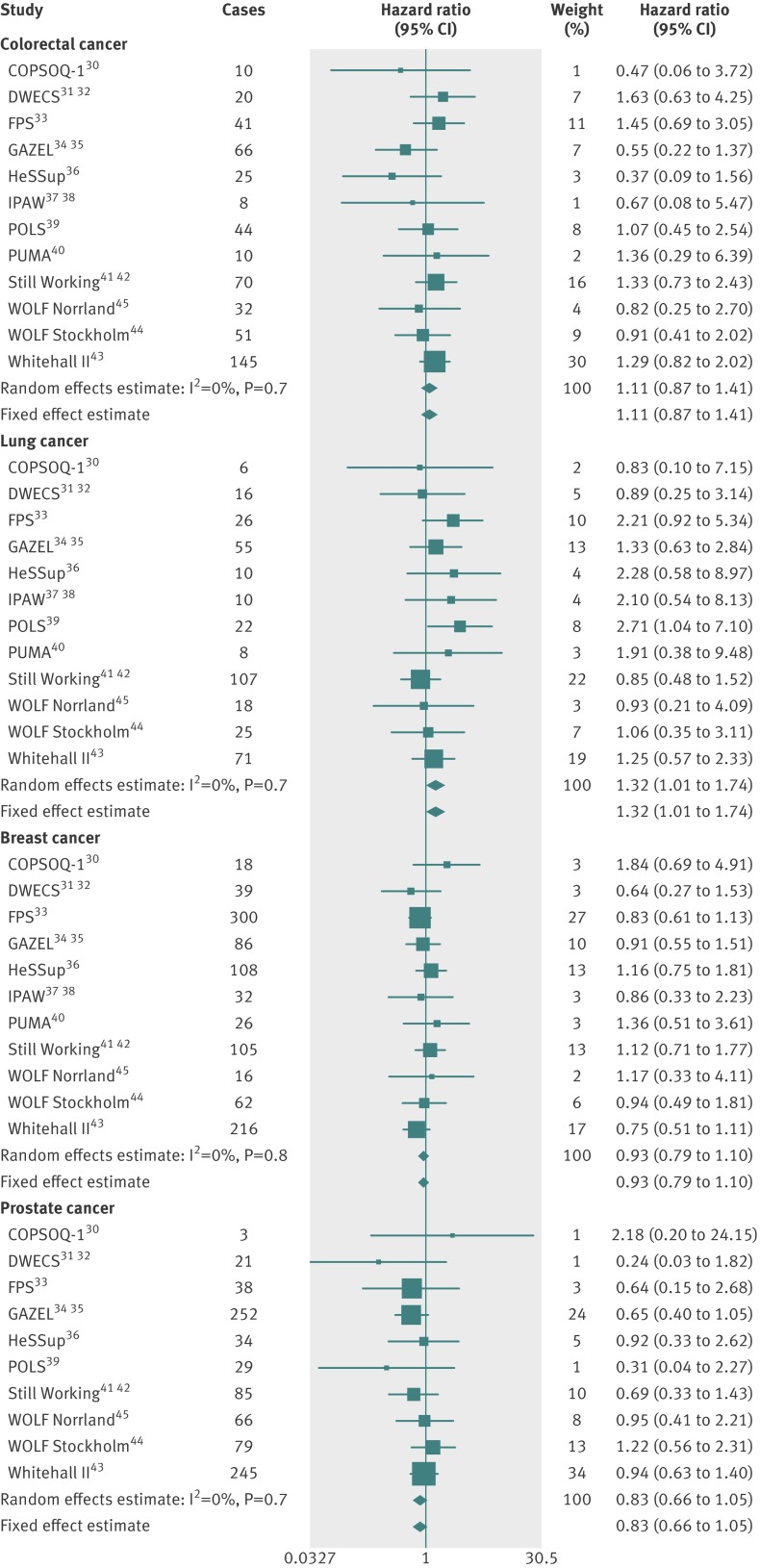
Figures 1 (age and sex adjusted analyses) and 2 (multivariable adjusted analyses) show the associations between job strain and the risk of the main cancer types . The point estimates from the meta-analyses suggested that job strain was associated with a slightly increased risk of colorectal and lung cancers and a slightly decreased risk of breast and prostate cancers, but the pooled 95% confidence intervals crossed the null value in all the multivariable adjusted analyses (fig 2). Thus, there was no clear evidence for an association between job strain and the risk of colorectal, lung, breast, or prostate cancers.
Fig 1 Age and sex adjusted associations between job strain and incident cancers
Fig 2 Multivariable adjusted associations between job strain and incident cancers
Associations between job strain model quadrants and cancer risk
The associations of the quadrants of the job strain model (high strain, active job, and passive job versus low strain) with the overall cancer risk are shown in appendix 4, figs S4A and S4B. Again, there was no evidence for an association between the quadrants (when compared with the low strain quadrant) and overall cancer risk in our meta-analysis. Exclusion of non-melanoma skin cancers (ICD-10 code C44) did not alter these findings (appendix 4, figs S5A and S5B). However, there was heterogeneity in the effect estimates for all job strain quadrants, and the study specific effect estimates varied in size and direction.
The associations between all the job strain quadrants and incident colorectal, lung, breast, and prostate cancers are shown in appendix 4, figs S6A-S9B. There was no consistent evidence for an association of any of the quadrants with the risk of these cancer types.
Discussion
Principal findings
We found no evidence for an association between job strain and the overall risk of cancer or the risk of colorectal, lung, breast, or prostate cancers in a meta-analysis of over 5700 incident cancer cases among over 116 000 men and women from six European countries. The study specific associations between job strain and cancer risk in our analyses varied in direction and magnitude, a result that was similar to previous studies, which have provided inconsistent association estimates.23 26 27 When we combined the study specific estimates in meta-analyses, there was no evidence for an association between job strain and cancer risk. This suggests that many of the previously reported associations (of varying directions and magnitudes) between work related stress and risk of cancer could have been influenced by chance, low power in some studies, different covariate adjustment, or residual confounding from possible unmeasured common causes of work stress and cancer.4 14 17 23 24 Such common causes could be include shift work (for which there is some evidence of an association with risk of breast cancer49) or other sources of stress, perhaps combined with one another.
Strengths and weaknesses
An important strength of our meta-analysis was that we used a large set of harmonised individual level data from 12 independent European studies. As far as we are aware, this is the largest study of the association between job strain and cancer risk and the only one conducted so far with individual participant data. A major advantage of individual participant meta-analysis of published as well as unpublished data, such as ours, is that it minimises publication bias, which is a concern in literature based meta-analyses.50 All studies in our analyses were prospective cohort studies, and the incident cancer outcomes were ascertained from national cancer, death, or hospital admission registers in all studies but the French study, GAZEL.34 35 As France has no national cancer register, incident cancer cases in GAZEL were ascertained from the employer’s medical register, which has been validated for accuracy and completeness,47 as well as from physician records of the individuals who self reported cancer and the national death register. The national registers are likely to capture at least 90-95% of incident cancer cases, and, importantly, such register data are generally not prone to recall or other biases.47 51 52 53 54
Most studies included in our analyses had average follow-up times exceeding 10 years, including two studies with median follow-up times of over 20 years. Sufficiently long follow-up times are important because most cancers have a latent period of years or even decades. If a true association between job strain and incident cancer existed because the physiological stress response has a role in cancer promotion or progression (for example, via the regulation of the inflammatory pathways), the follow-up periods in our analyses should have been long enough to detect such an association. However, it is not known how long the exposure to job strain needs to be to induce inflammatory or other physiological changes.
Exposure to work related stress was measured on the basis of a single baseline measure of job strain (high demands and low control at work). The length of the job strain exposure within the exposed group varied, however, which could have introduced regression dilution bias in our study specific models and heterogeneity in some of our meta-analyses. It remains unknown whether long term exposure to job strain or other indicators of work related stress, such as effort-reward imbalance at work or job insecurity, might contribute to cancer risk. In the present pooled dataset, however, we found an association between job strain and an increased risk of coronary heart disease, which suggests that a single validated measure of job strain exposure is capable of showing an association with chronic disease.29
The number of cases of lung cancer in our analyses was slightly smaller than would be expected based on the latest cancer incidence statistics. According to the International Association for Research on Cancer, the incidence of lung cancer in the European Union was 43.8 per 100 000. The corresponding statistics were 47.5 for colorectal cancer, 103.7 for breast cancer, and 105.6 for prostate cancer.55 The disparity in the lung cancer incidence probably relates to the socioeconomic patterning of this cancer. Our analyses were based on men and women who were working at baseline, and though some could have been made redundant during the follow-up, our data probably include fewer unemployed people than the general population. Unemployed people have a larger risk of many cancers, particularly lung cancer, than working people.56 This could be at least partly because smoking is more common among unemployed people than among those who work.57
We cannot exclude the possibility that residual confounding, such as from low intake of dietary fibre, shift or night time work, or exposure to pesticides, noxious fumes, dusts, or solvents, has influenced our estimates, though it is unlikely that residual confounding would have masked a strong association between job strain and cancer.
Interpretation of the findings
Our findings show that job strain is unlikely to be an important risk factor for cancer overall or for colorectal, lung, breast, and prostate cancers. This does not preclude other types of psychosocial stress (such as stress from adverse life events) or physiological stress from being linked to cancer risk.4 17 58 For example, in a meta-analysis of different types of stress and risk of breast cancer, stress from adverse life events was consistently associated with an increased risk, whereas the study specific findings on work related, care giving, and everyday stress varied considerably.4 In a French study, people with brain cancer were more likely to report adverse life events than controls without cancer, but there was no clear evidence for a difference in terms of stress at work between these groups.17 We did not investigate the possible influence of non-work related stress or its co-occurrence with work stress in our meta-analyses, but future studies would do well to examine whether these have an effect on risk. It is also possible that work related psychosocial stress could be related to the risk of some other cancers.
Conclusions
Our meta-analyses provided no evidence for an association between job strain and overall cancer risk or the risk of colorectal, lung, breast, or prostate cancers. These findings suggest that work related psychosocial stress is unlikely to be an important risk factor for these cancers. Thus, though reducing work stress would undoubtedly improve the psychological and physical wellbeing of the working individuals as well as the working population, it is unlikely to have an important impact on cancer burden at a population level.
What is already known of this topic
Work related stress is associated with many adverse health outcomes, such as coronary heart disease and depression
Work stress has been suggested to increase the risk of cancer, though studies of this association have had inconsistent (positive, negative, and null) findings, depending on the characteristics of the study and participants and type of cancer
What this study adds
Job strain, a widely used measure of work related stress, is not associated with the overall risk of cancer or the risk of the most common cancer types: colorectal, lung, breast, or prostate
Based on a large study of European men and women in many different work place settings, our findings suggest that stress at work, measured and defined as job strain, is unlikely to be an important risk factor for cancer
Contributors: All authors participated in designing the study, generating hypotheses, interpreting the data, and writing and critically reviewing the paper. KH analysed the data and, with help from MK, wrote the first draft. KH, STN, and MK had full access to anonymised data from all studies, with the exception of data from POLS, COPSOQ-I, DWECS, IPAW, and PUMA. WEH had full access to anonymised data from POLS and IEHM had full access to the anonymised data from COPSOQ-I, DWECS, IPAW, and PUMA. KH is guarantor.
Funding: The IPD-Work Consortium is supported by the EU New OSH ERA research programme (funded by the Finnish Work Environment Fund, Finland, the Swedish Research Council for Working Life and Social Research, Sweden, the Danish National Research Centre for the Working Environment, Denmark), the Academy of Finland (grant 132944), the BUPA Foundation (grant 22094477), and the Economic and Social Research Council, UK. POLS is funded by the Ministry of Social Affairs and Employment, Netherlands. MK is supported by the Medical Research Council and a professorial fellowship from the Economic and Social Research Council, UK. AS is a British Heart Foundation professor. At the time of preparation of the manuscript, GDB was a Wellcome Trust Fellow. The funding bodies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Each constituent study in the IPD-Work consortium was approved by the relevant local or national ethics committees and all participants gave informed consent to take part. Details of the ethical approval are provided in appendix 1.
Data sharing: No additional data available.
Cite this as: BMJ 2013;346:f165
Web Extra. Extra material supplied by the author
Appendix 1: Full details of studies and participants
Appendix 2: Supplementary information on potential confounders
Appendix 3: Flow charts and participants’ characteristics by study
Appendix 4: Meta-analyses
References
- 1.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberg RA. The biology of cancer. Garland Science, Taylor and Francis Group, LLC, 2007.
- 3.Agents classified by the IARC monographs, volumes 1-105. IARC, 2012.
- 4.Antonova L, Aronson K, Mueller CR. Stress and breast cancer: from epidemiology to molecular biology. Breast Cancer Res 2011;13:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansel A, Hong S, Camara RJ, von Kanel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci Biobehav Rev 2010;35:115-21. [DOI] [PubMed] [Google Scholar]
- 6.Heikkilä K, Nyberg ST, Fransson EI, Alfredsson L, De Bacquer D, Bjorner JB, et al. Job strain and tobacco smoking: an individual-participant data meta-analysis of 166 130 adults in 15 European studies. PLoS ONE 2012;7:e35463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyberg ST, Heikkila K, Fransson EI, Alfredsson L, De Bacquer D, Bjorner JB, et al. Job strain in relation to body mass index: pooled analysis of 160 000 adults from 13 cohort studies. J Intern Med 2011;272:65-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heikkila K, Nyberg ST, Fransson EI, Alfredsson L, De Bacquer D, et al. Job strain and alcohol intake: a collaborative meta-analysis of individual-participant data from 140 000 men and women. PLoS ONE 2012;7:e40101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765-78. [DOI] [PubMed] [Google Scholar]
- 10.Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW Jr, et al. Alcohol, tobacco and breast cancer—collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer 2002;87:1234-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. Alcohol consumption and the risk of cancer: a meta-analysis. Alcohol Res Health 2001;25:263-70. [PMC free article] [PubMed] [Google Scholar]
- 12.Fedirko V, Tramacere I, Bagnardi V, Rota M, Scotti L, Islami F, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol 2011;22:1958-72. [DOI] [PubMed] [Google Scholar]
- 13.Huncharek M, Haddock KS, Reid R, Kupelnick B. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health 2010;100:693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol 2008;5:466-75. [DOI] [PubMed] [Google Scholar]
- 15.Alleyne R. Stress could cause cancer claim scientists. The Telegraph 2010. January 14.
- 16.Wu M, Pastor-Pareja JC, Xu T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature 2010;463:545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabaniols C, Giorgi R, Chinot O, Ferahta N, Spinelli V, Alla P, et al. Links between private habits, psychological stress and brain cancer: a case-control pilot study in France. J Neurooncol 2011;103:307-16. [DOI] [PubMed] [Google Scholar]
- 18.Nakaya N, Bidstrup PE, Saito-Nakaya K, Frederiksen K, Koskenvuo M, Pukkala E, et al. Personality traits and cancer risk and survival based on Finnish and Swedish registry data. Am J Epidemiol 2010;172:377-85. [DOI] [PubMed] [Google Scholar]
- 19.Santos MC, Horta BL, Amaral JJ, Fernandes PF, Galvao CM, Fernandes AF. Association between stress and breast cancer in women: a meta-analysis. Cad Saude Publica 2009;25(suppl 3):S453-63. [DOI] [PubMed] [Google Scholar]
- 20.Kojima M, Wakai K, Tokudome S, Tamakoshi K, Toyoshima H, Watanabe Y, et al. Perceived psychologic stress and colorectal cancer mortality: findings from the Japan Collaborative Cohort Study. Psychosom Med 2005;67:72-7. [DOI] [PubMed] [Google Scholar]
- 21.Kruk J, Aboul-Enein HY. Psychological stress and the risk of breast cancer: a case-control study. Cancer Detect Prev 2004;28:399-408. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen NR, Zhang ZF, Kristensen TS, Netterstrom B, Schnohr P, Gronbaek M. Self reported stress and risk of breast cancer: prospective cohort study. BMJ 2005;331:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansson C, Jeding K, Lagergren J. Job strain and risk of esophageal and cardia cancers. Cancer Epidemiol 2009;33:473-5. [DOI] [PubMed] [Google Scholar]
- 24.Spiegelman D, Wegman DH. Occupation-related risks for colorectal cancer. J Natl Cancer Inst 1985;75:813-21. [DOI] [PubMed] [Google Scholar]
- 25.Karasek R, Brisson C, Kawakami N, Houtman I, Bongers P, Amick B. The Job Content Questionnaire (JCQ): an instrument for internationally comparative assessments of psychosocial job characteristics. J Occup Health Psychol 1998;3:322-55. [DOI] [PubMed] [Google Scholar]
- 26.Kuper H, Yang L, Theorell T, Weiderpass E. Job strain and risk of breast cancer. Epidemiology 2007;18:764-8. [DOI] [PubMed] [Google Scholar]
- 27.Schernhammer ES, Hankinson SE, Rosner B, Kroenke CH, Willett WC, Colditz GA, et al. Job stress and breast cancer risk: the nurses’ health study. Am J Epidemiol 2004;160:1079-86. [DOI] [PubMed] [Google Scholar]
- 28.Fransson EI, Nyberg ST, Heikkilä K, Alfredsson L, De Bacquer D, Batty GD, et al. Agreement between alternative versions of the job demand-control questionnaire in 70,800 participants in six European cohort studies. BMC Public Health 2012;12(62) 10.1186/1471-2458-12-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kivimaki M, Nyberg ST, Batty GD, Fransson E, Heikkila K, Alfredsson L, et al. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet 2012;380:1491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kristensen TS, Hannerz H, Hogh A, Borg V. The Copenhagen Psychosocial Questionnaire—a tool for the assessment and improvement of the psychosocial work environment. Scand J Work Environ Health 2005;31:438-49. [DOI] [PubMed] [Google Scholar]
- 31.Burr H, Bjorner JB, Kristensen TS, Tüchsen F, Bach E. Trends in the Danish work environment in 1990-2000 and their associations with labor-force changes. Scand J Work Environ Health 2003;29:270-9. [DOI] [PubMed] [Google Scholar]
- 32.Feveile H, Olsen O, Burr H, Bach E. Danish Work Environment Cohort Study 2005: from idea to sampling design. Stat Trans 2007;8:441-58. [Google Scholar]
- 33.Kivimaki M, Lawlor DA, Smith GD, Kouvonen A, Virtanen M, Elovainio M, et al. Socioeconomic position, co-occurrence of behavior-related risk factors, and coronary heart disease: the Finnish Public Sector Study. Am J Public Health 2007;97:874-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldberg M, Leclerc A, Bonenfant S, Chastang JF, Schmaus A, Kaniewski N, et al. Cohort profile: the GAZEL Cohort Study. Int J Epidemiol 2007;36:32-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zins M, Leclerc A, Goldberg M. The French GAZEL cohort study: 20 years of epidemiological research. Adv Life Course Res 2009;14:135-46. [Google Scholar]
- 36.Korkeila K, Suominen S, Ahvenainen J, Ojanlatva A, Rautava P, Helenius H, et al. Non-response and related factors in a nation-wide health survey. Eur J Epidemiol 2001;17:991-9. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen M, Kristensen T, Smith-Hansen L. The intervention project on absence and well-being (IPAW): design and results from the baseline of a 5-year study. Work Stress 2002;16:191-206. [Google Scholar]
- 38.Nielsen ML, Rugulies R, Christensen KB, Smith-Hansen L, Bjorner JB, Kristensen T. Impact of the psychosocial work environment on registered absence from work: a two-year longitudinal study using the IPAW cohort. Work Stress 2004;18:323-35. [Google Scholar]
- 39.De Groot W, Dekker R. The Dutch System of Official Social Surveys. EuReporting Working Paper. Mannheim Centre for European Social Research, 2001.
- 40.Borritz M, Rugulies R, Bjorner JB, Villadsen E, Mikkelsen OA, Kristensen TS. Burnout among employees in human service work: design and baseline findings of the PUMA study. Scand J Public Health 2006;34:49-58. [DOI] [PubMed] [Google Scholar]
- 41.Kalimo R, Toppinen S. Organizational well-being: ten years of research and development: in a forest industry corporation. In: Kompier M, Cooper C, eds. Preventing stress, improving productivity: European case studies in the workplace. Routledge, 1999:52-85.
- 42.Vaananen A, Murray M, Koskinen A, Vahtera J, Kouvonen A, Kivimaki M. Engagement in cultural activities and cause-specific mortality: prospective cohort study. Prev Med 2009;49:142-7. [DOI] [PubMed] [Google Scholar]
- 43.Marmot MG, Smith GD, Stansfeld S, Patel C, North F, Head J, et al. Health inequalities among British civil servants: the Whitehall II study. Lancet 1991;337:1387-93. [DOI] [PubMed] [Google Scholar]
- 44.Peter R, Alfredsson L, Hammar N, Siegrist J, Theorell T, Westerholm P. High effort, low reward, and cardiovascular risk factors in employed Swedish men and women: baseline results from the WOLF Study. J Epidemiol Community Health 1998;52:540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alfredsson L, Hammar N, Fransson E, de Faire U, Hallqvist J, Knutsson A, et al. Job strain and major risk factors for coronary heart disease among employed males and females in a Swedish study on work, lipids and fibrinogen. Scand J Work Environ Health 2002;28:238-48. [DOI] [PubMed] [Google Scholar]
- 46.Karasek R, Theorell T. Healthy work: stress, productivity, and the reconstruction of working life. Basic Books, 1990.
- 47.Chevalier A, Goldberg M, Gonard C, Guenel P, Callet B, Antonini B, et al. Cancer incidence among active male workers at Electricite de France-Gaz de France. Rev Epidemiol Sante Publique 1996;44:25-36. [PubMed] [Google Scholar]
- 48.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang XS, Armstrong ME, Cairns BJ, Key TJ, Travis RC. Shift work and chronic disease: the epidemiological evidence. Occup Med 2011;61:78-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet 1993;341:418-22. [DOI] [PubMed] [Google Scholar]
- 51.Summary Quality Report for Cancer Registration Statistics. Information Paper. Office for National Statistics, 2011.
- 52.Barlow L, Westergren K, Holmberg L, Talback M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol 2009;48:27-33. [DOI] [PubMed] [Google Scholar]
- 53.Coleman MP, Demaret E. Cancer registration in the European community. Int J Cancer 1988;42:339-45. [DOI] [PubMed] [Google Scholar]
- 54.Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol 1994;33:365-9. [DOI] [PubMed] [Google Scholar]
- 55.WHO. EUCAN cancer factsheets. IARC, 2012.
- 56.Lynge E, Andersen O. Unemployment and cancer in Denmark, 1970-1975 and 1986-1990. IARC Sci Pub 1997;138:353-9. [PubMed] [Google Scholar]
- 57.Henkel D. Unemployment and substance use: a review of the literature (1990-2010). Curr Drug Abuse Rev 2011;4:4-27. [DOI] [PubMed] [Google Scholar]
- 58.Russ TC, Stamatakis E, Hamer M, Starr JM, Kivimäki M, Batty GD. Association between psychological distress and mortality: individual participant pooled analysis of 10 prospective cohort studies. BMJ 2012:e4933. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Full details of studies and participants
Appendix 2: Supplementary information on potential confounders
Appendix 3: Flow charts and participants’ characteristics by study
Appendix 4: Meta-analyses