Abstract
Serrated polyposis (SP) is a clinically defined syndrome characterized by the occurrence of multiple serrated polyps in the large intestine. Individuals with SP and their relatives are at increased risk of colorectal carcinoma (CRC). We aimed to determine the pathologic and molecular profiles of CRCs in individuals fulfilling World Health Organization criteria for SP. A total of 45 CRCs were obtained from 38 individuals with SP (27 females, 11 males; median age at CRC diagnosis 58.5 years) attending genetics clinics. Tumor samples were pathologically reviewed, screened for somatic BRAF and KRAS mutations, and underwent immunohistochemistry analysis for mismatch repair protein (MMR) expression. Tumors were spread throughout the large intestine, with 64% located in the proximal colon. Mutations in BRAF, KRAS and immunohistochemical evidence of MMR deficiency were found in 46%, 5%, and 38%, respectively. Nearly half of CRCs were BRAF/KRAS wildtype, and these were associated with distal location (63%) and MMR proficiency (84%). Overexpression of p53 and/or evidence of β-catenin activation were identified in 13 CRCs. Ten patients (26%) had synchronous or metachronous CRCs. In conclusion, the majority of CRCs arising in individuals with SP do not harbor molecular hallmarks of serrated pathway CRCs but show a diverse range of molecular profiles. The high proportion of multiple CRCs suggests that individuals with SP would benefit from frequent colonoscopic surveillance and from a consideration of a more extensive colectomy at the time of CRC diagnosis.
Keywords: Serrated polyposis, Molecular pathway, Colorectal cancer
INTRODUCTION
Predisposing factors to colorectal carcinoma (CRC), the third most commonly diagnosed cancer worldwide, include increasing age, family history and environmental risk factors (1, 2). A familial component has been reported in 15–30% of patients with CRC, depending on age of diagnosis (3, 4). For example, individuals with first-degree relatives affected by CRC are 2–3 times as likely to be diagnosed with CRC compared to individuals without CRC-affected first degree relatives (1). Only 2–5% of CRCs are currently explained by known genetic syndromes: Lynch syndrome caused by mutation in DNA mismatch repair genes, familial adenomatous polyposis (FAP) caused by mutation in APC, familial adenomatous polyposis type 2 caused by mutations in MUTYH, various hamartomatous polyposis syndromes caused by mutations in STK11, SMAD4, PTEN or GREM1 (5, 6). The balance of unexplained CRCs with a familial component may be the result of a combination of unknown minor genetic defects, mutations in yet to be identified susceptibility genes, and environmental risk factors shared by relatives.
Serrated polyposis (SP) is a CRC predisposition syndrome (7–12) with an established familial risk for CRC but without a known genetic basis (7, 13). The hallmark of SP is the presence of multiple serrated polyps (hyperplastic polyps and sessile serrated adenomas/polyps) in the large intestine. It was previously suggested that the polyp burden and the co-occurrence of conventional adenomas increased the risk of CRC in patients with SP (8, 9, 14).
CRCs develop through different molecular pathways that involve sequences of genomic and epigenomic alterations associated with pathologic and clinical features: the serrated neoplasia pathway and the traditional adenoma pathway. Mutation in BRAF, along with CpG island methylator phenotype (CIMP), is the molecular marker of serrated neoplasia pathway CRC, in which serrated polyps are thought to be the precursor lesions (15, 16). Alternatively, WNT/CTNNB1 alterations, KRAS and TP53 mutations are common genetic events in traditional adenoma pathway CRC. Another major molecular classifier of CRC is microsatellite instability (MSI), present in most but not all serrated neoplasia pathway CRCs and, less frequently, in Lynch syndrome-associated CRC. Similar to FAP representing a model of CRC evolving through the traditional adenoma pathway, SP is a reasonably suggested model for CRC evolving through the serrated neoplasia pathway. However, despite the presence of multiple serrated polyps, small numbers of conventional adenomas are often also documented in SP individuals. Although they are not the predominant polyp type, the presence of conventional adenomas in SP is associated with an increased risk of CRC in these individuals (9, 14). Hence, different to FAP, SP may not be a model for CRC evolving purely via the serrated neoplasia pathway. In this study, we sought to characterize the pathologic and molecular features of CRC in a large cohort of well-documented individuals with SP in order to identify their lesion of origin for CRC in SP, to improve our understanding of the disorder and facilitate the development of prevention and surveillance strategies.
MATERIAL AND METHODS
Patient selection
A cohort of individuals with at least 5 serrated polyps proximal to the rectosigmoid region was selected from patients recruited to a registry between 2000 and 2011 in Australia, New Zealand, Canada and the USA. These individuals were referred by gastroenterologists to tertiary center genetics clinics with a suspected polyposis syndrome and/or family history of CRC. Written informed consent from all individuals was obtained to take part in research and the study was approved by the Human Research Ethics Committee of Queensland Institute of Medical Research under the Genetics of Serrated Neoplasia Project (QIMR HREC P912). From this registry, we sought for all CRC cases. Clinical data were collected from medical charts, colonoscopy reports and pathology reports. Standard white light colonoscopy was used in all patients. The cumulative number and distribution of polyps were recorded from colonoscopy and pathology reports on polyp biopsies and surgical resection specimens. To be selected for the study, patients had to meet either criterion (1) or (3) of the 2010 World Health Organization (WHO) criteria for SP (17): (1) at least five serrated polyps proximal to the sigmoid colon with two or more of these being >10 mm; or (3) >20 serrated polyps of any size but distributed throughout the colon. No patient was selected purely on the basis of criterion (2) (any number of serrated polyps proximal to the sigmoid colon in an individual who had a first-degree relative with SP). Only CRC cases with available tissue for pathology review were included in the series. For statistical analysis, location in the colon was designated as proximal for tumours located in the caecum, ascending colon and transverse colon and as distal in the descending colon, sigmoid colon and rectum.
Tissue specimens
Formalin-fixed paraffin-embedded (FFPE) tissue blocks were retrieved for all selected CRC specimens. A pathology review from H&E-stained tissue sections was performed by 3 specialist gastrointestinal pathologists (Jeremy Jass, NIW and CR). The CRCs were assessed histologically for the following features: histologic type (including serrated adenocarcinoma subtype), tumor grade, presence of tumor infiltrating lymphocytes, and residual polyp adjacent to the carcinoma. Pathologic staging was performed according the American Join Committee on Cancer (AJCC) system, 7th edition, 2010. Polyps were classified into conventional adenomas or different categories of serrated polyps according to the WHO criteria (17).
Molecular Analysis
Genomic DNA was extracted from FFPE tissue using QIAamp DNA Micro Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. V600E BRAF mutation analysis was performed by a fluorescent allele specific PCR assay to detect the somatic T>A mutation at nucleotide 1799 in exon 15, as previously described (9). Somatic mutations in codons 12 and 13 of KRAS were screened using real-time PCR with high resolution melting analysis in the presence of the SYTO9 fluorescent intercalating dye followed by direct Sanger sequencing on cases with differential melting profiles as previously described (14).
Immunohistochemistry
CRC specimens were evaluated for MMR protein expression (MLH1, MSH2, MSH6 and PMS2) by immunohistochemistry (IHC) as described previously (18). Patients with CRC that demonstrated loss of expression of MSH2 and/or MSH6 or MLH1/PMS2 with wildtype BRAF underwent germline mutation testing (Sanger sequencing and MLPA) of the MMR gene indicated by the pattern of loss from IHC (19).
When available, additional tissue sections were used to assess for immunohistochemical expression of p53 (clone DO7, Cell Marque, Rocklin, CA, USA; dilution 1:100), and β-catenin (clone 14, Cell Marque, Rocklin, CA, USA; dilution 1:600). Heat induced epitope retrieval was performed for both immunostains using Reveal Decloaker RTU solution (Biocare Medical, Concord, CA, USA). The Dako autostainer was used for all immunostains. Expression of p53 proteins in tumor cells was semi-quantitatively scored as 0, no expression; 1, <10%, 2, 11–25%; 3, 26–50%; 4, 51–75%; and 5, >75%. Only tumors scoring 3 or more were considered positive for p53 overexpression. Assessment of β-catenin expression was undertaken as follow: lateral membrane expression (score 0 if present and 1 if absent), cytoplasmic staining (score 0, 1, or 2 for none, weak, and strong expression, respectively), and nuclear expression (score 0, 1, or 2 for none, weak, and strong expression, respectively). Tumors showing a combined score of 4 or 5 were classified as positive altered expression of β-catenin.
Statistical Analysis
We used Pearson’s chi-square test or Fisher’s exact test as appropriate to compare categorical variables. Student’s t-test was used for continuous variables. A two-tailed P value was used for all analyses and a P value of less than 0.05 was used to determine statistical significance. All statistical analyses were performed using SPSS statistics software version 17.0 (SPSS Inc., Chicago, IL).
RESULTS
Characteristics of SP Patients with CRC
Forty-six cases of CRC were identified from 39 patients fulfilling at least WHO criterion 1 or 3 for SPS. One male patient, 19 years old at time of CRC diagnosis, was found to carry an MSH2 mutation carrier, identified after immunohistochemistry demonstrated loss of MSH2/MSH6 expression in tumor cells. This patient also had 28 histologically documented serrated polyps in the large intestine (WHO criterion 3 for SP) and was excluded from the study. No other patient was found to carry a germline mutation in an MMR gene. The final patient cohort comprised 45 CRCs from 38 patients. Twenty-two of these patients overlapped with our previous study which focused on describing the histologic and molecular characteristics of polyps associated with SP individuals (14). The additional 16 patients included in this cohort were newly enrolled individuals or individuals not initially selected due to lack of sufficient information at the time of the previous study. There were 9 patients meeting criterion 1 only, with serrated polyp counts ranging from 6 to 20 (Table 1). The remaining patients had a serrated polyp count between 21 and 109, fulfilling criterion 3. A family history of CRC in first-degree or second degree relatives was reported in 16 of 27 patients (59.3%) for whom this information was available. At least one conventional adenoma was present in 40 patients (88.9%). The mean number of conventional adenomas per patient was 4.7 (standard deviation 3.1). None of the patients was diagnosed with a colonic hamartomatous polyp, making hereditary mixed polyposis syndrome unlikely. The median age of SP patients at diagnosis of first CRC was 58.5 years, ranging from 18 to 76 years. The female to male sex ratio was 2.4:1.
Table 1.
Clinical characteristics of 38 patients with serrated polyposis
| Characteristics | N (%) |
|---|---|
| Age (years) at diagnosis of first CRC | |
| Mean ± SD | 55.7 ± 14.4 years |
| Median, range | 58.5, 18 – 76 years |
| Sex | |
| Male | 11 (28.9) |
| Female | 27 (71.1) |
| WHO criteria | |
| Criterion 1 only | 9 (23.7) |
| Criterion 3 | 29 (76.3) |
| Serrated polyp count, Mean, range | 47.6, 6 – 109 |
| Synchronous CRC | 6 (15.8) |
| Metachronous CRC | 5 (13.2) |
| Family history of CRC | 16/27 (59.3) |
Synchronous CRCs occurred in 4 patients, and 3 patients had metachronous CRC with an interval of 10, 11 and 16 years respectively between both malignancies. Multiple CRCs were reported in 3 additional patients: one patient had a total of 4 carcinomas with a surgical resection for a CRC, followed by 2 synchronous CRCs and then the carcinoma 7 years later that was reviewed and analyzed; one patient had 2 previous CRCs 13 and 19 years before the CRC that was reviewed; and one patient had a synchronous, stage pT1, CRC arising from a sessile serrated adenoma with cytological dysplasia described in the pathology report, for which no tissue was available. Altogether, 10 of 38 patients (26.3%) in this series, comprising 6 females and 4 males, had synchronous or metachronous CRCs. The median interval between metachronous CRC was 10.5 years, ranging from 6 to 13 years. Synchronous CRCs among the 6 patients with a total of 15 tumors were confined to the proximal colon for 11 (73%) and the distal colon for 4 (27%). This distribution was not statistically different from the distribution of non-synchronous CRCs (P=0.52). There was no association between the presence of family history and the occurrence of multiple CRCs.
Pathologic Characteristics of CRC
All 38 patients with CRCs underwent partial or total colectomy. The distribution of the 45 CRCs is represented in figure 1 and table 2. There were 29 CRCs (64%) located in the proximal colon and 16 CRCs in the distal colorectum. As displayed in table 2, the main histologic subtype was adenocarcinoma of no specific type (71.1%), followed by mucinous adenocarcinoma (22.2%) and serrated adenocarcinoma (6.7%). A minor mucinous component (<50% of tumor analyzed) was present in 6 non-mucinous adenocarcinoma, including in the 3 serrated adenocarcinomas. The majority of tumors were of low grade histology (82.2%). Tumor size ranged from 5 to 150 mm. In 6 cases, the CRC size was <10 mm. A contiguous residual polyp was identified in 13 CRCs (28.9%): these comprised 1 tubular adenoma, 3 tubulovillous adenomas, 4 traditional serrated adenomas, and 5 sessile serrated adenomas. Tumor stage, according to the AJCC system 7th edition, was stage 0 for 2, stage I for 15, stage IIA for 15, stage IIIA for 2, stage IIIB for 5, stage IIIC for 2, unknown for 4.
Figure 1.
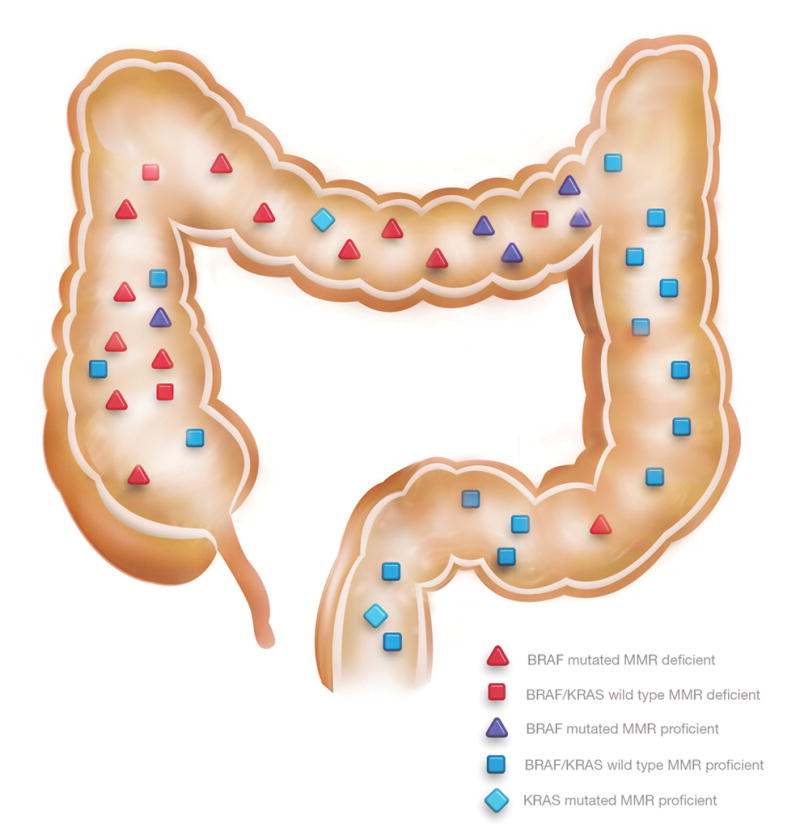
Location of colorectal carcinomas with molecular characteristics
Table 2.
Pathologic characteristics of 45 colorectal carcinomas.
| Characteristics | N (%) |
|---|---|
| Location | |
| Caecum | 5 (11.1) |
| Ascending colon | 12 (26.7) |
| Transverse colon | 12 (26.7) |
| Descending colon | 5 (11.1) |
| Sigmoid colon | 8 (17.8) |
| Rectum | 3 (8.7) |
| Histologic type | |
| Adenocarcinoma | 32 (71.1) |
| Mucinous adenocarcinoma | 10 (22.2) |
| Serrated adenocarcinoma | 3 (8.7) |
| Histologic grade | |
| Low | 37 (82.2) |
| High | 8 (17.8) |
| Size in mm, mean, range | 37.8, 5 – 150 |
| Contiguous polyp present | 13/32 (28.9) |
| Conventional adenoma present | 40 (88.9) |
| Tumor stage | |
| 0 | 2 (4.4) |
| I | 15 (33.4) |
| IIA | 15 (33.4) |
| IIIA | 2 (4.4) |
| IIIB | 5 (11.2) |
| IIIC | 2 (4.4) |
| Unknown | 4 (8.8) |
| Tumor infiltrating lymphocytes | 19 (42.2) |
Molecular phenotype of CRC
In order to classify CRC molecularly, we performed screening for BRAF and KRAS somatic mutations and immunohistochemical analysis for expression of MMR proteins MLH1, PMS2, MSH2 and MSH6. In a subset of tumors (n=25), additional unstained paraffin sections were available to stain for p53 and/or β-catenin. A V600E mutation in BRAF was identified in 18 of 39 CRCs (46.2%) and a KRAS mutation was found in 2 of 40 tumors (5.0%) with sequence variants of c.34G>C and c.35G>A respectively. Loss of immunohistochemical expression of MLH1 and PMS2 proteins was found in 17 of 45 tumors samples (37.8%). Tumors with the BRAF mutation were more likely to have MMR deficiency compared with BRAF-wildtype CRCs (76.5% vs. 14.3%, P<0.001).
Results were available for BRAF, KRAS and MMR testing on 38 tumor samples (Table 3). As displayed in figure 2, CRCs were classified in 5 categories according to their status for combinations of BRAF mutation, KRAS mutation and MMR deficiency. The largest group of tumors (40%) had a BRAF and KRAS wildtype/MMR proficient phenotype, followed by BRAF mutated/MMR deficient phenotype (34%).
Table 3.
Molecular findings stratified by mismatch repair deficiency status and colon location in 38 colorectal carcinomas for which information for all features were available.
| BRAF mutated CRC | KRAS mutated CRC | BRAF/KRAS wildtype CRC | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Prox. colon | Distal colon | Total | Prox. colon | Distal colon | Total | Prox. colon | Distal colon | Total | ||
| MMRD | 11 | 1 | 13 | 0 | 0 | 0 | 3 | 0 | 3 | 16 (42.1%) |
| MMRP | 5 | 0 | 5 | 1 | 1 | 2 | 4 | 12 | 15 | 22 (57.9%) |
|
| ||||||||||
| Total | 16 | 1 | 18 (47.3%) | 1 | 1 | 2 (5.4%) | 7 | 12 | 18 (47.3%) | 38 |
MMRD: Mismatch repair deficient; MMRP: Mismatch repair proficient; Prox.: Proximal; CRC: Colorectal carcinoma.
Figure 2.
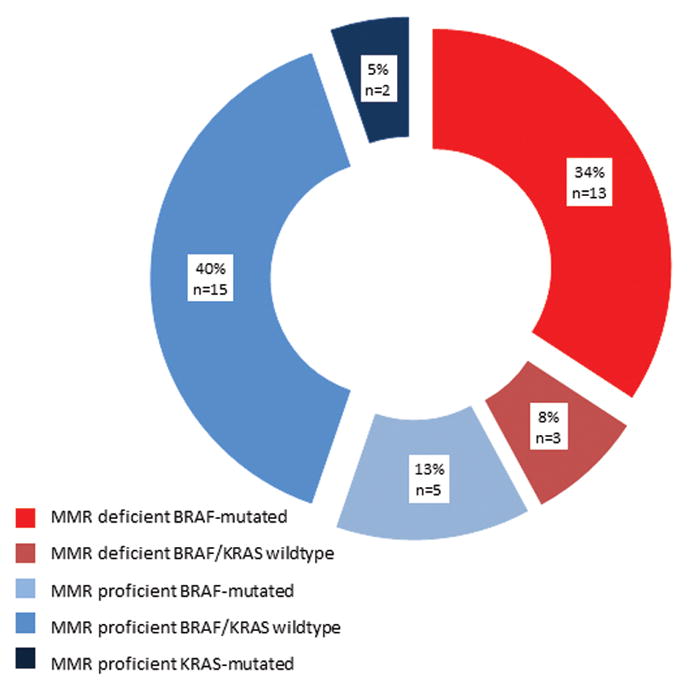
Molecular classification of colorectal carcinomas according to BRAF, KRAS and mismatch repair deficiency status.
Immunohistochemical overexpression of p53 was identified in 8/25 cases (32%), including 2 BRAF-mutated/MMR deficient CRCs, 4 BRAF and KRAS wildtype/MMR proficient CRCs, and 2 MMR proficient CRCs with unknown KRAS or BRAF status. Immunohistochemical evidence of β-catenin activation was identified in 8/24 CRCs (33.3%). Two cases were BRAF-mutated/MMR deficient CRCs (1 with p53 overexpression and 1 with normal p53) and the other 6 were BRAF and KRAS wild-type/MMR proficient CRCs. Combined p53 overexpression and β-catenin activation was present in one BRAF-mutated/MMR deficient CRC and 2 BRAF and KRAS wildtype/MMR proficient CRCs.
Correlation between molecular findings and clinico-pathologic characteristics
A positive correlation was found between BRAF-mutated CRC and female gender (P=0.05), proximal tumor location (P<0.001), mucinous or serrated adenocarcinoma histologic type (P<0.001), and the presence of tumor infiltrating lymphocytes (P=0.04) (Table 4). Similarly, MMR-deficient CRC correlated positively with proximal tumor location (P=0.001), mucinous or serrated adenocarcinoma histologic type (P=0.001), the presence of tumor infiltrating lymphocytes (P<0.001), and lower tumor stage (P=0.02). The BRAF mutation was present in 5/9 (56%) of CRCs with a contiguous serrated polyp and in none of the 4 CRCs with a contiguous conventional adenoma. For 4 patients who developed 2 synchronous CRC, molecular testing was available for all 8 tumours. In 2 patients, both tumors had a similar molecular profile (MMR-proficient/BRAF and KRAS wildtype for both), whereas the synchronous tumors from 2 patients had a different profile: MMR-deficient/BRAF-mutated and MMR-proficient/BRAF-mutated in 1 patient; MMR-deficient/BRAF-mutated and MMR-deficient/BRAF-wild-type in 1 patient.
Table 4.
BRAF and mismatch repair status of colorectal carcinomas according to clinicopathologic features.
| BRAF | Mismatch Repair | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Wildtype | Mutated | P | Proficient | Deficient | P | |
|
| ||||||
| Age (years), mean±SD | 59.5±16.7 | 53.2±11.6 | 0.18 | 51.6±13.8 | 65.8±8.1 | <0.001 |
| Sex | 0.05 | 0.51 | ||||
| Male | 10 | 3 | 10 | 4 | ||
| Female | 11 | 15 | 18 | 13 | ||
| Family history | 0.59 | 0.76 | ||||
| Present | 11 | 8 | 13 | 7 | ||
| Absent | 4 | 6 | 8 | 4 | ||
| Location | <0.001 | 0.001 | ||||
| Proximal | 8 | 17 | 13 | 16 | ||
| Distal | 13 | 1 | 15 | 1 | ||
| Histologic type | <0.001 | 0.001 | ||||
| Adenocarcinoma | 20 | 7 | 25 | 7 | ||
| Mucinous and serrated adenocarcinoma | 1 | 11 | 3 | 10 | ||
| Histologic grade | 0.11 | 0.45 | ||||
| Low | 19 | 12 | 24 | 13 | ||
| High | 2 | 6 | 4 | 4 | ||
| Tumour infiltrating lymphocytes | 0.04 | <0.001 | ||||
| Present | 6 | 11 | 4 | 15 | ||
| Absent | 15 | 7 | 24 | 2 | ||
| Stage | 0.40 | 0.02 | ||||
| 0–I | 9 | 5 | 13 | 4 | ||
| IIA | 5 | 8 | 5 | 10 | ||
| IIIA-B-C | 5 | 4 | 7 | 2 | ||
The characteristics of CRCs showing a contiguous residual polyp are displayed in table 5. While the numbers are small for statistical analysis, CRCs with an adjacent traditional serrated adenoma or a sessile serrated adenoma demonstrated more frequent mucinous differentiation, BRAF mutation and MMR deficiency.
Table 5.
Characteristics of the 13 colorectal carcinomas with a contiguous residual polyp
| Polyp type | Proximal colon location | Histologic type | BRAF mutation | KRAS mutation | MMR deficiency | Others |
|---|---|---|---|---|---|---|
| Tubular or tubulovillous adenoma (n=4) | 4/4 (100) | 4 AC | 0/4 (0) | 0/3 (0) | 0/4 (0) | 0/1 β-catenin +; 0/1 p53+ |
| Traditional serrated adenoma (n=4) | 3/4 (75) | 3 MC; 1 AC | 3/4 (75) | 0/4 (0) | 2/4 (50) | 0/2 β-catenin +; 0/2 p53+ |
| Sessile serrated adenoma (n=5) | 4/5 (80) | 2 AC, 2 MC, 1 SC | 3/4 (75) | 0/4 (0) | 3/5 (60) | 2/3 β-catenin +; 1/4 p53+ |
MMR: Mismatch repair; AC: Adenocarcinoma of no specific type; MC: Mucinous carcinoma; SC: Serrated carcinoma
DISCUSSION
An increased risk of CRC is seen in individuals with SP, and has been associated with the presence of conventional adenoma (7, 8, 13, 14). Therefore, in this study, we sought to understand the developmental sequence of the CRC observed in SP patients. The reported incidence of CRC from published series of SP patients (>20 patients) varies from 14% to 54% (7, 9, 12, 14, 20–22). Whilst this range is wide, even at its lower limit, it represents a significant increase in risk over the general population. The differences between series may be at least partially explained by the type of population studied. The lowest incidence has been reported from unselected population cohorts while the highest incidence has been found from cohorts of patients referred to genetic clinics. The latter may represent a recruitment bias for patients with strong family history and CRC at time of polyposis diagnosis. However, the actual risk of CRC in SP patients is still uncertain for several reasons. Firstly, the prevalence of SP in the general population is unknown, and likely to be under-recognized, as most patients with uncomplicated colorectal serrated polyps, even if multiple, may remain asymptomatic. In a large population-based screening trial of asymptomatic patients aged between 55–64 years, SP was prospectively identified at a rate of 1 in 3,000 individuals (23). However, most published series of SP patients reported a broad range of age distribution (17 to 85 years) and a mean age at SP diagnosis of between 47.7 years to 56 years (7–9, 11, 12). Therefore, it is possible that the prevalence of SP in the general asymptomatic population, that is including those above and below 55–64 years of age, may be higher than 1 in 3,000 currently reported. Moreover, it has recently been shown SP is under-diagnosed due to lack of awareness of the syndrome among gastroenterologists and surgical pathologists (24, 25). Secondly, a large proportion of SP patients are likely to have a first diagnosis of SP at the time of presentation with CRC and this will precipitate referral to genetics clinics, thereby inflating the reported rate of CRC from this biased population. Finally, it is difficult to identify patients with SP and conduct prospective studies without a genetic alteration to define this condition. While the genetic basis for SP has yet to be discovered, a body of evidence suggests an inherited genetic basis: common ethnicity background found in many SP patients with a northwestern European ancestry (10, 11, 22), frequent familial clustering of SP individuals (11, 12, 21, 26), and the recently described increased risk of colorectal and pancreatic cancers in first-degree and second-degree relatives of SP patients (13).
Studying SP patients referred to genetics clinics allows researchers to collect a unique cohort of symptomatic SP patients with CRC. In this study, we aimed to understand the origin of CRC arising in SP patients and selected individuals with SP who likely represented the most severe clinical phenotype. This allowed us to readily obtain a large number of individuals with SP and CRC, which would not be available in a population study.
The rate of BRAF mutation in SP CRC (46.2%) was higher than the mutation rate reported in CRC from the general population (10–20%) (27–29). As previously reported for population studies, BRAF-mutated CRCs in SP were associated with female gender, proximal colonic location, mucinous histological subtype and presence of tumor infiltrating lymphocytes (30, 31). In contrast, the rate of KRAS mutation was considerably lower than that seen in the general population (5% vs >30%). However, the majority of CRC in SP patients were wildtype for both BRAF and KRAS. Moreover, we found molecular alterations commonly associated with the traditional adenoma carcinoma pathway in 13 CRCs demonstrating immunohistochemical overexpression of p53 and/or evidence of β-catenin activation. These results are consistent with Boparai et al. (32) who reported in a series of 19 CRCs from SP patients BRAF mutation in 53%, β-catenin activation in 26% and p53 overexpression in 42%. Though the expression of β-catenin has been previously reported in serrated polyps (33), it was always associated with BRAF mutation. In our study, however, activation of β-catenin was found in CRCs with or without BRAF mutation.
These findings demonstrate that a large proportion of CRC from SP patients do not develop through the canonical serrated neoplasia pathway (driven by BRAF mutation) but show various molecular phenotypes including those more likely to be associated with the common CRC traditional adenoma pathway. Though KRAS mutation was rare in this series, these few may represent fusion pathway CRCs (30). This poorly recognized subgroup of CRCs is reportedly associated with low levels of CIMP and MSI, downregulation of MGMT by methylation and frequent KRAS mutation (16, 30). Pai et al. described a new polyp entity, the so-called atypical conventional adenoma, found in individuals who also have at least one sessile serrated adenoma (34). These polyps share some morphological characteristics with serrated polyps and were all BRAF/KRAS wild-type. This polyp subtype may possibly be the precursor lesion of the vast majority of CRCs in SP patients. Subsequent morphologic studies should be undertaken to test this hypothesis.
Sessile serrated adenomas are characterized by a high rate of BRAF mutation, up to 95% (35), and are likely to be the precursor lesion of most CRCs evolving through the serrated neoplasia pathway. In our series, 5 of the 9 CRCs (55.5%) with a residual contiguous serrated polyp harbored the V600E mutation in BRAF. We previously showed that the presence of conventional colorectal adenomas in SP was associated with the occurrence of CRC, suggesting that at least some CRC may develop from conventional adenomas (9, 14). In our current series, the majority of patients (88.9%) had at least one conventional adenoma identified separate from the CRC. However, only 4 CRCs had a residual contiguous conventional adenoma, a relatively low number, possibly secondary to overgrowth of the precursor polyp by the carcinoma in some cases or insufficient tumor sampling. This suggests that CRC in SP patients may develop from non-serrated polyps through either a derivative of the traditional adenoma pathway. SP could therefore, potentially, be considered a disorder associated with a hypermature or inappropriately aged colonic mucosa, possibly secondary to an alteration in DNA methylation, with a resultant propensity to the development of early onset multiple serrated polyps and conventional adenomas, as described by Pai et al. (34). The molecular subtype of CRC which may develop in SP patients will reflect the histologic type of the precursor polyp, with a skewed distribution toward a higher frequency of BRAF-mutated serrated pathway CRC compared to the general population where serrated polyps are not as common. We also demonstrated heterogeneity of CRC within a single patient who had synchronous CRCs showing different molecular profiles.
Another important finding of our study was the frequent occurrence of multiple CRCs identified in 26.3% of SP patients. One or multiple metachronous CRCs were present in 13.2% of patients who have had initial segmental resections, with a median interval of 10.5 years between CRC diagnoses. In general, patients with a diagnosis of CRC are at increased risk of developing metachronous CRCs in comparison with the general population (standardized incidence ratio 1.3, 95% CI 1.1–1.5) (36). Fante et al. reported that 7% of all CRCs from a CRC registry were multiple (37). Metachronous CRCs were present in 1.5% of patients with a higher prevalence in Lynch patients compared to non-Lynch patients (5.8% vs. 1.3%). Synchronous CRCs were reported in 2.5% of all patients. Multiple CRCs have been described from the outset in SP-associated CRC (38, 39). Our data provides additional evidence that, as in other high risk CRC syndromes (40), when SP patients present with CRC, more extensive colonic resection should be considered for both the subsequent risk of metachronous cancer and future control of polyps. This is particularly important because several studies have reported that a high proportion of interval CRCs, defined as CRCs diagnosed within 5 years of a complete colonoscopy, are serrated neoplasia pathway CRCs (41, 42). This may be explained by the lower polyp detection rate reported for right-sided serrated polyps (43) or by the rapid progression of sessile serrated adenomas with dysplasia to adenocarcinoma, even for a polyp <10 mm in size (44, 45). As nearly half of the CRCs in SP patients are serrated neoplasia pathway CRCs, they would be anticipated to have a significant risk of interval cancers. Boparai et al. reported 5 CRCs in SP patients diagnosed at follow-up intervals as short as 1–2 years (8). Moreover, not uncommonly the invasive component was found in serrated polyps <10 mm in size. In our series, 6 of the CRC (13.3%) were <10 mm in size, thus supporting consideration of extensive colectomy when SP patients present with newly diagnosed CRCs. With multiple polyps and potentially unachievable colonoscopic removal of all polyps >5 mm, an alternative, as previously suggested (46), is referral to a specialist tertiary center for endoscopic surveillance, with advanced imaging techniques, every 6–12 months (12, 47, 48).
As a retrospective study from multiple genetic clinics, our study has several limitations. Patients in our cohort likely represent the most severe phenotype of this heterogeneous polyposis syndrome with high polyp burden, strong family history and multiple CRCs. The availability of uneven tissue sections and varied quality of tumor DNA limited our ability to perform a wider range of molecular testing on all samples. Furthermore, until a genetic presymptomatic, predictive, test is available, clinical studies of SP, with adequate numbers of cases, will continue to be limited by retrospective recruitment bias. In summary, we report the largest series of CRCs diagnosed in the context of SP patients. These tumors are characterized by diversity in their molecular alteration profiles, encompassing features of at least serrated neoplasia pathway, and the traditional adenoma pathway. In addition, residual polyp histology showed lesions of both pathways in equal measure. These results suggest that serrated polyps are not the only precursor lesions of CRC in SP patients, and the role of atypical conventional adenomas requires consideration. Following segmental colonic resection for CRC in SP patients, annual colonoscopic surveillance to remove all polyps > 5mm should be considered to reduce the risk of metachronous and interval CRCs. Advanced imaging techniques, though not routinely available (49), may aid polyp detection (50). If endoscopic control of polyps is not achievable and sessile serrated adenomas with cytological dysplasia or conventional adenomas are present, subtotal colectomy should be considered (12).
Acknowledgments
Funding: This work was supported by National Cancer Institute, National Institutes of Health under 1R01CA123010 (Genetics of Serrated Neoplasia) and RFA # CA-95-011 and through cooperative agreements with the Australasian Colorectal Cancer Family Registry (U01 CA097735). During this work, JY was a Cancer Council Queensland Senior Research Fellow. CR is a Jass Pathology Fellow. MJ is a NHMRC Senior Research Fellow and JLH is an Australian fellow of the NHMRC.
The authors thank all study participants of the Australasian Colon Cancer Family Registry and Study Co-ordinator Judi Maskiell, Data Managers Erika Pavluk, David Packenas, Belinda Nagler and Diane McKeone, and participant interviewers for their contributions to this project. The authors also acknowledge the contributions of the late Professor Jeremy Jass to the study including performing pathology reviews for cases.
Footnotes
Conflicts of interest: Authors declare no conflict of interest
References
- 1.Taylor DP, Burt RW, Williams MS, et al. Population-based family history-specific risks for colorectal cancer: a constellation approach. Gastroenterology. 2010;138:877–885. doi: 10.1053/j.gastro.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. 2011 Available at: http://www.dietandcancerreport.org/
- 3.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 4.Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42:216–227. doi: 10.1016/j.ejca.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Jass JR. Colorectal polyposes: from phenotype to diagnosis. Pathol Res Pract. 2008;204:431–447. doi: 10.1016/j.prp.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Jaeger E, Leedham S, Lewis A, et al. Hereditary mixed polyposis syndrome is caused by a 40-kb upstream duplication that leads to increased and ectopic expression of the BMP antagonist GREM1. Nat Genet. 2012;44:699–703. doi: 10.1038/ng.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boparai KS, Reitsma JB, Lemmens V, et al. Increased colorectal cancer risk in first-degree relatives of patients with hyperplastic polyposis syndrome. Gut. 2010;59:1222–1225. doi: 10.1136/gut.2009.200741. [DOI] [PubMed] [Google Scholar]
- 8.Boparai KS, Mathus-Vliegen EM, Koornstra JJ, et al. Increased colorectal cancer risk during follow-up in patients with hyperplastic polyposis syndrome: a multicentre cohort study. Gut. 2010;59:1094–1100. doi: 10.1136/gut.2009.185884. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan DD, Sweet K, Drini M, et al. Risk factors for colorectal cancer in patients with multiple serrated polyps: a cross-sectional case series from genetics clinics. PLoS ONE. 2010;5:e11636. doi: 10.1371/journal.pone.0011636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchanan DD, Sweet K, Drini M, et al. Phenotypic diversity in patients with multiple serrated polyps: a genetics clinic study. Int J Colorectal Dis. 2010;25:703–712. doi: 10.1007/s00384-010-0907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalady MF, Jarrar A, Leach B, et al. Defining phenotypes and cancer risk in hyperplastic polyposis syndrome. Dis Colon Rectum. 2011;54:164–170. doi: 10.1007/DCR.0b013e3181fd4c15. [DOI] [PubMed] [Google Scholar]
- 12.Edelstein DL, Axilbund JE, Hylind LM, et al. Serrated polyposis: rapid and relentless development of colorectal neoplasia. Gut. 2012 doi: 10.1136/gutjnl-2011-300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Win AK, Walters RJ, Buchanan DD, et al. Cancer risks for relatives of patients with serrated polyposis. Am J Gastroenterol. 2012;107:770–778. doi: 10.1038/ajg.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosty C, Buchanan DD, Walsh MD, et al. Phenotype and polyp landscape in serrated polyposis syndrome: a series of 100 patients from genetics clinics. Am J Surg Pathol. 2012;36:876–882. doi: 10.1097/PAS.0b013e31824e133f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jass JR, Whitehall VL, Young J, et al. Emerging concepts in colorectal neoplasia. Gastroenterology. 2002;123:862–876. doi: 10.1053/gast.2002.35392. [DOI] [PubMed] [Google Scholar]
- 16.Leggett B, Whitehall V. Role of the Serrated Pathway in Colorectal Cancer Pathogenesis. Gastroenterology. 2010;138:2088–2100. doi: 10.1053/j.gastro.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 17.Snover DC, Ahnen DJ, Burt RW, et al. Serrated polyps of the colon and rectum and serrated polyposis. In: Bosman FT, Carneiro F, Hruban RH, et al., editors. WHO Classification of Tumours of the Digestive System. Lyon, France: IARC Press; 2010. pp. 160–165. [Google Scholar]
- 18.Walsh MD, Cummings MC, Buchanan DD, et al. Molecular, pathologic, and clinical features of early-onset endometrial cancer: identifying presumptive Lynch syndrome patients. Clin Cancer Res. 2008;14:1692–1700. doi: 10.1158/1078-0432.CCR-07-1849. [DOI] [PubMed] [Google Scholar]
- 19.Newcomb PA, Baron J, Cotterchio M, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2331–2343. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 20.Carvajal-Carmona LG, Howarth KM, Lockett M, et al. Molecular classification and genetic pathways in hyperplastic polyposis syndrome. J Pathol. 2007;212:378–385. doi: 10.1002/path.2187. [DOI] [PubMed] [Google Scholar]
- 21.Chow E, Lipton L, Lynch E, et al. Hyperplastic polyposis syndrome: phenotypic presentations and the role of MBD4 and MYH. Gastroenterology. 2006;131:30–39. doi: 10.1053/j.gastro.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 22.Yeoman A, Young J, Arnold J, et al. Hyperplastic polyposis in the New Zealand population: a condition associated with increased colorectal cancer risk and European ancestry. N Z Med J. 2007;120:U2827. [PubMed] [Google Scholar]
- 23.Lockett MJ, Atkin WS. Hyperplastic polyposis: prevalence and cancer risk. Gut. 2001;48:A4. [Google Scholar]
- 24.Crowder CD, Sweet K, Lehman A, et al. Serrated Polyposis Is an Underdiagnosed and Unclear Syndrome: The Surgical Pathologist has a Role in Improving Detection. Am J Surg Pathol. 2012;36:1178–1185. doi: 10.1097/PAS.0b013e3182597f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vemulapalli KC, Rex DK. Failure to recognize serrated polyposis syndrome in a cohort with large sessile colorectal polyps. Gastrointest Endosc. 2012;75:1206–1210. doi: 10.1016/j.gie.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 26.Buchanan DD, Roberts A, Walsh MD, et al. Lessons from Lynch syndrome: a tumor biology-based approach to familial colorectal cancer. Future Oncol. 2010;6:539–549. doi: 10.2217/fon.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dahlin AM, Palmqvist R, Henriksson ML, et al. The Role of the CpG Island Methylator Phenotype in Colorectal Cancer Prognosis Depends on Microsatellite Instability Screening Status. Clin Cancer Res. 2010;16:1845–1855. doi: 10.1158/1078-0432.CCR-09-2594. [DOI] [PubMed] [Google Scholar]
- 28.English DR, Young JP, Simpson JA, et al. Ethnicity and risk for colorectal cancers showing somatic BRAF V600E mutation or CpG island methylator phenotype. Cancer Epidemiol Biomarkers Prev. 2008;17:1774–1780. doi: 10.1158/1055-9965.EPI-08-0091. [DOI] [PubMed] [Google Scholar]
- 29.Ogino S, Shima K, Meyerhardt JA, et al. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: results from intergroup trial CALGB 89803. Clin Cancer Res. 2012;18:890–900. doi: 10.1158/1078-0432.CCR-11-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 31.Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–6069. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 32.Boparai KS, Dekker E, Polak MM, et al. A serrated colorectal cancer pathway predominates over the classic WNT pathway in patients with hyperplastic polyposis syndrome. Am J Pathol. 2011;178:2700–2707. doi: 10.1016/j.ajpath.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yachida S, Mudali S, Martin SA, et al. Beta-catenin nuclear labeling is a common feature of sessile serrated adenomas and correlates with early neoplastic progression after BRAF activation. Am J Surg Pathol. 2009;33:1823–1832. doi: 10.1097/PAS.0b013e3181b6da19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pai RK, Mackinnon AC, Joseph L, et al. Identification of histologically distinct conventional adenomas that arise predominately in patients with sessile serrated adenomas. Am J Surg Pathol. 2010;34:355–363. doi: 10.1097/PAS.0b013e3181c6b9dd. [DOI] [PubMed] [Google Scholar]
- 35.Rosty C, Parry S, Young JP. Serrated polyposis: an enigmatic model of colorectal cancer predisposition. Patholog Res Int. 2011;2011:157073. doi: 10.4061/2011/157073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulder SA, Kranse R, Damhuis RA, et al. The Incidence and Risk Factors of Metachronous Colorectal Cancer: An Indication for Follow-up. Dis Colon Rectum. 2012;55:522–531. doi: 10.1097/DCR.0b013e318249db00. [DOI] [PubMed] [Google Scholar]
- 37.Fante R, Roncucci L, Di Gregorio C, et al. Frequency and clinical features of multiple tumors of the large bowel in the general population and in patients with hereditary colorectal carcinoma. Cancer. 1996;77:2013–2021. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2013::AID-CNCR8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 38.Jass JR, Cottier DS, Pokos V, et al. Mixed epithelial polyps in association with hereditary non-polyposis colorectal cancer providing an alternative pathway of cancer histogenesis. Pathology. 1997;29:28–33. doi: 10.1080/00313029700169494. [DOI] [PubMed] [Google Scholar]
- 39.Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110:748–755. doi: 10.1053/gast.1996.v110.pm8608884. [DOI] [PubMed] [Google Scholar]
- 40.Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut. 2011;60:950–957. doi: 10.1136/gut.2010.228056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 2010;105:1189–1195. doi: 10.1038/ajg.2009.699. [DOI] [PubMed] [Google Scholar]
- 42.Bressler B, Paszat LF, Chen Z, et al. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96–102. doi: 10.1053/j.gastro.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 43.Kahi CJ, Hewett DG, Norton DL, et al. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–46. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein NS. Small colonic microsatellite unstable adenocarcinomas and high-grade epithelial dysplasias in sessile serrated adenoma polypectomy specimens: a study of eight cases. Am J Clin Pathol. 2006;125:132–145. [PubMed] [Google Scholar]
- 45.Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology. 2005;47:32–40. doi: 10.1111/j.1365-2559.2005.02180.x. [DOI] [PubMed] [Google Scholar]
- 46.Young JP, Parry S. Risk factors: Hyperplastic polyposis syndrome and risk of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2010;7:594–595. doi: 10.1038/nrgastro.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.East JE, Saunders BP, Jass JR. Sporadic and syndromic hyperplastic polyps and serrated adenomas of the colon: classification, molecular genetics, natural history, and clinical management. Gastroenterol Clin North Am. 2008;37:25, 46, v. doi: 10.1016/j.gtc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 48.Lynch PM. Hyperplastic polyposis: semantics, biology, and endoscopy. Gut. 2010;59:1019–1021. doi: 10.1136/gut.2009.195032. [DOI] [PubMed] [Google Scholar]
- 49.Burke CA, Snover DC. Editorial: sessile serrated adenomas and their pit patterns: we must first see the forest through the trees. Am J Gastroenterol. 2012;107:470–472. doi: 10.1038/ajg.2011.468. [DOI] [PubMed] [Google Scholar]
- 50.Boparai KS, van den Broek FJ, van Eeden S, et al. Increased polyp detection using narrow band imaging compared with high resolution endoscopy in patients with hyperplastic polyposis syndrome. Endoscopy. 2011;43:676–682. doi: 10.1055/s-0030-1256447. [DOI] [PubMed] [Google Scholar]


